Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5760
Research Article(ISSN: 2690-5760) 
A-28-Years-Old Immunocompetent Man Having Post Prosthetic Aortic Valve Infective Endocarditis with Severe Aortic Regurgitation Due to Multi-Drug Resistant Burkholderia Cepacia: Lessons Learned Volume 5 - Issue 4
Khin Phyu Pyar1*, Kyaw Thura2, Min Ko Ko Tun3, Ye Ko Ko Htet3, Myo Min Aung4, Min Hein Myint Shein5, Thant Zaw Maung6, Aung Zaw Htet7, Kyaw Thurein Lwin7, Zaw Min Htike8, Nyan Naing Soe9 and Ye Min Thu9
- 1Professor and Head/ Senior Consultant Physician and Nephrologist, Department of Medicine/Nephrology, Defence Services Medical Academy/ No. (1) Defence Services General Hospital (1000-Bedded), Yangon, Myanmar.
- 2Senior Consultant Cardiovascular Surgeon, Department of Cardiovascular Surgery, No. (1) Defence Services General Hospital (1000-Bedded), Yangon, Myanmar
- 3Consultant Cardiovascular Surgeon, Department of Cardiovascular Surgery, No. (1) Defence Services General Hospital (1000-Bedded), Yangon, Myanmar.
- 4Consultant Anesthetist, Department of Anaesthesia, No. (1) Defence Services General Hospital (1000-Bedded), Yangon, Myanmar.
- 5Senior Perfusionist, Department of Anaesthesia, No. (1) Defence Services General Hospital (1000-Bedded), Yangon, Myanmar
- 6Senior Consultant Cardiologist, Department of Cardiology, No. (1) Defence Services General Hospital (1000-Bedded), Yangon, Myanmar.
- 7Senior Consultant Nephrologist, Department of Nephrology, No. (1) Defence Services General Hospital (1000-Bedded), Yangon, Myanmar
- 8Consultant Microbiologist, Department of Microbiology, No. (1) Defence Services General Hospital (1000-Bedded), Yangon, Myanmar
- 9Consultant Physician/Lecturer, Department of Medicine, Defence Services Medical Academy, Yangon, Myanmar.
Received: May 24, 2024; Published: June 12, 2024
Corresponding author: Khin Phyu Pyar, Professor and Head/ Senior Consultant Physician and Nephrologist, Department of Medicine/ Nephrology, Defence Services Medical Academy/ No. (1) Defence Services General Hospital (1000-Bedded), Yangon, Myanmar.
DOI: 10.32474/JCCM.2023.05.000217
Case summary
A-28-years-old immunocompetent man had prolonged fever for two months. He had prosthetic valve replacement to aortic valve and mitral valve repair (ring annuloplasty) for rheumatic mixed aortic and mitral valve disease (severe aortic regurgitation and mitral regurgitation); and, surgical closure to congenital ventricular septal defect 5 years ago. He was treated as infective endocarditis with ceftazidime and Pipercillin/ Tazobactam according to first blood culture and sensitivity which revealed Acinectobacter spp. For para-valvular leakage in aortic valve and failed medical treatment, previous prosthetic aortic valve was replaced again with mono-leaflet mechanical valve. Second blood culture revealed Burkholderia cepacia, resistance to all antibiotics (quinolone, cephalosporin, aminoglycosides, trimethoprim- sulphamethoxazole, tigecycline and colistin). Autopsy findings were reported.
Keywords:infective endocarditis; Burkholderia cepacian; prosthetic aortic valve; mitral valve repair; ventricular septal defect
Introduction
Infective endocarditis is a life-threatening microbial infection of native and prosthetic heart valves, endocardial surface, and/ or indwelling cardiac device. Despite technological advances, the prevalence of infective endocarditis is increasing; and its mortality has not significantly improved.
Burkholderia cepacia is a rare cause of infective endocarditis. Burkholderia cepacia prosthetic valve endocarditis (PVE) is extremely rare, with few cases in the literature [1].
Case presentation
A-28-years-old man had prolonged fever for two months. He suffered central chest pain at the peak of fever; he also had dyspnea on exertion and palpitation. He had surgical aortic valve replacement (SAVR) (mechanical) for severe aortic regurgitation (rheumatic etiology); mitral valve repair (ring annuloplasty) for rheumatic mitral regurgitation; and, surgical closure to congenital ventricular septal defect 5 years ago. There was no history of orthopnea, paroxysmal nocturnal dyspnea and dyspnea at rest. There was no recent surgery or dental procedure. As his nasopharyngeal swab for SARSCoV-2 was positive, he received remdesivir for 5 days.
He continued to have undue tachycardia and fever; temperature 101°F; tachycardia 106/minutes; blood pressure 110/60 mmHg; wide pulse pressure; SaO2 96% on air; apex beat displaced and heaving; and, early diastolic murmur was heard in the aortic area.
Transthoracic echocardiogram revealed severe aortic regurgitation, prosthetic aortic valve was functioning well; vegetation (0.5 x 0.9 cm) was found; para-valvular leakage was seen; and, LVEF was 66%. Blood culture done on Day ‘0’ showed Acinectobacter spp; and sensitive to Ceftazidine, Cotrimazole, Pipercillin/ Tazobactam. Vigorous medical treatment was given according to blood culture report.
Regarding the progress of disease, temperature was swinging; the patient was unwell; and, serum creatinine was rising. Therefore, consultation with nephrologist was done; he suggested conservative management. Two sets of blood culture were repeated on Day ‘6’ and Day ‘24’; and, they were sent to two separate microbiology laboratories. And both were sterile.
In view of refractory fever, para-valvular leakage of aortic prosthetic valve with vegetation, severe aortic regurgitation and sterile culture, previous prosthetic aortic valve was replaced on Day ‘42’. The endothelial surface of aorta was normal. Valve dehiscence was found at NCC and RCC annular area measuring 30 % of the circumference. There was no vegetation over the previous prosthetic aortic valve. Old prosthetic valve was excised and removed. Calcified debris were removed too. Then, it was replaced with mono-leaflet mechanical valve (TTK Chitra 25 mm).
Hemodynamic status was stable during surgery as well as post-operative period. However, the patient continued to have fever with chills and rigors. Blood culture done on Day ‘46’ showed Burkholderia cepacia, it was resistance to all antibiotics (quinolone, cephalosporin, aminoglycosides, trimethoprim-sulfamethoxazole trimethoprim- sulfamethoxazole, tigecycline and colistin. Blood cultures on Day ‘48’and Day ‘54’ did not isolate organism.
Fever was swinging. One week after surgery, he had febrile fits; and, temperature was 104°F. He was managed with multidisciplinary team- cardiac surgeon, cardiologist, intensive care physician and neurologist. Non-contrast CT Head was normal. Blood biochemistry was normal.
Echocardiogram showed as follows: LVEF was 55%; left ventricular hypertrophy; moderate to severe mitral regurgitation; prosthetic aortic valve was functioning well; no leakage; no vegetation; and, no pericardial effusion.
Urine culture report Day ‘48’ isolated Candida Albican; the duration of indwelling urinary catheter was only five days. Blood culture done on Day ‘57’ showed growth of Enterococcus spp; they were sensitive to ampicillin and levofloxacin. Therefore, levofloxacin was added. In spite of all efforts, the patient was deteriorating; hemoglobin dropped. Two units of packed cell was transfused.
On Day ‘62’, the patient suffered sudden onset of excruciating central chest pain, palpitation and dyspnea. It was followed by fit attack; and, he became unconscious suddenly. Advance cardiopulmonary resuscitation was done; nonetheless, the patient did not recover. The immediate cause of death was possibly due to ventricular tachyarrhythmia; it provoked non-coronary angina.
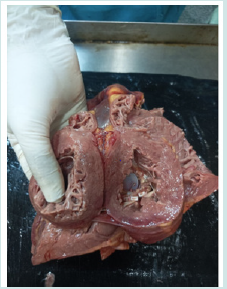
Autopsy revealed a large area of ecchymosis over pericardium as shown in photo (1) Figure 1; it was related with cardiopulmonary resuscitation. Photo (2) Figure 2 demonstrates mitral valve; no vegetation. Photo (3) Figure 3 shows intact aortic prosthetic valve. Autopsy of brain, kidneys and coronary ostia were normal.
Discussion
Prosthetic valve endocarditis (PVE) is one type of infectious endocarditis (IE); it accounts for 20% of all cases of endocarditis. Epidemiology of the aortic PVE is different if the valve replacement was done surgically (surgical aortic valve replacement SAVR) or transcatheter aortic valve replacement (TAVR). In SAVR, the rate of incidence of PVE is 6 per 1000 cases. The rate of PVE is higher in patients who had bioprosthetic SAVR than patients with mechanical SAV. This patient had SAVR (mechanical) five years ago and he had good immunity; one reason for case reporting Table 1.
It is important to get an early diagnosis and treatment in PVE. This patient came to hospital late; only after two months history of fever. It may be one reason for having complications and mortality. Both echocardiographic and intraoperative findings pointed out poorly functioning prosthetic mechanical aortic valve; para-valvular leakage in transthoracic echocardiogram and prosthetic mechanical aortic valve dehiscence occupying 30 % of the circumference in intraoperative findings. As the patient had surgery 5 years ago and he was doing well till 2 months prior to admission, aortic valve dysfunction might be related with infective endocarditis. If the patient came early, one week after onset of fever, his prosthetic valve would not be damaged.
In the diagnosis of infective endocarditis, positive blood cultures are crucial; demonstrating the presence of bacteria [2]. At least two positive blood cultures are mandatory; they must be taken within at least 12-hour interval. Furthermore, the microorganisms identified in the culture must be typically known to cause infective endocarditis. Infective endocarditis is an infection of the cardiac native or prosthetic valves commonly caused by Staphylococcus aureus, viridans streptococci group, and coagulase-negative staphylococci [3,4]. In this patient, blood culture done outside hospital on Day ’0’ showed Acinectobacter spp and sensitive to Ceftazidine, Cotrimazole, Pipercillin/ Tazobactam. Therefore, he was treated with Ceftazidine, and Pipercillin/ Tazobactam. This patient had serial blood culture; they did not grow common organism. In this patient, Acinectobacter spp was grown in Day ‘0’; Enterococcus spp was detected in Day ‘57’; and, Burkholderia cepacia was demonstrated in Day ‘46’. Infective endocarditis is typically caused by Staphylococcus aureus, viridans streptococci group, and coagulase-negative staphylococci. Staphylococci, streptococci, and enterococci bacterial species are estimated to account for ∼80% of all cases [5]. It is another reason for case reporting a case of IE with unusual organism.
Risk factors for IE include congenital heart disease, structural and valvular heart disease, implantation of prosthetic heart valves, and intravenous drug abuse [6]. This patient had open cardiac surgery at 3 areas of heart 5 years ago: repair of ventricular septal defect, mitral valvuloplasty for rheumatic mitral regurgitation and aortic prosthetic valve replacement for severe aortic regurgitation. He recovered well in previous surgery; he only had fever 2 months prior to admission to hospital. There was limited report on patient who developed IE several years after repair for both congenital and acquired (valvular) heart disease. He had clinical evidence as well as echocardiographic evidence of IE. This is another reason for sharing experience.
The blood culture was sterile on two different timing in this patient; Day ‘6’ and Day ‘24’. According to Brouqui & Raoult, the chances of false negative blood culture results ranges from 2.5 to 31% [7]. False negative test results may be due to the followings: (1) use of antibiotic therapy prior to blood sample collection; (2) bacteria that are difficult to culture like Coxiella Burnetii; (3) fungal infective endocarditis [8]. Negative blood cultures are found in approximately 24% of all cases of infective endocarditis; that of prosthetic valve endocarditis is 23% [9,10]. Therefore, having negative blood culture in this patient in Day ‘6’ and Day ‘24’may be due to the use of antibiotic therapy prior to blood sample collection. We should have done serological testing [2] and tissue cultures during cardiac surgery in this case.
Clinically the patient was having fever, undue tachycardia, unexplained anemia and rising creatinine although his blood culture was sterile. Then, the treating team decided to remove and replace poorly functioning prosthetic valve with vegetation. Therefore, clinical parameters are paramount important in assessing the response to treatment in infective endocarditis. This is the lesson we would like to share.
In this patient, blood culture was done 5 times within hospital stay (62 days). Among them, blood culture was positive in 3 different sets done at Day’0’, Day’46’ and Day’57’. The organisms were as follows: Acinectobacter spp in Day ‘0’; Enterococcus spp in Day ‘57’; and, Burkholderia cepacia in Day ‘46’. Burkholderia cepacia is known to be multi-drug resistant bugs; and, is rarely found in infective endocarditis. However, Burkholderia cepacia prosthetic valve endocarditis in a 38-year-old man with poor dentition and a history of intravenous drug use and mitral valve replacement was reported [11,12]. Moreover, Burkholderia cepacia-induced native valve endocarditis in patient without any predisposing factors was mentioned in rare reports [13,14]. In addition, Falcão Pedrosa Costa et al described Burkholderia cepacia-induced infective endocarditis in a renal transplant patient; it was associated with an intracardiac fragment of a catheter inserted 16 years before [15]. This patient had IE due to multi-drug resistant Burkholderia cepacia [16-19]; anti-microbial resistant lead to fatal sequelae. It was reported that endocarditis due to Burkholderia cepacia was rare but serious, leading to valve dysfunction and heart failure [19].
Another point to discussed is that ‘Is Redo prosthetic aortic valve indicated?’. Early surgical intervention for PVE is indicated if the patient develops heart failure due to valve dehiscence, the development of intracardiac fistula, or the presence of severe prosthetic valve dysfunction. Therefore, ‘Redo’ prosthetic aortic valve was not wrong. The patient’s hemodynamic status was stable during and immediately after cardiac surgery.
Two sets of blood cultures on different days, Day ‘6’ and Day ‘24’, at two different laboratories were sterile. Nonetheless, the patient was not responding clinically; fever was swinging; undue tachycardia persisted; anemia increasing. Therefore, the prosthetic aortic valve was replaced again with mono-leaflet mechanical valve on Day ‘42’. The blood culture on Day ‘46’ showed Burkholderia cepacia; it was resistance to all antibiotics (quinolone, cephalosporin, aminoglycosides, trimethoprim- sulfamethoxazole, tigecycline and colistin). Although blood cultures on Day ‘48’ & Day ‘54’ were sterile, the clinical condition was deteriorating. Day ‘57’ blood culture result was Enterococcus spp and sensitive to Ampicillin and Levofloxacin; they had been given previously. It can be concluded that this patient had unusual microorganisms like Acinectobacter spp, Burkholderia cepacia and Enterococcus spp; Burkholderia cepacia was multi-drug resistant.
Conclusion
Prosthetic valve endocarditis is a catastrophic complication of cardiac valve replacement and is associated with high mortality rates. It is important to achieve an early diagnosis and initiate the treatment as early as possible. Early referral to tertiary center is important. Blood culture as well as serological testing for PCR should be encouraged. Infective endocarditis caused by multidrug resistant organism like Burkholderia cepacia is potentially fatal.
Acknowledgements
The authors would like to thank patient’s family for giving consent to this article. Also, to all doctors and nursing team for making great efforts in caring him. The authors acknowledged the following team; Professor Yu Aye Latt and ICU team, Professor Tin Moe Mya and pathology team, Professor Thet Naing, Professor Myint Zaw, Professor Kyaw Zay Ya and Professor Ko Ko Lwin for administrative support.
Declaration of conflict of interest
The authors declared no potential conflicts of interests with respect to authorship and publication of this article.
Ethical approval
Our institution does not require ethical approval for reporting cases.
References
- Yonas E, Damay V, Pranata R, Nusarintowati N (2018) Infective endocarditis due to Burkholderia cepacia in a neonate: A case report. Journa 12(1): 120.
- Rajani R, Klein J L (2020) Infective endocarditis: A contemporary update. Clinical Medicine (London, England) 20(1): 31-35.
- Liesman R M, Pritt B S, Maleszewski J J, Patel R (2017) Laboratory Diagnosis of Infective Endocarditis. Journal of Clinical Microbiology 55(9): 2599-2608.
- Hubers S A, De Simone D C, Gersh B J, Anavekar N S (2020) Infective Endocarditis: A Contemporary Review. Mayo Clinic Proceedings 95(5): 982-997.
- Chambers H F and Bayer A S (2020) Native-Valve Infective Endocarditis. The New England Journal of Medicine 383(6): 567-576.
- Nnaoma C, Chika Nwosuh O, Sossou C (2019) A Rare Culprit of Infective Endocarditis in an IV Drug User: Burkholderia cepacia. Case Reports in Medicine pp.6403943.
- Brouqui P, Raoult D (2001) Endocarditis due to rare and fastidious bacteria. Clinical Microbiology Reviews 14(1): 177-207.
- Houpikian P and Raoult D (2005) Blood culture-negative endocarditis in a reference center: Etiologic diagnosis of 348 cases. Medicine, 84(3): 162-173.
- Jędrzejczyk Patej E, Mazurek M, Kowalski O, Sokal A, Liberska A, et al. (2021) Clinical manifestations of device-related infective endocarditis in cardiac resynchronization therapy recipients. Archives of Medical Science : AMS 17(3): 638-645.
- Michałowska I, Stokłosa P, Miłkowska M, Zakrzewski D, Nieznańska M, et al. (2021) The role of cardiac computed tomography in the diagnosis of prosthetic valve endocarditis—A comparison with transthoracic and transesophageal echocardiography and intra-operative findings. European Journal of Radiology 138(1): 109637-109637.
- Gonzalez J M, Lowenhaar G, Ramgopal M, Chalasani P (2024) Burkholderia cepacia: A Rare Source of Endocarditis. Rhode Island Medical Journal 107(1): 23-25.
- Dellalana L E, Byrge K C, Gandelman J S, Lines T, Aronoff D M, et al. (2019) A Unique Case of Burkholderia cepacia Prosthetic Mitral Valve Endocarditis and Literature Review. Infectious Diseases in Clinical Practice (Baltimore, Md.) 27(3): 123-125.
- Ki H K, Kim S H, Han S W, Cheong H S (2011) A case of native valve endocarditis caused by Burkholderia cepacia without predisposing factors. BMC Infectious Diseases 11(1): 114-114.
- Pyar K P, Hla S, Aung Z N H, Lin M, Kyaw A, et al. (2021). Infective endocarditis involving native aortic valve due to Burkholderia cepacia presenting as refractory heart failure in immunocompetent host: A case report 2(5): 1-4.
- Falcão Pedrosa Costa A, Castelo Branco Cavalcanti F, Modesto dos Santos V (2014) Endocarditis due to Burkholderia cepacia and an intracardiac foreign body in a renal transplant patient. Revista Portuguesa de Cardiologia (English Edition), 33(2): 117.e1-117.e4.
- Moore J E, Crowe M, Shaw A, McCaughan J, Redmond A O B, et al. (2001) Antibiotic resistance in Burkholderia cepacia at two regional cystic fibrosis centres in Northern Ireland: Is there a need for synergy testing? Journal of Antimicrobial Chemotherapy 48(2): 319-321.
- Rhodes K A, Schweizer H P (2016) Antibiotic resistance in Burkholderia species. Drug Resistance Updates : Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy 28(1): 82-90.
- Scoffone V C, Barbieri G, Buroni S, Scarselli M, Pizza M, et al. (2020) Vaccines to Overcome Antibiotic Resistance: The Challenge of Burkholderia cenocepacia. Trends in Microbiology 28(4): 315-326.
- Bhojraj S, Hamdulay Z, Ali M, Kumar P (2007) Prosthetic valve endocarditis secondary to Burkholderia cepacia. Indian Journal of Thoracic and Cardiovascular Surgery 23(1):25-27.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...