Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
The Copolymerization of CO2 and Cyclohexene Oxide, Catalyzed by a Tridentate Schiff Base Chromium(III) Complex Volume 4 - Issue 3
Adrian Pap, Mark Esposito, James D Woodyard, David R Khan and Jason C Yarbrough*
- Department of Chemistry and Physics, West Texas A&M University, USA
Received: March 06, 2020; Published: May 14, 2020
*Corresponding author: Jason C Yarbrough,Department of Chemistry and Physics, West Texas A&M University, USA
DOI: 10.32474/AOICS.2020.04.000190
Abstract
The catalytic copolymerization reaction of carbon dioxide and cyclohexene oxide (CHO) using the single-site chromium (III) Schiff base catalyst, [Cr (k3-C24H29NO2) Cl] (complex 1) has been investigated according to gravimetric, IR and NMR spectral and GPC analysis of the resulting poly(cyclohexene carbonate) polymers (PCHC). Typical copolymerization reactions were carried out in bulk CHO monomer, utilizing a 25 ml stainless steel autoclave reactor, pressurized to 52 bar in CO2 and maintained at 80 °C. Complex 1 proved to be selective toward production of polymer and exhibited an average turnover frequency of TOF = 12.8(±1.1) hr-1. Nevertheless, limited CO2 incorporation was observed, resulting in polymers that were ~40-50% polycarbonate at most. Introduction of a neutral phosphine (tricyclohexylphosphine, PCy3) was observed to increase CO2 incorporations to as high as 82% based on 1H NMR analysis. GPC analysis showed the polymers exhibited number-average-molecular-weights with a mean value of Mn= 2.6(±0.4) kDa under optimal copolymerization conditions (with 1 equivalent PCy3relative to chromium) and fairly broad molecular weight distributions with polydispersity indices (PDI) ranging from 3.6 to 12.0.
Keywords: CO2Capture; CO2Utilization; CO2Epoxide Copolymerization; Polycarbonate; Homogeneous Catalysis
Abbreviations: CO2: Carbon Dioxide; CHO: Cyclohexene Oxide; H2L: (1S,2R)-1-[(3,5-di-tert-butyl salicylidine)imino]-2-indanol; H2L’: 1-[(3-tert-butyl-5-bromosalicylidine)imino]-2-indanol; TOF: Turnover Frequency; PCHC: Poly(cyclohexene carbonate); PCy3: Tricyclohexylphosphine; PDI: Polydispersity Index
Introduction
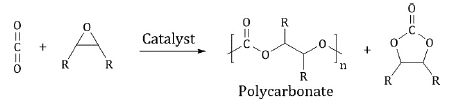
Carbon dioxide has long been considered a major contributor to climate change due to its behavior as a “greenhouse gas” and its accumulation in the atmosphere [1]. Indeed, atmospheric CO2 levels have increased from approximately 315 ppm in 1960 to 415.67 ppm as of February 10, 2020 [2]. Further, the rise in anthropogenic CO2 emissions is expected to continue and to expand over the next several decades [1]. One of the possible measures which may mitigate this growing problem is CO2capture and utilization [1, 3-8]. In this context, the use of CO2 as a renewable, raw material holds tremendous potential and has, therefore, been a goal of synthetic chemists for many years [9-11]. For these reasons, the metal-catalyzed coupling of CO2and epoxides to produce cyclic carbonates [12-15] and polycarbonates [16-32] is the subject of considerable interest (Scheme I). This is particularly so in the preparation of aliphatic polycarbonates as possible alternatives to petrochemical materials in end uses such as films, packaging and rigid plastics applications [33].
Most of the early work in the catalytic coupling of CO2and epoxides focused on zinc compounds[25, 34-39] stemming from Inoue’s findings in 1969 [40]. Since then, several other metals have been reported for this application. Among these are numerous chromium (III) and cobalt (III) coordination compounds based on the well-known Schiff base “salen” ligand and derivatives thereof (salen = N, N’-bis (salicylidene)-1, 2- ethylene diamine). The first example of a chromium (III) salen catalyst used for the copolymerization of CO2and epoxides was reported in the patent literature by Jacobsenandcoworkers [41].In this account, there was little reported in the way of relevant details regarding copolymerization beyond stating that poly(propylene carbonate) was prepared under 1 atm CO2. Independently, and unaware of the work detailed in patent literature, Darensbourg and Yarbrough, inspired by Jacobsen’s work on asymmetric epoxide ring-opening, reported the use of a salen chromium(III) chloride catalyst which exhibited high activity toward copolymerization of CO2with CHO, resulting in polymer with very uniform molecular weight distributions[42]. Around this same time Nguyen and coworkers at Northwestern University independently reported similar results with Jacobsen’s salen chromium (III) chloride catalyst [43]. This sparked a new direction in the investigation of CO2/epoxide copolymerization from zinc to chromium (III) and cobalt (III) salen and porphyrin catalysts and related derivatives. Indeed, these have become the basis for nearly all of the CO2/epoxide work conducted since 2001 as detailed in the literature, including numerous comprehensive reviews on the subject[17, 30, 39,44-52].
In spite of the inherent versatility in the synthesis of Schiff base ligands, tetra dentate salens, porphyrins and their derivatives represent the bulk of the chromium (III) and cobalt (III) catalysts employed for this purpose [46]. Relatively few chromium species have been investigated with markedly different ligand architectures. Notable exceptions include the work of Duchateau and coworkers concerning the use of chromium(III) iminopyrrole, aminopyrrole and aminophosphine complexes [53] as well as that of Kazak and coworkers who have developed chromium(III) diaminebisphenolate catalysts for this purpose [54]. Here in we report the activity toward CO2/CHO copolymerization of a chromium(III) catalyst using a tridentate Schiff base ligand, (1S,2R)-1-[(3,5-di-tert-butylsalicylidine)imino]-2-indanol (H2L) the synthesis of which has been published previously [55,56]. We report its effectiveness as a catalyst for the bulk, solvent lesscopolymerization of CO2and CHO with and without the assistance of a neutral phosphine donor, tri-cyclohexyl phosphine (PCy3).
Materials and Methods
Unless otherwise indicated, all manipulations were carried out under inert conditions using standard Schlenk and dry box techniques. Additionally, all reagents were purchased from Sigma Aldrich and used as received unless otherwise stated. Priortouse, THF was distilled from sodium/benzophenoneketyl still and store Dover 3 Å molecular sieves. CHO was purchased from Across Organics and freshly distilled over CaH2 prior to use. Sure/SealTM a hydrous ethanol was purchased from Sigma Aldrich and used as received. The ligand (1S, 2R)-1-[(3,5-di-tert-butylsalicylidine)imino] -2-indanol, C24H31NO2(H2L) was purchased from Sigma Aldrich and used as received. Complex1, [Cr(k3O,N,O-L)Cl] was prepared according to a previously published procedure [56]. All 1H and 13C NMR spectra were recorded using a Bruker Avance III HD Spectrometer (AscendTM400 Magnet) at ambient temperature. FTIR spectra were recorded on a Nicolet iS5 spectrometer. Much of the syntheses and data collection described herein was recently reported in the MSc. Thesis of Adrian Pap at West Texas A&M University [57]. GPC analysis was performed by EAG Laboratories (Maryland Heights, MO, USA).
Typical Copolymerization of CO2 and Cyclohexene Oxide
In a typical copolymerization experiment approximately 11-12 g of freshly distilled CHO monomer is charged into a 25-ml, stainless-steel reactor equipped with a magnetic stirring bar, a two-way inlet valve and a pressure gauge. To this, an appropriate amount of complex 1 (typically 15 mg) is added along with tri cyclohexylphosphine (typically between 0 and 18 mg). The reactor is then sealed, removed to a fume hood, connected via inlet valve to a purged, “bone dry” CO2 line and placed in a temperature-controlled oil bath. The reactor is then pressurized to approximately 34.5-37.5 bar in CO2and heated to temperature (typically80°C). At 80°C a pressure of approximately 50-57 baris achieved.Thereactor is then maintained at these conditions with stirring for a reaction time of 17-18 hours. Following each copolymerization a small a liquor to the crude reaction mixture is removed and analyzed by FTIR spectroscopy to determine the presence of either polycarbonate (n(CO2) = 1742-1748 cm-1) or the cyclic carbonate, 7,9- dioxabicyclo[4.3.0]nonan-8-one (n(CO2) = 1802 cm-1). The reaction mixture is then diluted in dichloromethane and precipitated in cold methanol and the crude polymer is then dried in vacuo to constant mass to determine turn over numbers and frequencies and analyzed by 1H and13C NMR spectroscopies.
Results and Discussion
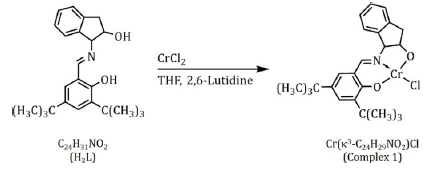
The ligand (1S, 2R)-1-[(3, 5-di-tert-butylsalicylidine) imino]-2-indanol, C24H31NO2 (H2L) contains 2 chiral centers at the first and second position of the indanol moiety and can be prepared according to a previously published procedure [56]. As such, a chiral ligand environment may be a source of stereo control in the ring- opening and enchainment of the CHO monomer during polymerization. A point of investigation described later in this treatment. Complex 1, [Cr (k3O, N, O-L) Cl], was readily synthesized from the ligand, H2L, as described previously in the literature and depicted in Scheme II [56]. The reaction of H2L with 1 equivalent of CrCl2 produced the dark brown, air-stable catalyst (complex 1) in reasonable yield. Further, a previously published XRD characterization of an analogous compound reported by Jacobsen and coworkers in the same 2002 publication[56], exhibited two hexacoordinate, octahedral chromium(III) ions in a dimeric complex ([Cr(H2O)(k3-O,N,O-m-O-L’)Cl]2(wherein H2L’= 1-[(3-tert-butyl-5-bromosalicylidine)imino]-2-indanol, C20H22NO2)[56]. Given the preference of Cr (III) for octahedral coordination geometry, this structure provides sound precedent for the suggestion of a dimeric structure for complex 1 in the solid state. However, to our knowledge, no structural determination of complex 1 has yet been reported in theliterature.
Scheme II: The synthesis of Complex 1, [Cr (k3-C24H29NO2) Cl], was carried out according to the procedure reported by Eric Jacobsen and coworkers in 2002[56].
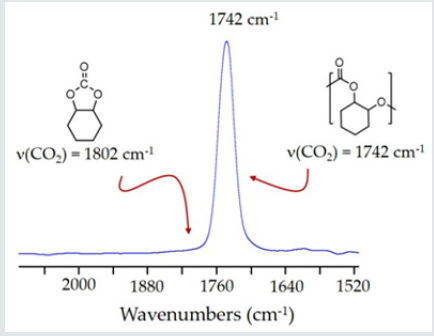
Complex 1 proved to be reasonably effective toward CO2/CHO copolymerization (Scheme III) as indicated by the results detailed in Table1. Typical copolymerization’s were carried out with7-15mg complex 1, in bulk CHO monomer at 80°C, and at ~52 bar in CO2 in a stainless-steel reactor, equipped with a magnetic stir bar and pressure gauge. Following each copolymerization reaction, the product was precipitated in cold methanol or hexane. The crude solid polymer precipitate was collected and dried in vacuo to constant mass, which was then used to calculate the turnover frequencies reported in Table 1. Complex 1 initially exhibited a turnover number of 236 mol CHO/mol Cr and a corresponding turnover frequency (TOF) of 13.9 hr-1 (Table 1, entry 1). Further, following each polymerization and prior to isolation of the PCHC product, a sample of the crude reaction mixture was taken and analyzed by FTIR spectroscopy (in CH2Cl2) to determine the extent of cyclic carbonate formation. Based on the FTIR data, complex 1 appeared to be nearly 100% selective toward the production of polymer under the reported reaction conditions as evidenced by the intense absorbance observed at approximately 1742-1748 cm-1 as seen in the FTIR spectrum illustrated in Figure 1. This is assigned to the n(C=O) stretching frequency of poly (cyclohexene carbonate) [45]. Very little if any signal was observed for the corresponding band assigned to the cyclic carbonate, 7,9-dioxabicyclo[4.3.0]nonan-8-one, at 1802 cm-1(Figure 1). Additionally, selectivity toward polymer was further supported by theabsence of the chemical shift assigned to the methine protons of the cyclic carbonate (d = 3.99 ppm) in the 1H NMR spectra recorded for the crude PCHC samples produced by complex 1 (Figure 2).
Figure 1: The n(C=O) region in a representative solution FTIR spectrum of poly (cyclohexene carbonate) prepared from copolymerization of CO2 and CHO mediated by complex 1.
aAmounts of PCy3are given in molar equivalents relative to chromium. b The turnover frequency is reported as moles of CHO consumed per mole of chromium per hour. C CO2 incorporation is determined by 1H NMR spectroscopy. D GPC analysis was conducted in THF against narrow molecular weight polystyrene standards. E The reactor pressure was observed to have reduced to ambient during the polymerization and the resulting polymer was predominantly CHO homopolymer.
Scheme III: The Copolymerization of CO2 and cyclohexene oxide, mediated by complex 1. All polymerizations were performed in a 25-ml stainless steel reactor equipped with magnetic stir bar, at 80°C and under ~52 bar CO2.
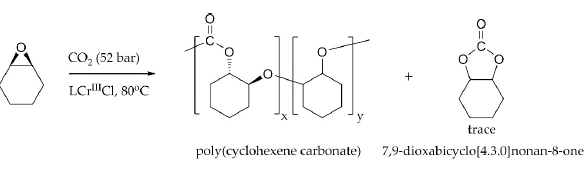
As suggested in Scheme III, the coupling of CO2 and CHO at a ratio of 1:1during the polymerization reaction is necessary for the realization of a purely polycarbonate product. The relative CO2 incorporation in a particular polymers ample is determined by1H NMR spectroscopy and taken based on relative integrations of the methane chemical shifts centered at d = 4.6 ppm and d = 3.4 ppm corresponding to carbonate and ether linkages respectively (Figure 2) [45]. While there is some variability concerning CO2incorporation in the polymers reported here in, the reisatr end worthy of remark. The initial CO2 incorporation observed for polymers produced using complex 1 as a catalyst was approximately ~40-50% relative to CHO monomer enchainment (Table 1, entries 1 and 2). However, given the precedent in the literature for the use of N-heterocyclic amines, bulky phosphines (Tolman cone angles – 145°-185°)[58] and anions derived from PPN+, as well as R4N+ salts[59-62], in concert with chromium (III) salen catalysts, we embarked on a study of the effect of the addition of various levels of tricyclohexylphosphine (PCy3) on CO2incorporations as well as turnover frequency for the polymerizations mediated by complex 1. What was observed was a marginal impact on TOF but a significant effect on CO2incorporation.
Figure 2: The n(C=O) region in a representative solution FTIR spectrum of poly (cyclohexene carbonate) prepared from copolymerization of CO2 and CHO mediated by complex 1.
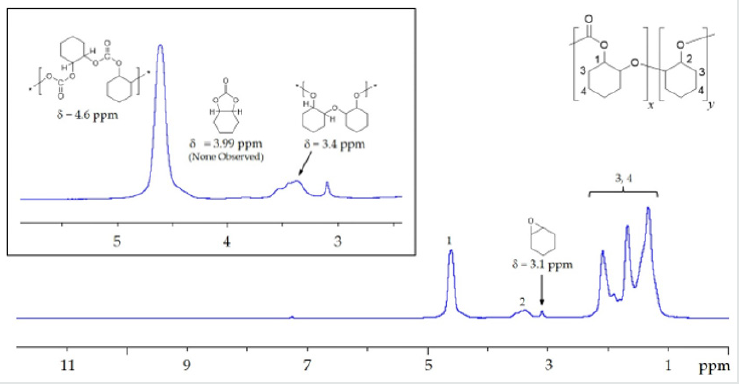
Table 1: Results of the copolymerization reactions of cyclohexene oxide and carbon dioxide catalyzed by complex 1.
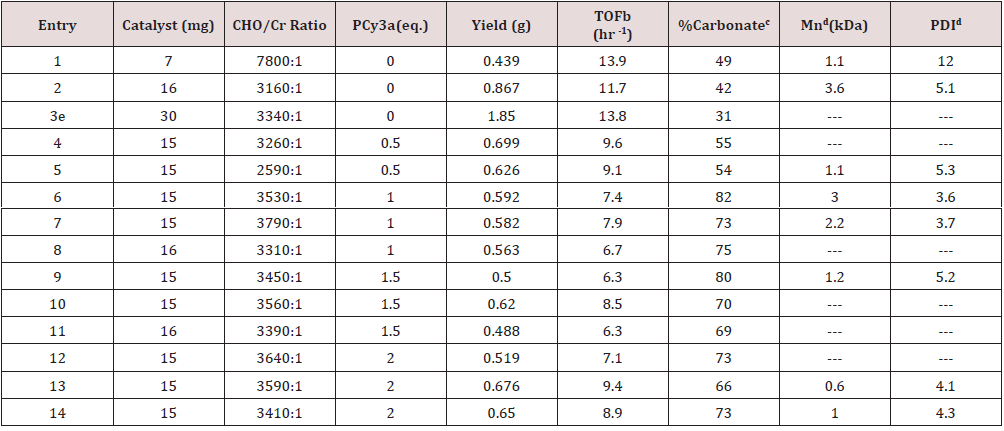
As the addition of PCy3was increased, in successive polymerizations, from 0 to 2 molar equivalents relative to chromium, CO2incorporation was observed to increase from an average of 45.5(±3.5)% with no added phosphine (Table1;entries1and2),to 76.7(s=3.9)% at 1eq. PCy3(Table 1;entries 6,7and8), and as high as 82% in one instance (Figure 3). However, there appears to be no statistically significant change in the CO2 incorporation with additional PCy3 beyond 1 equivalent (Figure 4). While there is insufficient data for a definitive conclusion regarding mechanism, these observations are consistent with literature accounts which describe the direct attack of a single PCy3molecule on a bound, activated CHO molecule, generating an activated, zwitterionic species [17, 39]. It is this species which can then bind the metal through its alkoxide oxygen donor, thus providing steric and electronic influence over the mechanism of CO2insertion and CHO enchainment. Darensbourg and coworkers have provided a detailed description of this mechanism in the literature concerning both Cr (III) and Co (III) salen catalysts [17, 39]. On such a basis, it isn’t necessarily surprising that additional phosphine (beyond 1 equivalent) would have little additional impact onCO2incorporation, as the bulk of the CHO homopolymerization would likely occur prior to activation of the zwitterionic, phosphonium species.
Figure 3: Stack plot of four representative 1H NMR spectra. Integration of the shifts centered at d = 4.6 ppm and d = 3.4 ppm provide relative quantification of carbonate and ether linkages respectively. The numbers adjacent to each shift are relative integrations normalized to 100%.
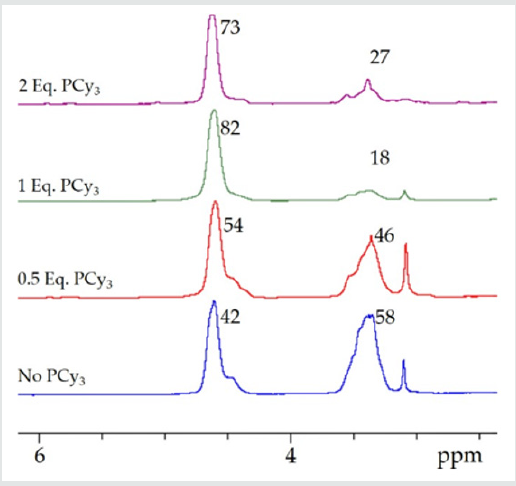
Figure 4: The average CO2 incorporations as determined by 1H NMR spectroscopy as described in Table 1 with increasing tricyclohexylphosphine (eq. = equivalents relative to chromium).

InordertoestablishthemolecularweightdistributionsofthePCHCsamplespreparedusingcomplex1,a selection of polymer samples was analyzed by gel permeation chromatography (GPC). The data collected revealed number-average-molecular-weights (Mn) of the resulting polymers ranging between 0.6 and 3.6 kDa (Table1). Additionally, the molecular weight distributions are broad with polydispersity’s ranging from 3.6to
12.0. This observation is not unprecedented in the coupling of CO2 and epoxides and has been reported to be a possible indication of either slow initiation of the active species, multiple initiation mechanisms (perhaps mono-vs. dinuclearinitiationsites), and/or the presence of trace amounts of protic impurities [39,63].Among the PCHC samples analyzed by GPC, was a set of two samples (entries 1 and 2 in Table 1) each prepared by copolymerization at 80°C and 52 bar CO2 and no added PCy3.These polymers were found to each have number average molecular weights (Mn) of 1.1 and 3.6k Dawith corresponding PDI’s of 12.0 and 5.3 respectively (Table 1). Further, number-average-molecular-weights were not observed to be significantly impacted from the addition of either 0.5 or 1 equivalent of PCy3 relative to chromium during copolymerization (entries 5, 6 and 7 in Table 1). The corresponding PDI’s were 5.3, 3.6 and 3.7 respectively. However, additional PCy3 resulted in a fairly linear decrease in Mn from 1 eq. to 1.5 and 2 eq. of PCy3 (Table 1). Therefore, just as observed for CO2 incorporation, there appears to be an optimum loading of 1 eq. of PCy3in terms its impact onMn.
Another important question we set out to investigate regarding this catalyst was that of stereo control. As stated previously, the ligand, H2L, contains two chiral centers and can be synthesized or purchased in an enantiomeric ally pure form [56]. To this point, 13C NMR spectroscopy was employed to evaluate the stereoregularity of the PCHC samples recovered from the polymerizations described herein. All 13C NMR spectra recorded for PCHC samples prepared in this study exhibited a set of chemical shifts centered at d=153.9pp man dd=153.1-153.4ppm which are assigned to isotactic, syndiotactican dheterotactic forms of the copolymer (Figure 5) [64]. As such, we observe all polymers to be atactic and therefore indicating no stereocontrol in the ring opening and enchainment of the CHO monomer.
Figure 5: The carbonate chemical shifts in are presentative 13CNMR spectrum of poly (cyclo hexene carbonate) prepared from copolymerization of CO2 and CHO, mediated by complex1.

Conclusion
We have shown complex 1 to be an effective catalyst in the coupling of CO2 and CHO to produce poly (cyclohexene carbonate) under reasonable conditions of temperature and subcritical CO2 pressure. The air-stable complex 1 was easily synthesized and characterized in this study and found to be nearly 100% selective toward copolymer under the conditions reported, as evidenced by the lack of any cyclic carbonate observed in the FTIR or NMR spectral data. Addition of tricyclohexylphosphine was shown to enhance the degree of CO2 incorporation, resulting in polymers which were found to be as high as 82% carbonate in contrast to initial values of ~40-50% carbonate for polymerization carried out in the absence of PCy3. Further, number average molecular weights and CO2 incorporations were highest for polymers prepared with addition of 1 equivalent of phosphine relative to chromium (Mn = 2.6 (±0.4) kDa; %CO2 = 76.7(s = 3.9)%). Given the results we report herein regarding the effect of PCy3 on the polymerization reactions, it is likely similar effects might be observed with addition of PPN+ salts or N-heterocyclic compounds such as imidazole and pyridine derivatives. This is will be the subject of future studies as we seek to explore the activity of other catalysts for this purpose.
Acknowledgement
The authors would like to thank Dr. Cathy Clewett and the West Texas A & M University NMR Facility for providing 1H and 13C NMR spectra for all samples prepared in this study. This research was funded by the Welch Foundation (grant number AE-0025), the Killgore Research Grant Program at West Texas A&M University and the Ross Wilson Endowed Chair in Chemistry (David R. Khan).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aresta M (2010) Carbon Dioxide: Utilization Options to Reduce its Accumulation in the Atmosphere. In: Carbon dioxide as a Chemical Feedstock, Aresta M (EdS.), Wiley-VCH: Weinheim, Germany 1-13.
- (2020) The Keeling Curve, Mauna Loa Laboratory Data.
- Song C (2006) Global challenges and strategies for control, conversion and utilization of CO2for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal Today 115(1-4): 2-
- Markewitz P, Kuckshinrichs W, Leitner W, Linssen J, Zapp P, et al. (2012) Worldwide innovations in the development of carbon capture technologies and the utilization ofCO2. Energ Environ Sci 5(6): 7281-7305.
- MacDowell N, Florin N, Buchard A, Hallett J, Galindo A, et (2010) An overview of CO2capture technologies. Energ Environ Sci 3(11): 1645-1669.
- Aresta M, Dibenedetto A (2007) Utilisation of CO2as a chemical feedstock: Opportunities and Dalton Trans (28): 2975-2992.
- Chapman AM, Keyworth C, Kember MR, Lennox AJJ, Williams CK, et al. (2015) Adding value to power station captured CO2: Tolerant Zn and Mg homogeneous catalysts for polycarbonate polyol production. ACS Catal 5(3): 1581-1588.
- Artz J, Muller TE, Thenert K, Kleinekorte J, Meys R, et al. (2018) Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment. Chem Rev 118(2): 434-504.
- Arakawa H, Aresta M, Armor JN, Barteau MA, Beckman EJ et al. (2001) Catalysis research of relevance to carbon management: progress, challenges, and opportunities. Chem Rev 101(4): 953-996.
- Sakakura T, Choi JC, Yasuda H (2007) Transformation of carbon dioxide. Chem Rev 107(6): 2365-2387.
- Peters M, Kohler B, Kuckshinrichs W, Leitner W, Markewitz P, et al. (2011) Chemical technologies for exploiting and recycling carbon dioxide into the value chain. ChemSus Chem 4(9): 1216-1240.
- Cokoja M, Wilhelm ME, Anthofer MH, Herrmann WA, Kuhn FE, et al. (2015) Synthesis of cyclic carbonates from epoxides and carbon dioxide by using organocatalysts. ChemSusChem 8(15): 2436-2454.
- Comerford JW, Ingram ID V, North M, Wu X (2015) Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem 17(4): 1966-1987.
- Martín C, Fiorani G, Kleij AW (2015) Recent advances in the catalytic preparation of cyclic organic ACS Catal 5(2): 1353-1370.
- Li P, Cao Z (2018) Catalytic preparation of cyclic carbonates from CO2and epoxides by metal–porphyrin and −corrole complexes: insight into effects of cocatalyst and meso-substitution. Organometallics 37(3): 406-414.
- Yang GW, Zhang YY, Wang Y, Wu GP, Xu ZK, et al. (2018) Construction of autonomic self-healing CO2- based polycarbonates via one-pot tandem synthetic strategy. Macromolecules 51(4):1308-1313.
- Lu XB, Darensbourg DJ (2012) Cobalt catalysts for the coupling of CO2and epoxides to provide polycarbonates and cyclic carbonates. Chem Soc Rev 41(4): 1462-1484.
- Pescarmona PP, Taherimehr M (2012) Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2. Catal Sci Technol 2(11): 2169-2187.
- Paul S, Zhu Y, Romain C, Brooks R, Saini PK, et (2015) Ring-opening copolymerization (ROCOP): synthesis and properties of polyesters and polycarbonates. Chem Commun 51(30): 6459-6479.
- Thevenon A, Cyriac A, Myers D, White AJP, Durr CB, et al. (2018) Indium catalysts for low-pressure CO2/epoxide ring-opening copolymerization: evidence for a mononuclear mechanism?. J Am Chem Soc 140(22): 6893-6903.
- Sugimoto H, Inoue S (2004) Copolymerization of carbon dioxide and J Polym Sci A: Polym Chem 42(22): 5561-5573.
- Poland SJ, Darensbourg DJ (2017) A quest for polycarbonates provided via sustainable epoxide/CO2 copolymerization processes. Green Chem 19(21): 4990-5011.
- Childers MI, Longo JM, Van Zee NJ, LaPointe AM, Coates GW, et al. (2014) Stereoselective epoxide polymerization and copolymerization. Chem Rev 114(16): 8129-8152.
- Moore DR, Cheng M, Lobkovsky EB, Coates GW (2003) Mechanism of the alternating copolymerization of epoxides and CO2 using beta-diiminate zinc catalysts: evidence for a bimetallic epoxide enchainment. J Am ChemSoc 125(39): 11911-11924.
- Cheng M, Lobkovsky EB, Coates GW (1998) Catalytic reactions involving C1feedstocks: new high-activity Zn(II)-based catalysts for the alternating copolymerization of carbon dioxide and epoxides. J Am Chem Soc 120(42): 11018-11019.
- D’Auria I, D’Alterio MC, Talarico G, Pellecchia C (2018) Alternating copolymerization of CO2and cyclohexene oxide by new pyridylamidozinc(II) catalysts. Macromolecules 51(23): 9871-9877.
- Chen K, Shi G, Li H, Wang C, Darensbourg DJ (2018) Design of betaine functional catalyst for efficient copolymerization of oxirane and CO2. Macromolecules 51(15): 6057-6062.
- Carrilho R, Dias L, Rivas R, Pereira M, Claver C, et al. (2017) Solventless coupling of epoxides and CO2in compressed medium catalysed by fluorinated metalloporphyrins. Catalysts 7(7):
- Schutze M, Dechert S, Meyer F (2017) Highly active and readily accessible proline-based dizinc catalyst for CO2/epoxide copolymerization. Chem Eur J 23(65): 16472-16475.
- Ni K, Kozak CM (2018) Kinetic studies of copolymerization of cyclohexene oxide with CO2by a diamino- bis(phenolate) chromium(III) complex. Inorg Chem 57(6): 3097-3106.
- Hatazawa M, Takahashi R, Deng J, Houjou H, Nozaki K, et al. (2017) Cationic co–salphen complexes bisligated by DMAP as catalysts for the copolymerization of cyclohexene oxide with phthalic anhydride or carbon dioxide. Macromolecules 50(20): 7895-7900.
- Ohkawara T, Suzuki K, Nakano K, Mori S, Nozaki K et al. (2014) Facile estimation of catalytic activity and selectivities in copolymerization of propylene oxide with carbon dioxide mediated by metal complexes with planar tetradentate ligand. J Am Chem Soc 136(30): 10728-10735.
- Luinstra G (2008) Poly(propylene carbonate), old copolymers of propylene oxide and carbon dioxide with new interests: catalysis and material properties. Polym Rev 48(1): 192-219.
- Darensbourg DJ, Holtcamp MW, Struck GE, Zimmer MS, Niezgoda SA, et (1999) Catalytic activity of a series of Zn(II) phenoxides for the copolymerization of epoxides and carbon dioxide. J Am Chem Soc 121(1): 107- 116.
- Darensbourg DJ, Wildeson, JR, Yarbrough JC, Reibenspies JH (2000) Bis2,6-difluorophenoxide dimeric complexes of zinc and cadmium and their phosphine adducts: lessons learned relative to carbon dioxide/cyclohexene oxide alternating copolymerization processes catalyzed by zinc phenoxides. J Am Chem Soc 122(50): 12487-12496.
- Darensbourg DJ, Zimmer MS, Rainey P, Larkins DL (2000) Solution and solid-state structures of phosphine adductsof monomeric zinc bisphenoxide importance of these derivatives in CO2/epoxide copolymerization processes. Inorg Chem 39(7): 1578-1585.
- Darensbourg DJ, Wildeson JR, Yarbrough JC (2002) Solid-state structures of zinc(II) benzoate complexes. catalyst precursors for the coupling of carbon dioxide and epoxides. Inorg Chem 41(4): 973-980.
- Super M, Berluche E, Costello C, Beckman E (1997) Copolymerization of 1,2-epoxycyclohexane and carbon dioxide using carbon dioxide as both reactant and solvent. Macromolecules 30(3): 368-372.
- Darensbourg DJ (2007) Making plastics from carbon dioxide: Salen metal complexes as catalysts for the production of polycarbonates from epoxides and CO2. Chem Rev 107(6): 2388-2410.
- Inoue S, Koinuma H, Tsuruta T (1969) Copolymerization of carbon dioxide and epoxide with organometallic compounds. Die Makromol Chem130(1): 210-220.
- Jacobsen EN, Tokunaga M, Larrow JF (2000) Stereoselective ring opening Patent Number 5929232.
- Darensbourg DJ, Yarbrough JC (2002) Mechanistic aspects of the copolymerization reaction of carbon dioxide and epoxides, using a chiral salen chromium chloride catalyst. J Am Chem Soc 124(22): 6335-6342.
- Paddock RL, Nguyen ST (2001) Chemical CO2fixation: Cr(III) salen complexes as highly efficient catalysts for the coupling of CO2 and epoxides. J Am Chem Soc 123(46): 11498-11499.
- Wang Y, Darensbourg DJ (2018) Carbon dioxide-based functional polycarbonates: Metal catalyzed copolymerization of CO2 and epoxides. Coord Chem Rev 372: 85-100.
- Darensbourg DJ, Andreatta J, Moncada AI (2010) Polymers from carbon dioxide: polycarbonates, polythiocarbonates and polyurethanes. In Carbon Dioxide as a Chemical Feedstock, Aresta M (Eds.), Wiley-VCH: Weinheim, Germany, 213-249.
- Kember MR, Buchard A, Williams CK (2011) Catalysts for CO2/epoxide copolymerisation. Chem Commun 47(1): 141-163.
- Coates GW, Moore DR (2004) Discrete metal-based catalysts for the copolymerization of CO2 and epoxides: discovery, reactivity, optimization, and mechanism. Angew Chem Int (edn), 43(48):6618-6639.
- Klaus S, Lehenmeier MW, Anderson CE, Rieger B (2011) Recent advances in CO2/epoxide copolymerization-New strategies and cooperative mechanisms. Coord Chem Rev 255(13-14):1460-1479.
- Darensbourg DJ, Wilson SJ (2012) What's new with CO2? Recent advances in its copolymerization with Green Chem 14(10): 2665-2671.
- Lu XB, Ren WM, Wu GP (2012) CO2copolymers from epoxides: catalyst activity, product selectivity, and stereochemistry control. Acc Chem Res 45(10): 1721-1735.
- Decortes A, Castilla AM, Kleij AW (2010) Salen-complex-mediated formation of cyclic carbonates by cycloaddition of CO2 to epoxides. Angew Chem Int Ed 49(51): 9822-9837.
- Xu Y, Lin L, Xiao M, Wang S, Smith AT et al. (2018) Synthesis and properties of CO2-based plastics: Environmentally-friendly, energy-saving and biomedical polymeric materials. Prog Polym Sci 80:163-182.
- Gurnham J, Gambarotta S, Korobkov I, Jasinska-Walc L, Duchateau R (2014) Chromium-catalyzed CO2– epoxide copolymerization. Organometallics 33(17): 4401-4409.
- Dean RK, Dawe LN, Kozak CM (2012) Copolymerization of cyclohexene oxide and CO2with a chromium diamine-bis(phenolate) catalyst. Inorg Chem 51(16): 9095-9103.
- Li Z, Fernández M, Jacobsen EN (1999) Enantioselective ring opening of meso aziridines catalyzed by tridentate Schiff base chromium(III) complexes. Org Lett 1(10): 1611-1613.
- Ruck RT, Jacobsen EN (2002) Asymmetric catalysis of hetero-ene reactions with tridentate Schiff base chromium(III) complexes. J Am Chem Soc 124(12): 2882-2883.
- Pap A (2018) Copolymerization of CO2and Epoxides Catalyzed by a Schiff-Base Chromium(III) Complex. MSc. Thesis, West Texas A&M University, Canyon,
- Tolman CA (1977) Steric effects of phosphorus ligands in organometallic chemistry and homogeneous Chem Rev 77(3): 313-348.
- Darensbourg DJ, Mackiewicz RM, Rodgers JL, Phelps AL (2004) (Salen)CrIIIX catalysts for the copolymerization of carbon dioxide and epoxides: role of the initiator and cocatalyst. Inorg Chem 43(6): 1831-
- Darensbourg DJ, Bottarelli P, Andreatta JR (2007) Inquiry into the formation of cyclic carbonates during the (salen)CrX catalyzed CO2/cyclohexene oxide copolymerization process in the presence of ionic initiators.Macromolecules 40(21): 7727-7729.
- Darensbourg DJ, Phelps AL (2005) Effective, selective coupling of propylene oxide and carbon dioxide to poly(propylene carbonate) using (salen)CrN3 Inorg Chem 44(13): 4622-4629.
- Darensbourg DJ, Mackiewicz RM, Rodgers JL (2005) Role of the cocatalyst in the copolymerization of CO2and cyclohexene oxide utilizing chromium salen complexes. J Am Chem Soc 127(40): 14026-
- Jutz F, Buchard A, Kember MR, Fredriksen SB, Williams CK et al. (2011) Mechanistic investigation and reaction kinetics of the low-pressure copolymerization of cyclohexene oxide and carbon dioxide catalyzed by a dizinc complex. J Am Chem Soc 133(43): 17395-17405.
- Nakano K, Nozaki K, Hiyama T (2001) Spectral assignment of poly[cyclohexene oxide-alt-carbon dioxide]. Macromolecules 34(18): 6325-6332.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...