Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Synthesis, Characterization and Antimicrobial Evaluation of Novel 2,5-Disubstituted 1,3,4-Oxadiazole Derivatives with Two Oxadiazole Rings Volume 4 - Issue 5
Joseph Adewuyi, Hamisu Ibrahim and Adebayo Ojo Oyewale*
- Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria
Received: August 18, 2020; Published: September 03, 2020
*Corresponding author: Adebayo Ojo Oyewale, Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria
DOI: 10.32474/AOICS.2020.04.000196
Abstract
Three new 2,5-disubstituted 1,3,4-oxadiazole derivatives from dicarboxylic acids were synthesized in a three-step synthesis procedure that involved formation of esters and hydrazides as intermediates. The final products; 1,4-bis(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl)benzene,1,4-bis(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)benzene and 1,2-bis(4-nitro-3-(5-phenyl-1,3,4-oxadiazol-2-yl)phenyl)disulfane; which all have a C2 symmetry and contain two oxadiazole rings were obtained in yields of 45.3 to 50%. The oxadiazoles were successfully characterized using Infrared Spectroscopy, Proton and Carbon Nuclear Magnetic Resonance Spectroscopy; and Gas Chromatography-Mass Spectrometry. The three synthesized compounds were tested for antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa and Candida albicans. Only 1,2-bis(4-nitro-3-(5-phenyl-1,3,4-oxadiazol-2-yl)phenyl)disulfane showed appreciable biological/ antimicrobial activity against these microorganisms with sensitivity zones of inhibition of 10 -19 mm, MIC of 0.37 - 1.11 mg/mL and MBC of 1.11 - 3.33 mg/mL at concentrations of 2.5 to 20 mg/mL.
Keywords: Disubstituted oxadiazole; heterocyclic compounds;C2 symmetry; antimicrobial activity
Graphical abstract:
Introduction
A wide variety of heterocyclic systems have been explored to develop molecules that are of pharmaceutical value. Some of the heterocyclic compounds are quinoline-based antibiotics and antimalarials such as quinine which have uses in medicinal chemistry [1], pesticide industry [2], and dyestuffs [3]. Heterocyclic class of organic compounds consisting of a five-membered ring containing one oxygen and two nitrogen atoms are called oxadiazoles, which are classified as 1,2,3-oxadiazole, 1,2,4-oxadiazole, 1,2,5-oxadiazole and 1,3,4-oxadiazole. Heterocyclic compounds of 5-membered rings have been synthesized and explored extensively for therapeutic activities [4]. Generally, triazoles, imidazole’s, pyrazoles, oxadiazoles, thiadiazoles, and pyrrole derivatives have been of interest. These heterocyclic molecules, which all contain at least one nitrogen atom, are among the largest group of chemical compounds in many natural products, fine chemicals, and biologically active pharmaceuticals [5]. Among them, the derivatives of oxadiazoles are of great importance in medicinal chemistry [5]. Of the possible four oxadiazole isomers, the most important and active isomer therapeutically is the 1,3,4-oxadiazole ring [6,7]. Usually, these 1,3,4-oxadiazole compounds are substituted either at the 2-position, 5-position or at both the 2- and 5-positions. The synthesis, transformation, and medicinal properties of 2,5-disubstituted 1,3,4-oxadiazole compounds have been of interest to researchers for a long time due to their pharmacological activities [8-17]. The common marketed antihypertensive agents such as Tiodazosin and Nesapidil, anticancer drug such as Zibotentan as well as antibiotics such as Furamizole all contain the 1,3,4-oxadiazole nucleus [18-20].
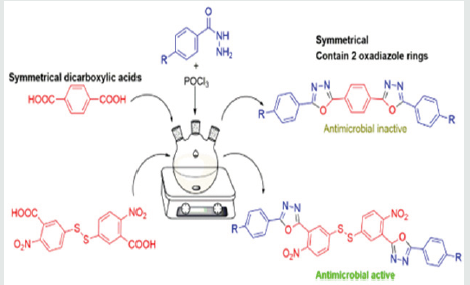
Several synthetic approaches have been utilized for the cyclization step in the formation of 1,3,4-oxadiazoles which include reflux, microwave assisted, mechanical grinding, and oxidative addition. Also, various mono carboxylic acids have been reacted with hydrazides in these cyclization reactions for the synthesis of 1,3,4-oxadiazoles [21,22]. However, interestingly, it was discovered that symmetrical dicarboxylic acids are rarely used for the synthesis with only one published paper having previously reported such synthesis [23]. Also, compounds are rarely synthesized with more than one 1,3,4-oxadiazole ring. Therefore, in this research, we decided to explore that. New series of symmetrical compounds containing two 2,5-disubstituted 1,3,4-oxadiazole rings were synthesized from symmetrical dicarboxylic acids and their biological activities against selected microorganisms were verified.
Materials and Methods
All reactions were carried out in reflux conditions. All reagents and solvents were purchased from local commercial sources and used without further purification. The synthesized products were characterized using IR, NMR (1H and 13C), and mass spectrometry. The NMR spectra were recorded in deuterated chloroform or DMSO at room temperature on a 400 MHz spectrometer for 1H and 13C using tetramethyl silane (TMS) as internal standard. The melting points were recorded in degree centigrade (uncorrected). Biological activities were carried out at Microbiology department, Ahmadu Bello University, Zaria, Nigeria (Tables 1-3).
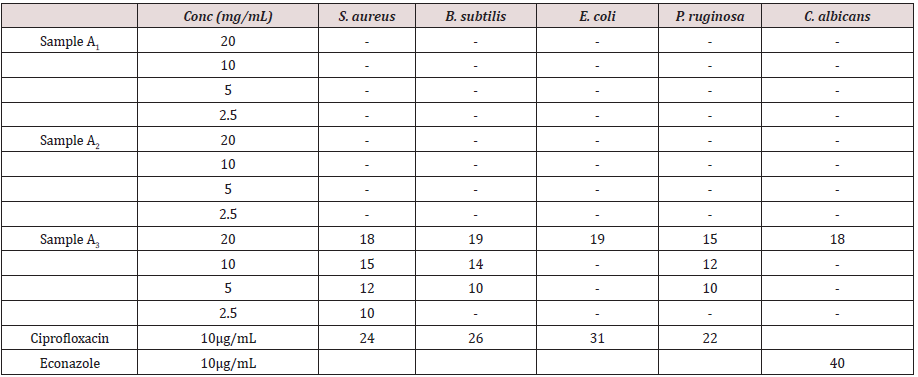
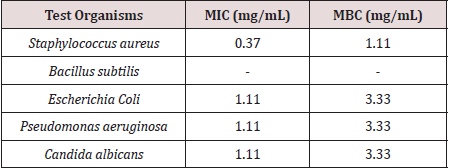
Table 3: Result of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Sample A3.
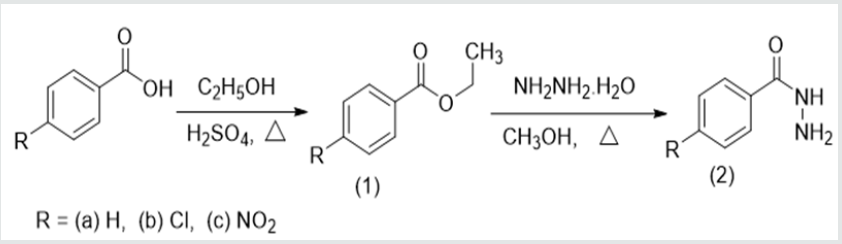
Synthesis of ethyl esters (1a-c)
The substituted benzoic acid (0.24 mol) with ethanol (80 mL) and concentrated H2SO4 (10 mL) were stirred and refluxed for 2 h in a round bottom flask (250 mL) with a reflux condenser. After completion of the reaction, excess ethanol was distilled off then the reaction was poured into distilled water (50 mL). Diethyl ether was used to extract the ester. The diethyl ether portion was concentrated leaving the ester as either a liquid or as a solid. The liquid esters were further purified by vacuum distillation using a Kugelrohr apparatus while the solid ester was recrystallized in ethanol.
Synthesis of acid hydrazides (2a-c)
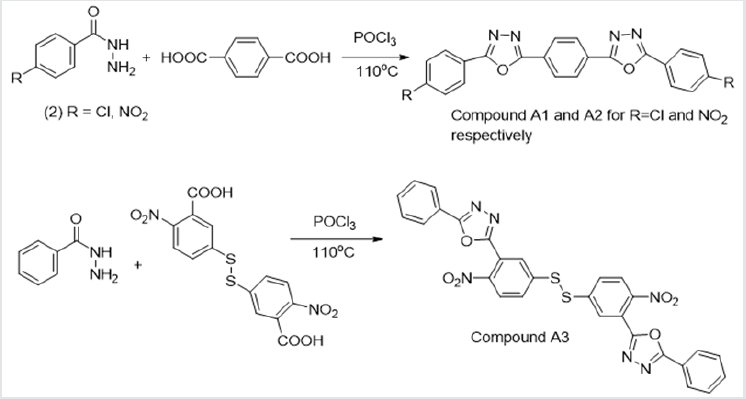
The synthetic procedure followed for the reaction was as reported by [21]. An equimolar mixture of the respective ester (0.05 mol) and 80 % hydrazine monohydrate (0.05 mol) was dissolved in methanol (30 mL) in a round bottom flask (100 mL). The mixture was stirred and refluxed for 5 h. After completion of the reaction, excess methanol was distilled off and the reaction mixture cooled to room temperature. Very cold distilled water was added along with shaking till the appearance of precipitates. Then diethyl ether (50 mL) was added to the mixture and transferred to a separatory funnel. The aqueous layer, containing the hydrazide, was collected, and cooled over ice till the solid product precipitates. The mixture was filtered and the solid product dried. It was then used for the next stage of synthesis without further purification. General procedure for the synthesis of the different 2,5-disubstituted 1,3,4-oxadiazoles (A1, A2, A3) The synthetic procedure followed a similar pathway reported by [21]. Stoichiometric amounts of the substituted acid hydrazides (0.006 mol) and the dicarboxylic acids (0.003 mol) were carefully dissolved in dry phosphorus oxychloride (10 mL). The resulting solution was refluxed at a temperature of 110 °C for 9 h. Excess of dry phosphorus oxychloride was then distilled off and the mixture was gradually poured into crushed ice with stirring. The separated solid was filtered, washed thoroughly with cold water and 20 % NaHCO3 solution [21]. The solid was then recrystallized using ethanol.
Antimicrobial screening of the synthesized compounds
The test microorganisms used for this analysis were clinical isolates of bacteria and a fungus obtained from Ahmadu Bello University Teaching Hospital, Zaria, Nigeria. The antimicrobial analysis was done in the Department of Microbiology, Ahmadu Bello University, Zaria, Nigeria. The isolates were: Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans. All media were prepared according to manufacturer’s instruction and sterilized by autoclaving at 121 °C for 15 minutes.
Determination of inhibitory activity (sensitivity test) of the synthesized compounds using agar well diffusion method
The standardized inoculate of both the bacterial and the fungal isolates were streaked on sterilized Muller Hinton and potato dextrose agar plates respectively with the aid of sterile swap sticks. Four wells were pouched on each inoculated agar plate with a sterile cork borer. The wells were properly labeled according to different concentrations of the extract prepared which were 20, 10, 5 and 2.5 mg/mL respectively. Each well was drilled up with approximately 0.2 mL of the synthesized compound dissolved in water. The inoculated plates with the compounds were allowed to stay on the bench for about one hour; this is to enable the compound to diffuse on the agar. The Muller Hinton agar plates were then incubated at 37 °C in an oven for 24 h while the plates of potato dextrose agar were incubated at room temperature for about 3-5 days. At the end of the incubation period, the plates were observed for any evidence of inhibition which appeared as a clear zone that was completely devoid of growth around the wells (zone of inhibition). The diameters of the zones were measured using a transparent ruler [24]. Ciprofloxacin was used as the standard for bacteria because it is a wide spectrum antibiotic that can be used for both gram-positive and gram-negative bacteria, while Econazole was the standard used for the fungus.
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) of the synthesized compounds was determined using tube dilution method with Muller Hinton broth used as diluents. The lowest concentration of the extract showing inhibition for each organism when the compound tested positive during sensitivity test was serially diluted in the test tubes containing Muller Hinton broth. The organisms were inoculated into each tube containing the broth in the compound. The inoculated tubes were then incubated at 37 °C for 24 h. At the end of the incubation period, the tubes were examined for the presence or absence of growth using turbidity as a criterion, the lowest concentration in the series without visible sign of growth (turbidity) was considered to be the minimum inhibitory concentration (MIC), and was recorded [24].
Determination of minimum bactericidal concentration (MBC)
The result from the minimum inhibitory concentration (MIC) was used to determine the minimum bactericidal concentration (MBC) of the synthesized compounds. A sterilized wire loop was dropped into the test tube(s) that did not show turbidity (clear) in the MIC test and a loopful was taken and streaked on sterile nutrient agar plates. The plates were incubated at 37 °C for 18-24 h. At the end of the incubation period, the plates were examined for the presence or absence of growth. This is to determine whether the antimicrobial effects of the extract are bacteriostatic or bactericidal [24].
Result and Dissection
Three different substituted aromatic esters (1) were synthesized from the corresponding aromatic carboxylic acids (Scheme 1). The esters were synthesized as liquids or solid in moderate to excellent yields. Ethyl benzoate (1a) was derived as a colorless oily liquid (80% yield) while ethyl 4-chlorobenzoate (1b) was derived as a light-yellow oily liquid (62% yield), and ethyl 4-nitrobenzoate (1c) as a pale white solid powder (41.7% yield, melting point = 56 °C). The low yield of 1c could be attributed to the polarity of the nitro group which may increase its solubility in the aqueous medium during extraction with ether. All the synthesized esters had characteristic sweet smell. The synthesis of substituted benzo hydrazides (2) was accomplished in low to moderate yields by reaction of (1) with hydrazine monohydrate. All the hydrazides synthesized from the esters were derived as solids; benzohydrazide (2a) (brown solid, 50% yield, melting point = 115 °C), 4-chlorobenzohydrazide (2b) (dirty-white needle-like solid, 32% yield, melting point = 164 °C), and 4-nitrobenzohydrazide (2c) (light brown solid, 31.4% yield, melting point = 210-214 °C).
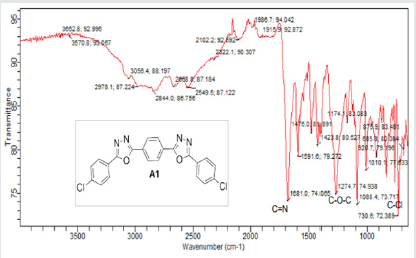
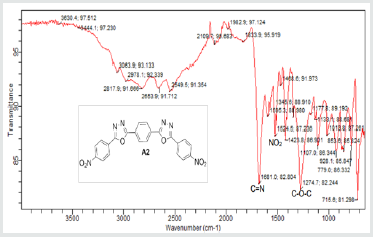
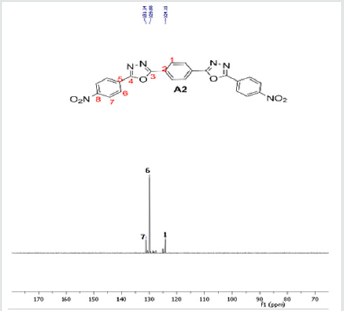
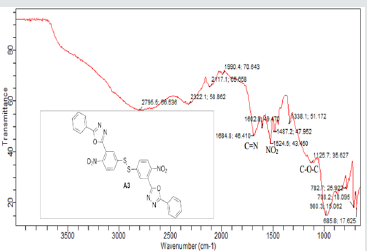
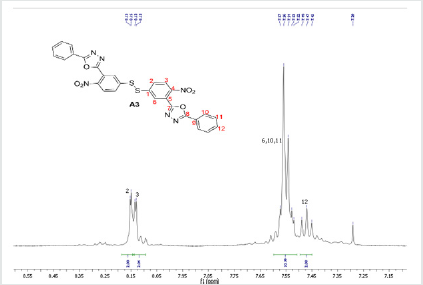
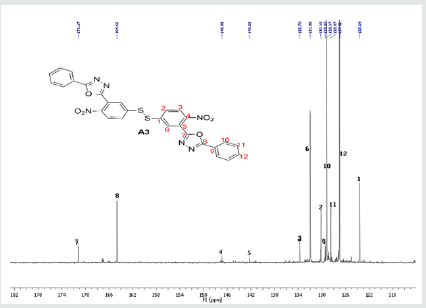
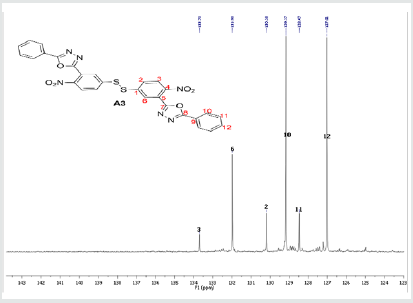
The hydrazides were then used to synthesize the substituted symmetrical oxadiazole derivatives A1, A2, and A3 containing two 2,5-disubstituted 1,3,4-oxadiazole rings. All substituents are in para-position to avoid steric hindrance/repulsion in the products that may occur at the ortho-position and to a lesser degree at the meta-position. The compounds were synthesized from the preceding hydrazides cyclized with various dicarboxylic acids in the presence of phosphorus oxychloride (Scheme 2). A1 was synthesized from 4-chlorobenzohydrazide (2b) and terephthalic acid. It was derived as a carton brown solid in 47.8% yield and melting point of 288-291 °C. Figures 1-5 show the characterization spectra for compound A1. Compound A2 was synthesized from 4-nitrobenzohydrazide (2c) and terephthalic acid. The presence of bulky nitro group did not affect the product yield as A2 was produced as a light yellow powdery solid with melting point of 131-133 °C and in 45.3% yield comparable to A1. Both A1 and A2 have strong IR bands at 1681 cm-1 and 1274 cm-1 corresponding to C=N and C-O-C groups respectively indicative of the formation of the oxadiazole ring. The 13C NMR and DEPT further confirmed this with peaks at 163.92 ppm and 166.82 ppm for the two inequivalent C=N carbons of the oxadiazole rings in A1 and 166.24 ppm and 167.11 ppm for those in A2. Figures 6-10 The 1H NMR accounted for all aromatic protons. The oxadiazole compound (A3) was synthesized from a rather unusual dicarboxylic acid, 5,5-dithio 2-nitrobenzoic acid) and benzohydrazide (Scheme 2). The choice of the dicarboxylic acid was due to the presence of sulphur. This is because the only reported case of a symmetrical compound with two oxadiazole rings [23] contains sulphur at the 2-position which we believe may be responsible for its moderate biological activity. A3 was synthesized in 50% yield as a chocolate/dark brown solid with melting point of 101-103 °C. The IR also showed characteristic bands at 1684 cm-1and 1126 cm-1 corresponding to C=N and C-O-C groups of the oxadiazole ring. This was further confirmed by 13C NMR and DEPT analysis which showed peaks at 164.65 ppm and 171.17 ppm indicative of the two inequivalent C=N carbon atoms of the oxadiazole ring. The 1H NMR accounted for all aromatic protons Figures 11-15.
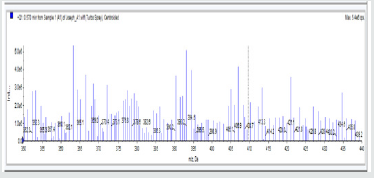
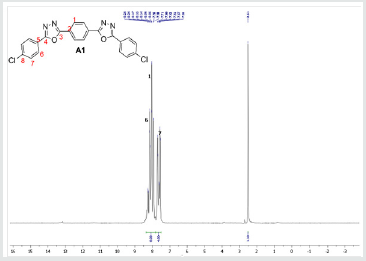
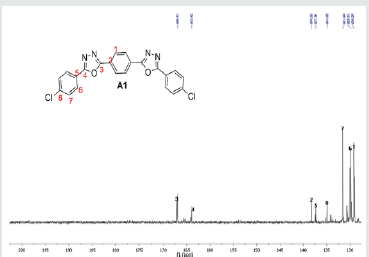
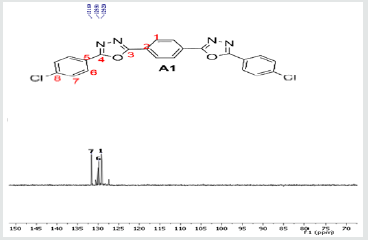
A1: 1,4-bis(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl)benzene, C22H12N4O2Cl2: Carton brown solid, 47.8% yield, melting point = 288-291 °C. IR: 1681 cm-1 (C=N), 730 cm-1 (C-Cl), 1274 cm-1 (C-O-C). 1H NMR (400MHz, DMSO-d6): 7.63 ppm (d, 4H), 8.15 ppm (d, 4H), 8.05 ppm (s, 4H). 13C NMR (400MHz, DMSO-d6): 129.23, 129.91, 131.60, 134.93, 137.36, 138.25, 163.92, and 166.82 ppm. MS: m/z 434.6 (M˙+) (M+4 peak also seen typical of compound with 2 Cl atoms)(Figures 1-5).
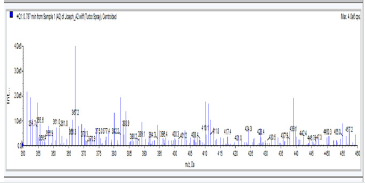
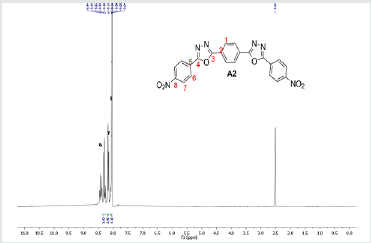
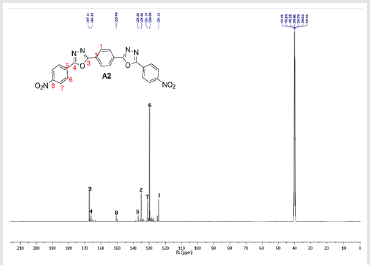
A2: 1,4-bis(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)benzene, C22H12N6O6: Light yellow powdery solid, 45.3% yield, melting point = 131-133 °C. IR: 1681 cm-1 (C=N), 1274 cm-1 (C-O-C), 1524 cm-1 (Aromatic NO2). 1H NMR (400MHz, DMSO-d6): 8.17 ppm (d, 4H), 8.32 ppm (d, 4H), 8.05 ppm (s, 4H). 13C NMR (400MHz, DMSO-d6): 124.15, 129.88, 131.39, 134.97, 137.36, 136.85, 150.49, 166.24, and 167.11 ppm. MS: m/z 457.2 (M+1˙+)(Figures 6-10).
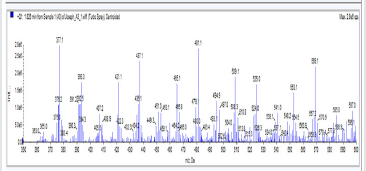
A3: 1,2-bis(4-nitro-3-(5-phenyl-1,3,4-oxadiazol-2-yl)phenyl)disulfane, C28H16N6O6S2: Chocolate/dark brown solid, 50% yield, melting point = 101-103 °C. IR: 1684 cm-1 (C=N), 1126 cm-1 (C-O-C), 1524 cm-1 (Aromatic NO2). 1H NMR (400MHz, CDCl3): 8.15 ppm (d, 2H), 8.13 ppm (d, 2H), 7.52-7.58 (m, 10H), 7.47 ppm (t, 2H). 13C NMR (400MHz, CDCl3): 123.59, 127.02, 128.47, 129.17, 129.38, 130.18, 131.98, 133.7, 142.23, 146.93, 164.65, and 171.17 ppm. MS: m/z 597.0 (M˙+)(Figures 11-15).
All the oxadiazoles synthesized were largely insoluble in water but moderately soluble in chloroform and ethanol.
Result from antimicrobial screening
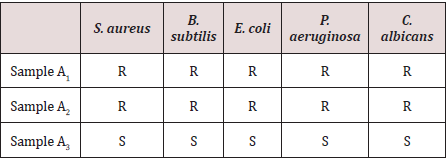
Tables 1 and 2 present the results of the antimicrobial screening which revealed that A1 and A2 at concentrations of 2.5 to 20 mg/mL do not have biological activity against the five test organisms used for the analysis. Compound A3 on the other hand showed appreciable biological activity against all the five test organisms compared to the standards (ciprofloxacin and econazole) used. The MIC of A3 was found to be 1.11 mg/mL on Pseudomonas aeruginosa, 0.37 mg/mL on Staphylococcus aureus, 1.11 mg/ml on Escherichia coli, and 1.11 mg/ml on Candida albicans. The MBC was found to be 3.33 mg/ml on Pseudomonas aeruginosa, 1.11 mg/ml on Staphylococcus aureus, 3.33 mg/ml on Escherichia coli, and 3.33 mg/ml on Candida albicans (Table 3). The activity may be due to the presence of sulphur in the compound. However, A3 is bacteriostatic towards Bacillus subtilis. In the only reference available for the synthesis of 1,3,4-oxadiazole derivatives using terephthalic acid, it reported the synthesis of 5,5’-benzene-1,4-diylbis-1,3,4-oxadiazole-2-thiol and tested in vitro activity against E. faecalis and E. Coli. The compound showed an intermediate biological effect on both bacteria [23]. Therefore, the presence of sulphur atoms in these symmetrical compounds (A3 and the previously reported compound [23] containing two 1,3,4-oxadiazole rings, could account for the observed biological activity against the test microorganisms.
Conclusion
This paper reports the synthesis of three new C2 symmetrical 2,5-disubstituted 1,3,4-oxadiazole derivatives, with two oxadiazole rings, from dicarboxylic acids (terephthalic acid and 5,5-dithiobis-(2-nitrobenzoic acid)) rather than the usual monocarboxylic acids. The final products, 1,4-bis(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl)benzene, 1,4-bis(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)benzene and 1,2-bis(4-nitro-3-(5-phenyl-1,3,4-oxadiazol-2-yl)phenyl)disulfane, each contain two 2,5-disubstituted 1,3,4-oxadiazole rings. The products were obtained in yields between 45.3% to 50% and were successfully characterized by spectroscopic methods. The compounds were tested for antimicrobial activity against five organisms namely Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans, of which only 1,2-bis(4-nitro-3-(5-phenyl-1,3,4-oxadiazol-2-yl)phenyl)disulfane showed appreciable biological/antimicrobial activity against these microorganisms at concentrations of 2.5 to 20 mg/ml. The “biological inactivity” of 1,4-bis(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl)benzene and 1,4-bis (5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)benzene may be due to the symmetry of the synthesized compounds and could be indicative of the reason symmetrical dicarboxylic acids are rarely used for the synthesis of 1,3,4-oxadiazole compounds as observed in the literature. For 1,2-bis(4-nitro-3-(5-phenyl-1,3,4-oxadiazol-2-yl)phenyl)disulfane, its biological activity is thought to be due to the presence of sulphur atoms in the molecule. Further experiments can be done to ascertain the effect of symmetry and the presence of certain atoms on the biological activity of oxadiazole compounds.
Conflicts of Interest
The authors have not declared any conflict of interest.
Acknowledgement
The authors thank the Microbiology Department, Ahmadu Bello University, Nigeria, for providing space and supplies for antimicrobial analyses.
References
- Vandekerckhove S, Dhooghe M (2015) Quinoline-based antimalarial hybrid compounds. Bioorganic and Medicinal Chemistry 23(16): 5098-5119.
- Alya MRE, Ibrahim MM, Okaeld AM, Gherbawy Y (2014) Synthesis, insecticidal and fungicidal screening of some new synthetic quinolone derivatives. Russian Journal of Bioorganic Chemistry 40(2): 214-217.
- Ezema BE, Ezema CG, Ugwu DI, Ayogu JI (2014) Synthesis of heterocyclic azo dyes from Quinolin-8-ol. Chemistry and Materials Research 6(9): ISSN2225-0956.
- Kerru N, Gummmidi L, Maddila S, Gangu KK, Jonnalagadda SB (2020) A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 25(8): 1909.
- Khalil MA, El-Khawass SM, Kassem MG (1980) Some novel 3,5-disubstituted oxadiazole-2-thiones as potential antimicrobial agents. Scientia Pharmaceutica 48: 344-350.
- Pidugu VR, Yarla NS, Pedada SR, Kalle AM Satya AK (2016) Design and synthesis of novel HDAC8 inhibitory 2,5-disubstituted-1,3,4-oxadiazoles containing glycine and alanine hybrids with anticancer activity. Bioorganic and Medicinal Chemistry 24: 5611-5617.
- Dabhi TP, Shah VH, Parikh AR (1992) Studies on 1,3,4-oxadiazoles: preparation and antimicrobial activity of 2-mercapto-/carboxy-methylthio-5-(30-arylaminosulfophenyl)-1,3,4-oxadiazoles. Indian Drugs 54: 98-154.
- Gul S, Rehman A, Abbasi MA, Khan KM, Nafeesa K, and et al., (2014) Synthesis, antimicrobial evaluation and hemolyticactivity of 2-[[5alkyl/aralkylsubstituted-1,3,4-oxadiazol-2-yl]thio]-N-[4-(4-morpholinyl)phenyl]acetamide derivatives. Journal of Saudi Chemistry 21: S425-S433.
- Zoumpoulakis P, Camoutsis Ch, Pairas G, Sokovic M, Glamoclija J, et al. (2012) Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Biological evaluation and conformational analysis studies. Bioorganic and Medicinal Chemistry 20: 1569-1583.
- Ingale N, Maddi V, Palkar M, Ronad P, Mamledesai S, et al. (2012) Synthesis and evaluation of anti-inflammatory and analgesic activity of 3-[(5-substituted-1,3,4-oxadiazol-2-yl-thio)acetyl]-2H-chromen-2-ones. Medicinal Chemistry Research 21: 16-26.
- Sriram D, Banerjee D, Yogeeswari P (2009) Efavirenz Mannich bases: Synthesis, anti-HIV and antitubercular activities. Journal of Enzyme Inhibition Medicinal Chemistry 24: 1-5.
- Macaev F, Rusu G, Pogrebnoi S, Gudima A, Stingaci E, et al. (2005) Synthesis of novel 5-aryl-2-thio-1,3,4-oxadiazoles and the study of their structure-anti-mycobacterial activities. Bioorganic & Medicinal Chemistry 13(16): 4842-4850.
- Palmer JT, Hirschbein BL, Cheung H, McCarte J, Janc JW, et al. (2006) Keto-1,3,4-oxadiazoles as cathepsin K inhibitors. Bioorganic and Medicinal Chemistry 16(11): 2909-2914.
- Khan MTH, Choudhary MI, Khan KM, Rani M, Atta-ur-Rahman (2005) Structure-activity relationships of tyrosinase inhibitory combinatorial library of 2,5-disubstituted-1,3,4-oxadiazole analogues. Bioorganic and Medicinal Chemistry 13(10): 3385-3395.
- Ke S, Li Z, Qian X (2008) 1,3,4-oxadiazole-3(2H)-carboxamide derivatives as potential novel class of monoamine oxidase (MAO) inhibitors: Synthesis, evaluation, and role of urea moiety. Bioorganic and Medicinal Chemistry 16(16): 7565-7572.
- Almasirad A, Tabatabai SA, Faizi M, Kebriaeezadeh, A, Mehrabi N, et al. (2004) Synthesis and anticonvulsant activity of 2-substituted-5-[2-(2-fluorophenoxy)phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorganic & Medicinal Chemistry Letters 14(24): 6057-6059.
- Bondock S, Adel S, Etman HA, Badria FA (2012) Synthesis and antitumor evaluation of some new 1,3,4-oxadiazole based heterocycle. European Journal of Medicinal Chemistry 48: 192- 199.
- Vardan S, Smulyan H, Mookherjee S, Eich R (1983) Effects of tiodazosin, a new antihypertensive, hemodynamics and clinical variables. Clinical Pharmacology and Therapeutics (34): 290-296.
- Schlecker R and Thieme PC (1988) The synthesis of antihypertensive 3-(1,3,4-oxadiazol-2-yl) phenoxypropanolahines. Tetrahedron 44(11): 3289-3294.
- Ogata M, Atobe H, Kushida H, Yamamoto K (1971) In vitro sensitivity of mycoplasmas isolated from various animals and sewage to antibiotics and nitrofurans. Journal of Antibiotics 24: 443-451.
- Malladi S, Isloor AM, Peethambar SK, Fun HK (2014) Synthesis and biological evaluation of newer analogues of 2,5-disubstituted 1,3,4-oxadiazole containing pyrazole moiety as antimicrobial agents. Arabian Journal of Chemistry 7(6): 1185-1191.
- Kumar RN, Poornachandra Y, Nagender P, Kumar GS, Swaroop DK, and et al., (2016) Synthesis of novel nicotinohydrazide and (1,3,4-oxadiazol-2-yl)-6-(trifluoromethyl) pyridine derivatives as potential anticancer agents. Bioorganic and Medicinal Chemistry Letters 26(19): 4829-4831.
- Datoussaida Y, Othmana AA, Kirschb G (2012) Synthesis and antibacterial activity of some 5,5’-(1,4-phenylene)-bis-1,3,4-oxadiazole and bis 1,2,4-triazole derivatives as precursors of new S-nucleosides. South African Journal of Chemistry 65: 30-35.
- Bradshaw LJ (1979) Laboratory Manual, third Edition. WB Saunders Company, London, UK.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...