Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Review Article(ISSN: 2637-4609) 
Synthesis and Antitubercular Acitivity of New Imidazo [2,1-B] [1,3,4] Thiadiazole-Phenothiazine Derivatives Volume 3 - Issue 4
Sunil Makwane1 and Rajiv Dua2*
- 1 Department of Chemistry, Dr. Harisingh Gour University, India
- 2 Department of Chemistry, Government of Madhya Pradesh, India
Received: August 21, 2018; Published: August 29, 2018
*Corresponding author:Rajiv Dua, Department of Chemistry, State Forensic Science Laboratory, Department of Home (Police), Government of Madhya Pradesh, India
DOI: 10.32474/AOICS.2018.03.000169
Abstract
New series of 10-(2-Styryl-5,6-dihydro-imidazo[2,1-b] [1,3,4] thiadiazole-6-yl)-10H-phenothiazine were synthesized by cyclisation of various carboxylic acid with thiosemicarbazide in presence of sulphuric acid was to get compound 1. Another way phenothiazine treated with chloroacetyl chloride yielded compound 2. Further cyclisation of compound 1 and 2 followed by refluxation about 18 hrs to get the final products 3 and 3a-3i of the series. The structures of compounds were confirmed by IR, 1H-NMR, 13C NMR and mass spectroscopy and by chemical analysis. All the final synthesized compounds 3 and 3a-3i were screened for their antitubercular activity screened against M. tuberculosis H37 Rv.
Keywords:Thiadiazole; Phenothiazine; Thiadiazole; Antitubercular Activity
Graphical Abstract
Scheme 1.
Introduction
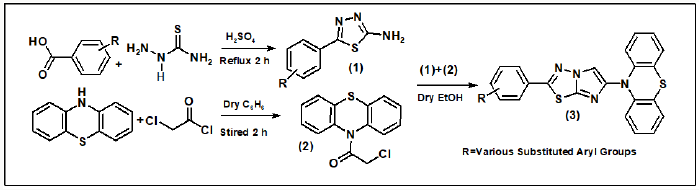
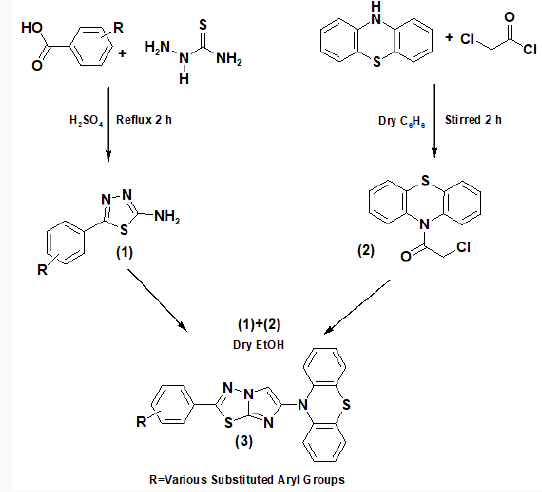
Tuberculosis (TB) is one of the dominant killer diseases [1] and it causes huge amount of human deaths despite the availability of more than 20 anti TB drugs and the Bacille Calmette Guerin (BCG) anti TB vaccine [2]. The emergence of the extensively drug-resistant tuberculosis (XDR-TB) and multidrug-resistant tuberculosis (MDR-TB), against which the traditional anti-TB drugs show limited efficacy,[3] further cause serious problem in TB control. With a population of 1.32 billion, India has the highest burden of tuberculosis (TB) and drug resistant TB (DR-TB) in the world. The Global TB Report 2017 published by World Health Organization (WHO) estimates that India contributes 27% (2.79 million) and 25% (147 000) of the global burden of TB and multi-drug resistant TB (MDR-TB), respectively. The Revised National Tuberculosis Control Programme (RNTCP) has notified 1.94 million patients in 2016. India has been locating and treating MDR-TB patients since 2007 and achieved complete geographical coverage of programmatic management of drug-resistant TB (PMDT) services in 2013 [4]. In the past years, the literature is enriched with progressive finding about the synthesis and pharmacological actions of fused heterocycles [5]. In the field of synthetic organic chemistry nitrogensulphur heteroatom containing aromatic molecules particularly 10-H-phenothiazine and 1,3,4-thiadiazole are becoming more popular as an area of research and provides a most valuable molecular template for the development of new molecule that can interact with a wide variety of biochemical processes. They have been shown to possess a broad spectrum of pharmacological activities such as anti-tubercular [6-7] anti-tumour [8] anti-inflammatory [9] antihyperlipidemic [10], cytotoxic [11], antimicrobial [12] and antiproliferative [13] agents. In continuation in our aim synthesis of new bioactive molecule by incorporating phenothiazine and 1,3,4 heterothiadiazole moieties in a single molecular framework, both molecules have broad biological spectrum such as antibacterial [14,15], antifungal [16,17] anticancer [18,19] anticonvulsant [20,21], antitubercular [22,23] and anti-inflammatory [24,25] herein, we carry out the synthesis and antimicrobial evaluation of some new synthesized molecule. Number of molecules have been claimed by researchers Imidazo [2,1-b]-1,3,4-thiadiazole all around the world because of its excellent biological profile. We have decided to synthesize a new series of 10-(2-Phenyl-imidazo [2,1-b] [1,3,4] thiadiazol-6-yl)-10H-phenothiazine shown in Scheme 1. The starting material, thiosemicarbazide undergoes cyclodehydration of acyl thiosemicarbazides treated with in situ by heating the various carboxylic acid in presence of H2SO4 yielded compound 1, 5-Phenyl-[1,3,4]thiadiazol-2-ylamine. In another separate reaction 10-H phenothiazine treated with chloroacetyl chloride yielded compound 2, 2-Chloro-1-phenothiazin-10-yl-ethanone. Further condensation reaction of compound 2 and compound 1 under reflux in dry ethanol 18 hrs yielded compound 3, 10-(2-Phenylimidazo[ 2,1-b] [1,3,4] thiadiazol-6-yl)-10H-phenothiazine, further compounds (3a-3i) synthesized by similar method as reported earlier. The structure of compounds 1 and (1a-1i), compound 2, and compound 3 and (3a-3i) were confirmed by IR, 1H NMR, 13C NMR, mass and chemical analysis. All the compounds 3 and 3a-3i were screened for their antitubercular activity screened against M. tuberculosis H37 Rv (Scheme 1).
Materials and Methods
All the chemicals and reagents were of analytical grade of sigma Aldrich, Merck, Chemi-loba and Himedia. The reagents and solvents were purified before using by standard methods. Melting points were taken in open capillaries and are uncorrected. Progress of reaction was monitored at various stages by silica gel-G coated TLC plates using MeOH: CHCl3 system. The spot was visualized by exposing dry plate at iodine vapour chamber and fluorescent indicator F 254 UV chamber. IR spectra were recorded in KBr disc on a Schimadzu 8201 PC, FTIR spectrophotometer (v max in cm-1) and 1H NMR and 13C NMR spectra were measured on a Brucker DRX- 300 spectrometer in CDCl3 at 500 and 75 MHz respectively using TMS as an internal standard. All chemical shifts were reported on δ scale. The mass spectra were recorded on a Jeol SX-102 GC-MS mass spectrometer. Elemental analyses were performed on a Carlo Erba-1108 analyzer. The analytical data of all the compounds were highly satisfactory. All the synthesized compounds were purified by column chromatography using Merck silica Gel 60 (230-400 Mesh). The reagent grade chemicals were purchased from the commercial sources.
Synthesis of 5-Phenyl- [1,3,4] thiadiazol-2-ylamine
Equimolar mixture of thiosemicarbazide (0.004 mole) and benzoic acid (0.004mole) in presence of H2SO4 in dry ethanol (25ml) was refluxed on a water bath for about 2hrs TLC was used to check reaction progress, then mixture was removed and poured in crushed ice to get a white precipitate, compound 1. A solid product was obtained which was purified over a silica gel column using chloroform: methanol (8:2 v/v) mixture as eluant. The elute was concentrated to get a solid product which was recrystallized from ethanol to yielded compound 1: White crystalline solid. M.P. 223- 225 0C,Yield 70%, IR( ν max cm-1): 1430 (νC-C), 1070 (νC-N) 763 (νC-S), 1454 (νC=C), 1585 (νN=C), 3378 (ν-NH2), 1H NMR: δ(ppm) 4.87 (2H, s, NH2) , 7.29 -7.73 (5H, m, Ar-H), 13C NMR : δ (ppm)126.9-131.01(C of aromatic ring), 169.4,163.8(C2,C5 of thiadiazole ring), Anal. Calcd. for C8H7N3S:C, 54.22, H, 3.98, N, 23.71% found C, 54.09, H, 3.70, N, 23.40%; MS 177.03 (M+).
The compounds 1a-1i were synthesized by the similar method as reported earlier.
a) 5-(2-chloro-phenyl)-[1,3,4]thiadiazole-2-ylamine: M.P.229-2300C, Yield 72%, IR( ν max cm-1): 1433(νC-C), 1072 (νCN ), 780 (νC-S),1541(νC=C), 1587 (νN=C), 745 (νC-Cl) 3380 (ν-NH2), 1H NMR δ(ppm) 4.73(2H, s, NH2)7.27-8.18 (4H, m, Ar-H), 13C NMR: δ(ppm) 127.5-133.1(C of aromatic ring),163.3,169.4(C2C5 of thiadiazole ring), Anal. Calcd. for C8H6 ClN3S: C, 45.39, H, 2.86, N,19.85% found C,45.09, H, 2.70, N,19.40%; MS 211.01 (M+)
b) 5-(3-chloro-phenyl)-[1,3,4]thiadiazole-2-ylamine: M.P.228-2300C, Yield 69%, IR( ν max cm-1): 1429 (νC-C), 1069 (νC-N), 779 (νC-S), 1537(νC=C), 1587 (νN=C), 737 (νC-Cl) 3382 (ν-NH2), 1HNMR: δ(ppm) 4.75 (2H, s, NH2), 7.35-7.68 (4H, m, Ar-H),13C NMR : δ (ppm) 126.91-31.01 (C of aromatic ring),169.2,162.9(C2C5 of thiadiazole ring), Anal. Calcd. for C8H6 ClN3S: C,45.39, H, 2.86, N, 19.85 % found C, 45.19, H, 2.65, N, 19.49%; MS 211.0 (M+)
c) 5-(4-chloro-phenyl)-[1,3,4]thiadiazole-2-ylamine: M.P.230-2320C,Yield73%, IR( ν max cm-1): 1427 (νC-C), 1050 (νCN ), 781 (νC-S), 1539 (νC=C), 1588 (νN=C), 749 (νC-Cl),3383 (ν-NH2), 1HMNR: δ(ppm) 4.89 (2H, s, NH2), 7.73-7.85 (4H, m, Ar-H), 13C NMR: δ (ppm) 129.1-135.6 (C of aromatic ring), 163.4,168.8( C2C5 of thiadiazole ring), Anal. Calcd. for C8H6 ClN3S: C,45.39, H, 2.86, N, 19.85% found C, 45.19, H,2.61, N, 19.38%; MS 211.02 (M+)
d) 5-(2-bromo-phenyl)-[1,3,4]thiadiazole-2-ylamine: M.P.228-2300C,Yield 68%, IR ( ν max cm-1): 1426 (νC-C),1055 (νC-N), 768 (νC-S), 1545 (νC=C), 1640 (νN=C), 545 (νC-Br) 3386 (ν- NH2), 1HNMR: δ(ppm) 4.80 (2H, s, NH2) 7.25-7.89 (4H, m, Ar- H), 13C NMR : δ (ppm) 163.1,169.5( C2 C5 of thiadiazole ring), 120.7- 132.1(C of aromatic ring), Anal. Calcd. for C8H6BrN3S: C, 37.52, H, 2.36, N, 16.41% found C, 37.18, H, 2.21, N, 16.35%; MS 254.94 (M+).
e) 5-(3-bromo-phenyl)-[1,3,4]thiadiazole-2-ylamine: M.P. 229-2300C, Yield 67%, IR ( ν max cm-1): 1431 (νC-C), 1052 (νC-N), 767 (νC-S), 1540 (νC=C), 1646 (νN=C), 536 (νC-Br), 3388 (ν-NH2), 1H NMR: δ(ppm) 4.84 (2H, s, NH2), 7.31-7.64 (4H, m, Hz, Ar-H), 13C NMR: δ (ppm): 118.1-132.9(C of aromatic ring),164.08,168.9(C2 C5 of thiadiazole ring), Anal. Calcd. for C8H6BrN3S: C, 37.52, H, 2.36, N, 16.41% found C, 37.28, H, 2.24, N, 16.25%; MS 254.82 (M+).
f) 5-(4-bromo-phenyl)-[1,3,4]thiadiazole-2-ylamine: M.P. 231-233 0C, Yield 69%, IR (ν max cm-1): 1429 (νC-C), 1041 (νC-N), 766 (νC-S), 1543 (νC=C), 1642 (νN=C), 541(νC-Br), 3390 (ν- NH2), 1H NMR: δ(ppm) 4.79(2H, s, NH2) ,7.68-7.79(4H, m, Ar-H), 13C NMR :δ (ppm) 124.0-131.0 (C of aromatic ring), 164.1- 169.5(C2 C5 of thiadiazole ring), Anal. Calcd. for C8H6BrN3S: C, 37.52, H, 2.36, N, 16.41% found C, 37.24, H, 2.20, N, 16.35%; MS 254 .86(M+).
g) 5-(2-nitro-phenyl)-[1,3,4]thiadiazole-2-ylamine: M.P. 257-2590C, Yield 78%, IR (ν max cm-1): 1428 (νC-C), 1053 (νC-N), 778 (νC-S), 1515 (νC=C), 1651 (νN=C), 1341(νC-NO2), 3391 (-νNH2), 1H NMR: δ(ppm) 4.90 (2H, s, NH2), 7.59-8.27 (4H, m, Ar- H), 13C NMR : δ (ppm) 127.5-148.3(C of aromatic ring), 164.01, 169.6(C2C5 of thiadiazole ring), Anal. Calcd. for C8H6N4O2S: C, 43.24, H, 2.72, N, 25.21% found C,43.14, H, 2.52, N, 25.8%; MS 222.02 (M+).
h) 5-(3-nitro-phenyl)-[1,3,4]thiadiazole-2-ylamine: M.P. 259-2610C, Yield 80%, IR: (ν max cm-1): 1426 (νC-C), 1048 (νC-N), 776 (νC-S), 1527 (νC=C), 1656 (νN=C), 1343 (νC-NO2), 3393 (-νNH2),1H NMR: δ(ppm) 4.78 (2H, s, NH2), 7.59-7.91 (4H, m, Ar- H),13CNMR: δ (ppm) 116.3-140.4 (C of aromatic ring), 164.2 169.3 (C2 C5 of thiadiazole ring), Anal. Calcd. for C8H6N4O2S: C, 43.24, H, 2.72, N, 25.21% found C, 43.16, H, 2.62, N, 25.10%; MS 222.22 (M+).
i) 5-(4-nitro-phenyl)-[1,3,4]thiadiazole-2-ylamine: M.P. 258-2600C, Yield 79%, IR(ν max cm-1): 1432 (νC-C), 1055(νCN ), 771 (νC-S), 1522 (νC=C), 1655 (νN=C), 1340 (νC-NO2), 3395 (ν-NH2), 1H NMR: δ(ppm) 4.81(2H, s, NH2) , 7.71-8.27 (4H, m, Ar-H), 13C NMR: δ (ppm)117.2-140.4( C of aromatic ring), 164.2-168.8 (C2C5 of thiadiazole ring), Anal. Calcd. for C8H6N4O2S: C, 43.24, H, 2.72, N, 25.21% found C, 43.26, H, 2.60, N, 25.12%; MS 222.19 (M+).
Synthesis of 2-Chloro-1-phenothiazin-10-yl-ethanone
Chloroacetyl chloride (0.06 mol) was added drop wise at 0.5 0C to phenothiazine (0.06 mol) in dry benzene (100 ml) and the mixture was stirred for 2 hrs. Reaction progress was checked by TLC during the reaction. After the completion of the reaction, the benzene was distilled off to get a solid product washed with petroleum ether which was purified over a silica gel column using chloroform: methanol (8:2 v/v) mixture as eluant. The elute was concentrated to give a product which was recrystallized from ethanol to yielded compound 2. M.P.190-1920C, Yield 94%, IR: (ν max cm-1) 1470 (νC-C), 2936 (ν C-H), 1333(νN-C),1552 (νC=C), 2836 (ν-CH2),1671(νC=O), 685 (ν C-S-C),735(ν C-Cl). 1H NMR: δ(ppm) 4.35(2H, s acyclic CH2), 7.14-7.40 (8H, m, Ar-H),13C NMR δ (ppm) 123.1-138.8 (C of phenothiazine ring), 165.5(C=O acyclic), 42.2 (CH2 acyclic), Anal. Calcd. for C14H10ClNOS: C, 60.98; H, 3.66, N, 5.08, found C, 60.76, H, 3.50, N, 5.01, MS 275.02 (M+).
10-(2-Phenyl-imidazo[2,1-b] [1,3,4] thiadiazol-6-yl)- 10H-phenothiazine
Equimolar amount of 5-Phenyl- [1,3,4] thiadiazol-2-ylamine, compound 1 (0.004 Mole) and Chloro-1-phenothiazin-10-ylethanone, compound 2 (0.004mol) in ethanol (20ml) was refluxed on a water bath for about 18 hr. After the completion of the reaction, the methanol was distilled off to get a solid product which was purified over a silica gel column using chloroform: methanol (8:2 v/v) mixture as eluant. The elute was concentrated to give a product which was recrystallized from ethanol to yielded compound 3. Light green shinny crystalline solid. Light green crystalline solid, M.P. 210- 2120C, Yield 70%, IR: (ν max cm-1) 1481 (νC-C), 3171(νC-H),1638(νC=N thiadiazole),1589(νC=N imidazole),1286(νN-C),772(νC-S),1495 (νC=C), 681 (νC-S-C phenothiazine). 1H NMR:δ(ppm) 6.77(1H, s, imidazole),7.1-7.4 (8H, m, Ar-H phenothiazine),7.45-7.46 (3H, m Ar-H thiadiazole),8.00(2H, d, J = 8.0, Hz, Ar-H), 13C NMR: δ(ppm) 125.9-130.4(C of aromatic ring), 124.2, 144.0 and 116.6-128.1(C of phenothiazine), 175.2,164.4 (C2,C5 thiadiazole), 100.9 and 150.6(C of imidazole), Anal. Calcd. for C22 H14 N4 S2: C, 61.03, H, 3.03, N, 12.94 %, found C, 61.00, H,3.01, N, 12.74%; MS 398.06 (M+).
The compounds 3a-3i were synthesized by the similar method as reported earlier
a. 10-[2-(2-Chloro-phenyl)-imidazo[2,1-b][1,3,4]thiadiazol- 6-yl]-10H-phenothiazine: M.P. 209-211 0C,Yield 71%, IR: (νmax cm-1)1479 (νC-C), 3172(νC-H), 1640 (νC=N thiadiazole), 1597(νC=N imidazole),1280(νN-C), 772(νC-S), 741(νC-Cl), 1493(νC=C),682(νC-S-C phenothiazine). 1H NMR: δ(ppm)6.71(1H, s, imidazole), 7.11-7.45 (8H, m, Ar-H phenothiazine), 7.40- 7.76 (4H, m, Ar-H aromatic ring),13C NMR:δ (ppm) 127.6- 133.2 (C of aromatic ring), 124.4,144.2 and 116.7-129.3 (C of phenothiazine),164.7,156.3 (C2,C5 thiadiazole), 100.8 and 150.4 (C of imidazole), Anal. Calcd. for C22H13Cl N4 S2, C, 61.03, H, 3.03, N,12.94%, found C, 61.00, H, 3.01, N, 12.74%; MS 432.03(M+).
b. 10-[2-(3-Chloro-phenyl)-imidazo[2,1-b][1,3,4] thiadiazol-6-yl]-10H-phenothiazine: M.P. 210-2110C, Yield 72%, IR:(ν max cm-1)1482 (νC-C), 3169 (νC-H), 1639 (νC=N thiadiazole), 1598 (νC=N imidazole), 1284(ν N-C),77(νC-S),737(νCCl ),1491(νC=C),679 (νC-S-C phenothiazine). 1H NMR: δ(ppm) 6.76(1H, s, imidazole), 7.42-7.71 (3H, m, Ar-H aromatic ring), 7.11-7.44 (8H, m Ar-H penothiazine), 7.95 (1H, t, J = 1.5, 0.4 Hz, Ar-H), 13C NMR: δ (ppm)126.9-131(C of aromatic ring), 124.1,145.1 and 116.6-128.0(C of phenothiazine), 157.7,164.3 (C2,C5 thiadiazole), 100.7 and 150.7 (C of imidazole), Anal. Calcd. for C22H13 ClN4S2: C, 61.03, H, 3.03, N, 12.94%, found C, 61.01, H, 3.00, N, 12.84%; MS 432.02 (M+).
c. 10-[2-(4-Chloro-phenyl)-imidazo[2,1-b][1,3,4]thiadiazol-6- yl]-10H-phenothiazine: M.P. 212-2140C, Yield 70%, IR:(νmax cm-1) 1480 (νC-C), 3176 (νC-H), 1638 (νC=N thiadiazole), 1589 (νC=N imidazole), 1285 (ν N-C), 770 (ν C-S), 742 (ν C-Cl), 1488 (νC=C), 682 (ν C-S-C phenothiazine).1H NMR: δ(ppm) 6.79(1H, s, imidazole),7.68-7.70 (4H, m, Ar-H aromatic ring), 7.12- 7.46 (8H, m Ar-H phenothiazine), 13C NMR : δ (ppm) 127.2- 135.7 (C of aromatic ring), 124.4,145.3 and 116.1-128.3(C of phenothiazine),157.7,156.3 (C2,C5 thiadiazole), 100.6 and 150.3 (C of imidazole), Anal. Calcd. for C22 H13 Cl N4S2:C, 61.03, H ,3.03, N, 12.94%, found C, 61.00, H, 3.02, N, 12.72%; MS 432.05 (M+).
d. 10-[2-(2-Bromo-phenyl)-imidazo[2,1-b][1,3,4] thiadiazol-6-yl]-10H-phenothiazine: M.P. 211-2120C Yield 70%, IR: (νmax cm-1) 1478 (νC-C),3175 (νC-H),1637(νC=N thiadiazole), 1588(νC=N imidazole), 1279(ν N-C) ,768 (νC-S), 542 (νC-Br), 1490 (νC=C), 685 (ν C-S-C phenothiazine). 1H NMR: δ(ppm) 6.73(1H, s, imidazole), 7.37-7.77 (4H, m, Ar-H aromatic ring),7.11-7.48 (8H, m Ar-H phenothiazine), 13C NMR: δ (ppm) 120.7-132.1 (C of aromatic ring),124.5,145.5 and 116.5- 128.3(C of phenothiazine),156.2,164.5(C2, C5 thiadiazole), 100.4 and 150.1 (C of imidazole), Anal. Calcd. for C22 H13 Br N4 S2: C, 55.35, H, 2.74, N,11.74%, found C, 55.20, H, 2.52, N, 11.62%, MS 475.96 (M+).
e. 10-[2-(3-Bromo-phenyl)-imidazo[2,1-b][1,3,4]thiadiazol- 6-yl]-10H-phenothiazine: M.P. 213-2140C, Yield 69%, IR:(ν max cm-1)1480(νC-C), 3174 (νC-H), 1638 (νC=N thiadiazole), 1590(νC=N imidazole),1281(νN-C)766 (νC-S), 540 (νC-Br) 1491 (νC=C), 683(νCS- C phenothiazine). 1H NMR: δ(ppm) 6.76 (1H, s, imidazole), 7.41-7.61 (3H, m, Ar-H aromatic ring), 7.10-7.49 (8H, m, Ar-H phenothiazine), 7.76 (1H, td, J = 1.5, Hz, Ar-H aromatic ring),13C NMR: δ(ppm)118.7-133.0 (C of aromatic ring), 124.6,145.7 and 116.7-128.4 (C of phenothiazine) , 175.1,164.4 (C2,C5 thiadiazole), 100.1 and 150.2(C of imidazole), Anal. Calcd. for C22 H13 Br N4 S2: C, 55.35%, H, 2.74, N,11.74 found C, 55.20, H, 2.52, N, 11.62%; MS 475.98(M+).
f. 10-[2-(4-Bromo-phenyl)-imidazo[2,1-b][1,3,4] thiadiazol-6-yl]-10H-phenothiazine: M.P. 215-2160C, Yield 68 %, IR:(ν max cm-1)1482 (νC-C), 3171 (νC-H), 1636 (νC=N thiadiazole), 1594 (νC=N imidazole), 1286 (νN-C) 769 (νC-S), 538 (νC-Br) 1489 (νC=C), 688 (νC-S-C phenothiazine), 1H NMR: δ(ppm) 6.72 (1H, s, imidazole), 7.69-7.78 (4H, m, Ar-H aromatic ring), 7.10-7.49 (8H, m Ar-H penothiazine), 13C NMR: δ(ppm)- 124- 131(C of aromatic ring), 124.2,145.8 and 116.8-128.7 (C of phenothiazine), 175.5,164.6 (C2,C5 thiadiazole), 100.3 and 150.7 (C of imidazole), Anal. Calcd. for C22H13BrN4S2: C, 55.35, H, 2.74, N, 11.74% found C, 55.19, H, 2.42, N, 11.72%; MS 475.99 (M+).
g. 10-[2-(2-Nitro-phenyl)-imidazo[2,1-b][1,3,4]thiadiazol- 6-yl]-10H-phenothiazine: M.P. 215-2170C, Yield 74%, IR :(ν max cm-1) 1487 (νC-C), 3170 (νC-H),1642(νC=N thiadiazole), 1598 (νC=N imidazole), 1287 (νN-C), 770 (νC-C), 1343 (νC-NO2) 1494 (νC=C), 689 (νC-S-C phenothiazine), 1H NMR: δ (ppm) 6.73 (1H, s, imidazole), 7.49-8.35 (4H, m, Ar-H aromatic ring), 7.11-7.48 (8H, m Ar-H phenothiazine), 13C NMR : δ (ppm) 127.6-148.4 (C of aromatic ring), 124.7,144.9 and 115.9-127.8( C of phenothiazine), 156.5,164.4 (C2,C5 thiadiazole), 100.2 and 150.6 (C of imidazole), Anal. Calcd. for C22H13N5O2S2: C, 59.58, H, 2.95, N, 15.79%, found C, 59.38, H, 2.85, N,15.59 %; MS 443.05 (M+).
h. 10-[2-(3-Nitro-phenyl)-imidazo[2,1-b][1,3,4]thiadiazol- 6-yl]-10H-phenothiazine (3h): M.P. 216-2180C, Yield 75 %,IR: (νmax cm-1)(νC-C) 1483, (νC-H) 3172, 1641 (νC=N thiadiazole), 1596 (νC=N imidazole), 1286 (νN-C), 773 (νC-S), 1340 (νC-NO2) ,1492 (νC=C), 685 (νC-S-C phenothiazine), 1H NMR: δ (ppm) 6.68 (1H, s, imidazole), 7.58-8.84 (4H, m, Ar-H aromatic ring), 7.10- 7.49 (8H, m Ar-H phenothiazine), 13C NMR : δ (ppm) 116.4- 140.5(C of aromatic ring), 124.8, 145.9 and 115.8-128.5(C of phenothiazine), 175.7,164.6 (C2,C5 thiadiazole), 100.1 and 150.9 C of imidazole, Anal. Calcd. for C22H13N5O2S2: C, 59.58, H, 2.95, N, 15.79 found C, 59.40, H, 2.81, N, 15.55; MS 443.04 (M+).
i. 10-[2-(4-Nitro-phenyl)-imidazo[2,1-b][1,3,4]thiadiazol- 6-yl]-10H-phenothiazine: M.P.220-2220C,Yield 76%, IR: (νmax cm-1) 1480 (νC-C), 3170 (νC-H), 1638 (νC=N thiadiazole), 1592 (νC=N imidazole), 1285 (νN-C), 770 (νC-S),1338 (νC-NO2), 1491 (νC=C), 685 (νC-S-C phenothiazine), 1H NMR: δ (ppm) 6.72 (1H, s, imidazole), 7.80-8.33 (4H, m, Ar-H aromatic ring), 7.11- 7.49 (8H, m Ar-H phenothiazine), 13C NMR : δ(ppm) 117.3- 140.5( C of aromatic ring), 124.9,145.6 and 116.8-128 (C of phenothiazine),175.1,163.4 (C2,C5 thiadiazole),100.5 and 150.8 (C of imidazole), Anal. Calcd. for C22H13N5O2S2: C, 59.58, H, 2.95, N,15.79%, found C, 59.37, H, 2.81, N, 15.59%; MS 443.02 (M+).
Results and Discussion
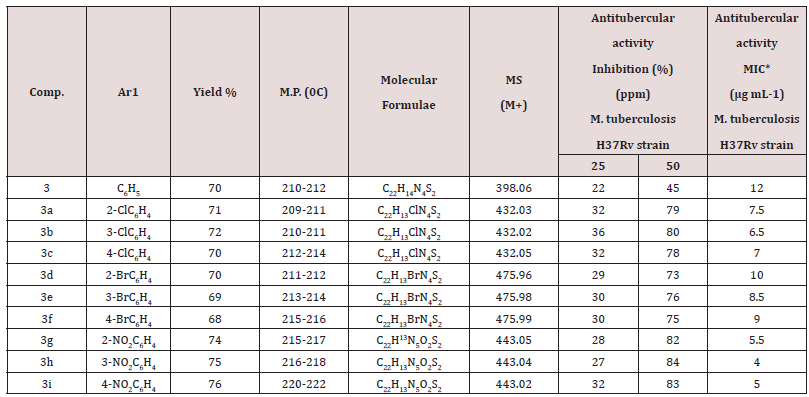
The reaction of thiosemicarbazide and benzoic acid afforded compound 1. The IR spectrum (ν cm-1) of compound 1 showed absorption peaks at 1560-1650 for (νN=C), 760-790 for (νC-S, thiadiazole ring) and peaks at 3350-3430 for (ν-NH2). The 1H NMR spectrum δ (ppm) exhibited the value at 3.9-4.9 (2H, s, NH2) ,7.28- 7.95 (5H, m, Ar-H) and 13C NMR spectrum gave δ (ppm) at 163-170 (C2,C5 of thiadiazole ring) supporting the structure of the compound 1. Similarly, the compounds 1 and 1a-1i have been synthesized by taking the various derivatives of benzoic acid. The IR spectra gave absorptions in the range of 1665-1675 cm−1 while strong signals appeared in the range of δ (ppm) 3.9-4.9 and 7.39-8.20 in the 1H NMR and δ(ppm)163-170 in the 13C NMR spectra supported the formation of compounds 1a-1i respectively. Another reaction was carried out between phenothiazine and chloroacetylchloride to give compound 2. The IR spectrum showed absorptions at 1671 cm-1 due to the presence of carbonyl function, 685 (ν C-S-C phenothiazine), 735(νC-Cl), 2836 (ν-CH2). In the 1H NMR, strong signals found at 4.35 (2H, s acyclic CH2), 7.14-7.40 (8H, m, Ar-H) and 13C NMR spectra gave signals at δ(ppm) 123.1-138.8 (C of phenothiazine ring), 165.5 (C=O acyclic), 42.2 (CH2 acyclic) supporting the confirmation of synthesis of compound 2. The compounds 1 and 1a-1i on reaction with equimolar amount of compound 2 in methanol gives the compounds 3 and 3a-3i. These compounds showed a characteristic IR absorption in the range of 1580-1600 cm−1 in the IR spectra showing the presence of N=C in the imidazole ring. The 1H NMR spectra clearly indicated the presence of one proton in imidazole ring in the range of δ(ppm) 6.68-6.79. The 13C NMR spectra of compound 3 and 3(a-i) also supported the formation of imidazole ring, δ(ppm) 100.1- 100.9 and 149.9-150.9 the above structures were supported by fact that the disappearance of NH2 proton and the appearance of N=CH proton in the range of δ(ppm) 6.68-6.79 (cyclic CH) in the 1H NMR spectra of compound 3 and 3a-3i. The compounds 2 and 2a-2i and 3 and 3a-3i were synthesized and compounds 3 and 3a-3i were screened for their antitubercular activity screened against M (Scheme 2). tuberculosis H37 Rv. Nitro group containing compounds showed higher activity in the order (3h> 3i > 3g) than chloro (3b>3a >3c) or bromo group containing compounds in the order (3e> 3f>3d). Based on structural activity relationship (SAR), concluded that the activity of compounds depends on electron withdrawing nature of the substituted groups, NO2 > Cl > Br > H. The MIC value of the synthesized compounds and standard drug showed in the Table 1
Antitubercular activity
The above synthesized compounds were screened against M. tuberculosis (H37Rv strain) using Lowenstein-Jensen (L.J.) Agar method at 50 and 100 μg/mL concentrations. The results were showing in Table 1. The standard antitubercular drugs isoniazid was taken as standards, showed 100% activity at both the above concentrations. The minimum inhibitory concentration (MIC) values of the synthesized compounds were determined.
Conclusion
In the conclusion we were successful in the initial hypothesis of synthesizing broad-spectrum antibiotics through experimentation. We report a successful effort to combine pharmacophoric groups; 5-Phenyl- [1,3,4] thiadiazol-2-ylamine and Chloro-1-phenothiazin- 10-yl-ethanone and the compounds were synthesised in good yield. The structures of compounds were established by FT-IR, 1H NMR, 13CNMR and Mass spectrometry techniques. The synthesized compounds posses antitubercular activity against Mycobacterium tuberculosis H37Rv strain.
Acknowledgement
The authors are thankful to SAIF, Punjab University, Chandigarh (India) And IISER Bhopal, (M.P.) India for providing spectral and analytical data of the synthesized compounds. We are also grateful to Mr. Vahid-Ul-Hassan, department of Zoology Dr. H.S. Gour University, Sagar [M.P.] for providing help in carrying out the antitubercular screening. We are also thankful to Head, Department of Chemistry, Dr. H. S. Gour Central University Sagar (India) for giving the facilities to carry out the work and UGC-New Delhi (India), for financial assistance as R.G.N.F. fellowship
References
- Herzog H (1998) History of tuberculosis. Respiration 65(1): 5-15.
- Tripathi RP, Tiwari N, Dwivedi N, Tiwari VK (2005) Fighting tuberculosis: an old disease with new challenges. Med Res Rev 25(1): 93-131.
- Koul A, Arnoult E, Lounis N, Guillemont J, Andries K (2011) The challenge of new drug discovery for tuberculosis. Nature 469 (7331): 483-490.
- (2017) Global Tuberculosis Report. World Health Organization, Geneva
- Tyagi MK, Srivastava SK, Srivastava SD (2014) Synthesis, Characterization of Some Novel Substituted Arylated Derivatives. J Applicable Chem 3(5): 1967-1972.
- Patel HM, Noolvi MN, Sethi NS, Gadad AK, Cameotra SS (2017) Synthesis and antitubercular evaluation of imidazo[2,1-b] [1,3,4] thiadiazole derivatives. Arabian Journal of Chemistry 10(Supp 1): 996-1002.
- Ramprasad J, Nayak N, Dalimba U, Yogeshwari P, Sriram D, et al. (2015) Synthesis and biological evaluation of new imidazo[2,1-b] [1,3,4] thiadiazole-benzimidazole derivatives. European Journal of Medicinal Chemistry 95: 49-63.
- Kumar S, Metikurki B, Bhadauria VS, Clercq ED, Schols D, et al. (2016) Synthesis of imidazo[2,1-b] [1,3,4] thiadiazole derivatives as possible biologically active agents. Acta Poloniae Pharmaceutica n Drug Research 73(4): 913-929.
- Jadhav VB, Kulkarni MV, Rasal VP, Biradar SS, Vinay MD (2008) Synthesis and anti-inflammatory evaluation of methylene bridged benzofuranyl imidazo [2,1-b] [1,3,4] thiadiazoles. European Journal of Medicinal Chemistry 43(8): 1721-1729.
- Patel HM, Noolvi MN, Goyal A, Thippeswamy BS (2013) 2,5,6-Trisubstituted Imidazo [2,1 b] [1,3,4] thiadiazoles: Search forantihyperlipidemic agent. European Journal of Medicinal Chemistry 65: 119-133.
- Kumar S, Hegde M, Gopalakrishnan V, Renuka VK, Ramareddy SA, et al. (2014) 2-(4-Chlorobenzyl)-6-arylimidazo[2,1-b] [1,3,4] thiadiazoles: Synthesis, cytotoxic activity and mechanism of action. European Journal of Medicinal Chemistry 84: 687-697.
- Alagawadi KR, Alegaon SG (2011) Synthesis, Characterization and Antimicrobial activity evaluation of new 2,4-Thiazolidinediones bearing imidazo [2,1-b] [1,3,4] thiadiazole moiety. Arabian Journal of Chemistry 4: 465-472.
- Romagnoli R, Baraldi PG, Prencipea F, Balzarinib J, Liekensb S, et al. (2015) Design, synthesis and antiproliferative activity of novel heterobivalent hybrids based on imidazo[2,1-b] [1,3,4] thiadiazole and imidazo[2,1b] [1,3] thiazole scaffolds. European Journal of Medicinal Chemistry 101(28): 205-217.
- Sharma R, Samadhiya P, Srivastava SD, Srivastava SK (2012) Synthesis and biological activity of 4-thiazolidinone derivatives of phenothiazine. J Serb Chem Soc 77(1): 17-26.
- Jalhan S, Jindal A, Gupta A, Hemraj (2012) Synthesis, Biological Activities and Chemistry of Thiadiazole Derivatives and Schiff Bases. Asian Journal of Pharmaceutical and Clinical Research 5(3):199-208.
- Kumar V, Upadhyay N, Manhas A (2015) Designing, syntheses, characterization, computational study and biological activities of silverphenothiazine metal complex. Journal of Molecular Structure 1099: 135-141.
- Li Q1, Ren J, Dong F, Feng Y, Gu G, et al. (2013) Synthesis and antifungal activity of thiadiazole-functionalized chitosan derivatives. Carbohydrate Research 373: 103-110.
- Abuhaie CM, Bicu E, Rigo B, Gautret P, Belei D, et al. (2013) Synthesis and anticancer activity of analogues of phenstatin, with a phenothiazine A-ring, as a new class of microtubule-targeting agents. Bioorganic & Medicinal Chemistry Letters 23(1): 147-152.
- Azaam MM, Kenawy ER, Badr El din AS, Khamis AA, El Magd MA (2018) Antioxidant and anticancer activities of aminophosphonates containing thiadiazole moiety. Journal of Saudi Chemical Society 22(1): 34-41.
- Laws ML, Roberts RR, Nicholson JM, Butcher R, Stables JP, et al. (1998) Synthesis, Characterization, and Anticonvulsant Activity of Enaminones. Part 5: Investigations on 3-Carboalkoxy-2- methyl-2,3-dihydro- NY-phenothiazin-4[1OH)-one Derivatives. Bioorganic & Medicinal Chemistry 6(12): 2289-2299.
- Harish KP, Mohana KN, Mallesha L (2013) Synthesis of indazole substituted-1,3,4- thiadiazoles and their anticonvulsant activity. Drug invention today 5(2): 92 -99.
- Sharma R, Samadhiya P, Srivastava SD, Srivastava SK (2011) Synthesis and biological activity of 2-oxo-azetidine derivatives of Phenothiazine. Org Commun 4(2): 42-51.
- Talath S, Gadad AK (2006) Synthesis, antibacterial and antitubercular activities of some 7-[4-(5-amino-[1,3,4] thiadiazole-2-sulfonyl)-piperazin-1- yl] fluoroquinolonic derivatives. European Journal of Medicinal Chemistry 41(8): 918-924.
- Sadanandam YS, Shetty MM, Bhaskar Rao A,RambabuY (2009) 10H-Phenothiazines: A new class of enzyme inhibitors for inflammatory diseases. European Journal of Medicinal Chemistry 44(1):197-202.
- Palaska E, Sahin G, Kelicen P, Durlu NT, Altinok G (2002) Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides,1,3,4- oxadiazoles,1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. II Farmaco 57(2): 101-107.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...