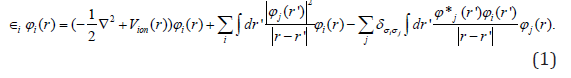Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Review Article(ISSN: 2637-4609) 
Spectroscopy and Dipole Moment of the Molecule C13H20BeLi2SeSi Via Quantum Chemistry using Ab Initio, Hartree-Fock Method in the Base Set CC-Pvtz and 6-311G**(3df, 3pd) Volume 3 - Issue 5
Ricardo Gobato1*, Marcia Regina Risso Gobato2, Alireza Heidari3 and Abhijit Mitra4
- 1 Laboratory of Biophysics and Molecular Modeling Genesis, State Secretariat for Education of Parana, Bela Vista do Paraiso, Parana, Brazil
- 2 Seedling Growth Laboratory, Green Land Landscaping and Gardening, Bela Vista do Paraiso, Parana, Brazil
- 3 Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA
- 4Department of Marine Science, University of Calcutta, West Bengal, India
Received: September 03, 2018; Published: September 24, 2018
*Corresponding author:Ricardo Gobato, Laboratory of Biophysics and Molecular Modeling Genesis, State Secretariat for Education of Parana, Bela Vista do Paraiso, Parana, Brazil
DOI: 10.32474/AOICS.2018.03.000171
Abstract
The work characterizes the electric dipole moment and the infrared spectrum of the molecule C13H20BeLi2SeSi. Calculations obtained in the ab initio RHF (Restrict Hartree-Fock) method, on the set of bases used indicate that the simulated molecule C13H20BeLi2SeSi features the structure polar-apolar-polar predominant. The set of bases used that have are CC-pVTZ and 6-311G** (3df, 3pd). In the CC-pVTZ base set, the charge density in relation to 6-311G** (3df, 3pd) is 50% lower. The length of the molecule C13H20BeLi2SeSi is of 15.799Å. The magnitude of the electric dipole moment || total obtained was p = 4.9771 Debye and p = 4.7936 Debye, perpendicular to the main axis of the molecule, for sets basis CC-pVTZ and 6-311**(3df, 3pd), respectively. The infrared spectra for absorbance and transmittance and their wavenumber (cm-1) were obtained in the set of bases used. The infrared spectrum for Standard CC-pVTZ shows peaks in transmittance with Intensity (I), at wavenumber 1,125.44cm-1, 1,940.70cm-1, 2,094.82cm-1, 2,178.43cm-1, 2,613.99cm-1 and transmittance 433.399km/mol, 399.425km/mol, 361.825km/mol, 378.993km/ mol, 433.774km/mol, respectively. While the infrared spectrum for Standard 6-311G** (3df,3pd), shows peaks in transmittance, at wavelengths 1,114.83cm-1, 1,936.81cm-1, 2,081.49cm-1, 2,163.23 cm-1, 2,595.24cm-1 and transmittance 434.556 km/mol, 394.430 km/mol, 345.287 km/mol, 375.381 km/mol, 409.232 km/mol, respectively. It presents “fingerprint” between the intervals (680cm- 1 and 1,500 cm-1) and (3,250cm-1 and 3,500cm-1). The dipole moments CC-pTZV are 3.69% bigger than 6-311G (3df, 3pd). As the bioinorganic molecule C13H20BeLi2SeSi is the basis for a new creation of a bio-membrane, later calculations that challenge the current concepts of biomembrane should advance to such a purpose.
Introduction
The work characterizes the electric dipole moment and the infrared spectrum of the molecule C13H20BeLi2SeSi [1]. Using a computational simulation using ab initio methods, RHF (Restrict Hartree-Fock), [2-9] set of basis CC-pVTZ [10-14] and 6-311G (3df, 3pd) [7,5-21]. Preliminary bibliographic studies did not reveal any works with characteristics studied here. There is an absence of a referential of the theme, finding only one work in [1]. To construct such a molecule, which was called a seed molecule, quantum chemistry was used by ab initio methods [2,3,15]. The equipment used was of the Biophysics laboratory built specifically for this task. The results were satisfactory. The ab initio calculations, by RHF [2-9] in the CC-pVTZ [10-14] and 6-311G (3df, 3pd) [7,15- 21], sets basis was shown to be stable by changing its covalent cyclic chain linkages, which was expected. The set of basis used was that of Ahlrichs and coworkers the TZVP keywords refer to the initial formations of the split valence and triple zeta basis sets from this group [22,23]. The structure of the C13H20BeLi2SeSi is a Bio-inorganic seed molecule for a biomembrane genesis that defies the current concepts of a protective mantle structure of a cell such as biomenbrane to date is promising, challenging. Leaving to the Biochemists their experimental synthesis. The quantum calculations must continue to obtain the structure of the bioinorganic biomenbrane. The following calculations, which are the computational simulation via Mm+, QM/MM, should indicate what type of structure should form. Structures of a liquid crystal such as a new membrane may occur, micelles [1,24-62].
Methods
Hartree-Fock Methods
Hartree-Fock theory is one the simplest approximate theories for solving the many-body Hamiltonian [2-9]. The full Hartree-Fock equations are given by
The vast literature associated with these methods suggests that the following is a plausible hierarchy:
The extremes of ‘best’, FCI, and ‘worst’, HF, are irrefutable, but the intermediate methods are less clear and depend on the type of chemical problem being addressed. [63,64] The use of HF in the case of FCI was due to the computational cost [1, 24-62].
Hardware and Software
For Calculations A Computer Models was Used: Intel® CoreTM i3-3220 CPU@3.3 GHz x 4 processors [65], Memory DDR3 4 GB, HD SATA WDC WD7500 AZEK-00RKKA0 750.1 GB and DVDRAM SATA GH24NS9 ATAPI, Graphics Intel® Ivy Bridge [66]. The ab initio calculations have been performed to study the equilibrium configuration of C13H20BeLi2SeSi molecule using the GAMESS [15,20]. The set of programs Gauss View 5.0.8 [67], Mercury 3.8 [68], Avogadro [69,70] are the advanced semantic chemical editor, visualization, and analysis platform and GAMESS [15,20] is a computational chemistry software program and stands for General Atomic and Molecular Electronic Structure System [15,20] set of programs. For calculations of computational dynamics, the Ubuntu Linux version 16.10 system was used [71].
Result
(Figures 1-5) and (Tables 1-3).
Figure 1: Representation of the molecular structure of C13H20BeLi2SeSi, obtained through computer via ab initio calculation method RHF [2-9], CC-pVTZ [10-14] sets basis obtained using computer programs GAMESS [15,20]. Images obtained in the software Mercury 3.8 [68]. Represented in bluish gray color the atom of silicon, in the purple color lithium, in the lemon yellow color beryllium, in the orange the selenium, in dark gray color carbon and in light gray color hydrogen. The image from left to right has a 90 degree rotation in the YZ plane, anti-clockwise.
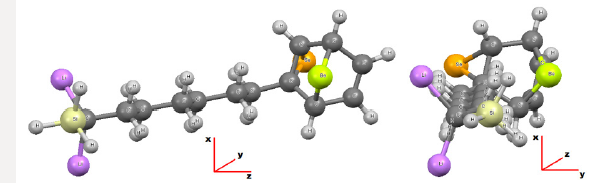
Figure 2: Molecule bio-inorganic C13H20BeLi2SeSi after dynamics obtained through computer via ab initio calculation method RHF [2-9] in sets of basis obtained using computer software GAMESS [15,20]. The length of the molecule C13H20BeLi2SeSi obtained in the base CC-pVTZ [10-14] is of 15.799Å. Represented in green color the positive charge, passing through the absence of color - black - zero charge, for the positive charge red color. A Δδ = 0.680 a.u. of CC-pVTZ [10-14] and Δδ = 1.366 a.u. of 6-311**(3df,3pd) [7,15-21], were the elemental charge e (e = ±1,607 x 10-19 C). Images obtained in the software Gaussview, Version 5, 2009 [67].
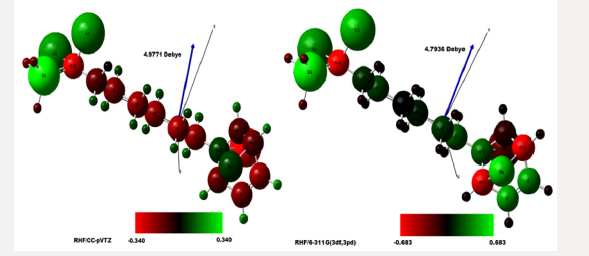
Figure 3: Characteristic infrared spectrum in absorbance and transmittance obtained using the ab initio HF method for the RHF [5-6,27-32] in sets of basis CC-pVTZ [10-14] obtained using computer software GAMESS [15,20]. Image created by Avogadro software [69,70].

Figure 4: Characteristic infrared spectrum in absorbance and transmittance obtained using the ab initio HF method for the RHF [2-9] in sets of basis RHF/6-311G**(3df,3pd) [7,15-21] obtained using computer software GAMESS [15,20]. Image created by Avogadro software [69,70].
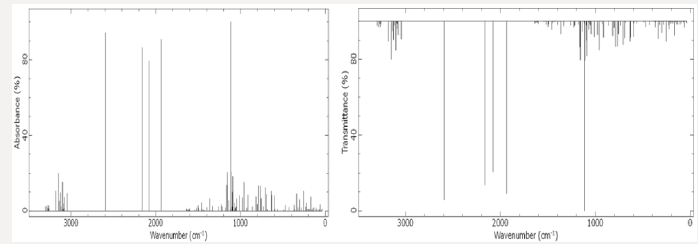
Figure 5: Infrared spectrum obtained using the ab initio for the RHF [2-9] method, in sets of basis RHF/CC-pVTZ [10-14] in black color and 6-311G**(3df, 3pd) [7,15-21] in red color, obtained using computer software GAMESS [15,20]. Graphic edited in origin software, for comparison of the spectra obtained in the set of bases used [72].
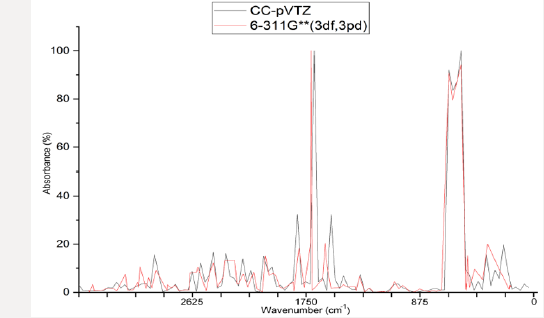
Table 1: Table containing the dipole moments of the C13H20BeLi2SeSi molecule via ab initio methods. [1,24-62].
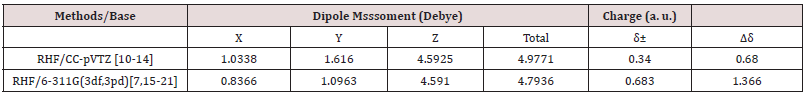
Selects Stuttgart potentials for Z > 2. MC-311G is a synonym for 6-311G [7]. The elemental charge e (e = ±1,607 x 10-19 C) [1,24-62].
Table 2: Table containing the frequency (cm-1) for Intensity (km/mol) of the C13H20BeLi2SeSi molecule via ab initio methods, set base RHF/CC-pVTZ [10-14] for the infrared spectrum.
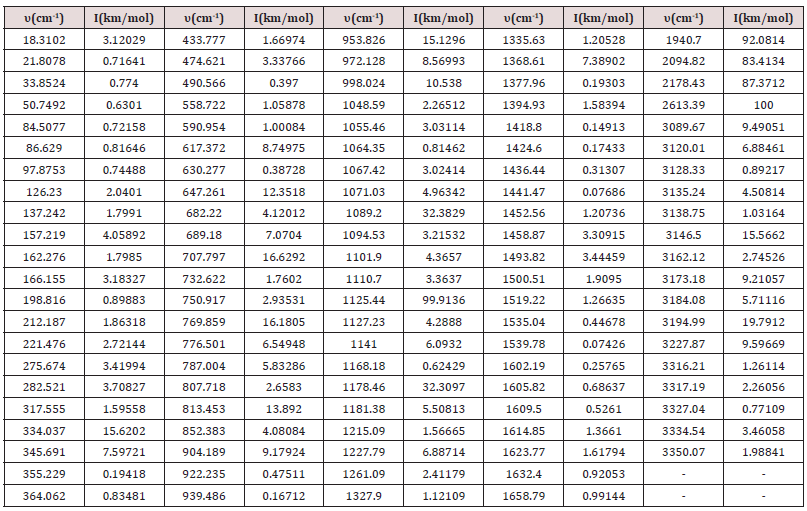
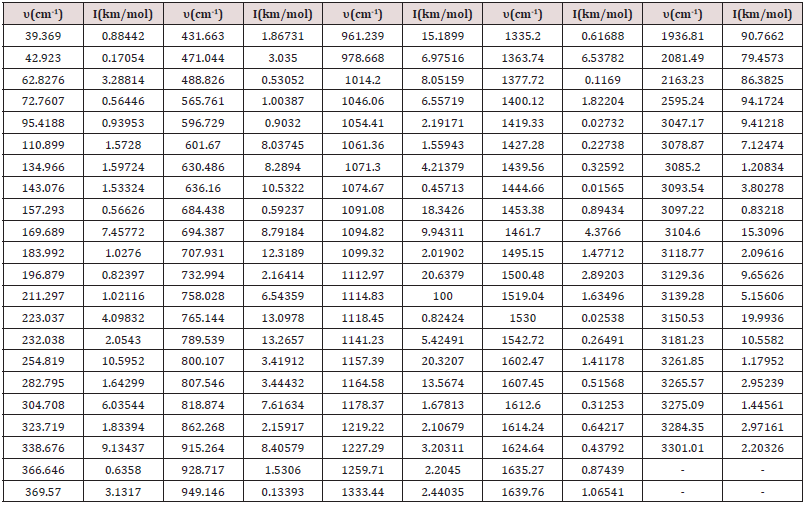
Table 3: Table containing the frequency (cm-1) for Intensity (km/mol) of the C13H20BeLi2SeSi molecule via ab initio methods, set base RHF/6-311G**(3df,3pd) [7,15-21] for the infrared spectrum.
Discussion
The Figure 1 shows the final stable structure of the bioinorganic C13H20BeLi2SeSi molecule obtained by an ab initio calculation with the method RHF (Restrict Hatrree-Fock), in sets of bases such as: 6-311G**(3df,3pd) and CC-pVTZ. As an example of analysis the set of bases CC-pVTV, with the charge distribution (Δδ) through it, whose charge variation is Δδ = 0.680 a.u. of elemental charge. In green color the intensity of positive charge displacement. In red color the negative charge displacement intensity. Variable, therefore, of δ- = 0.340 a.u. negative charge, passing through the absence of charge displacement, represented in the absence of black - for the green color of δ+ = 0.340 a.u. positive charge. The magnitude of the electric dipole moment || total obtained was p =4.9771 Debye, perpendicular to the main axis of the molecule, for sets basis CC-pVTZ. By the distribution of charge through the bioinorganic molecule it is clear that the molecule has a polar-apolarpolar structure, Figure 2 and Table 1. An analysis of the individual charge value of each atom of the molecule could be made, but here it was presented only according to Figure 2, due to the objective being to determine the polar-apolar-polar, the polar characteristic of the molecule, whose moment of dipole is practically perpendicular to the central axis of the molecule. In Figure 2 the dipole moment is visualized 6-311G**(3df,3pd) and CC-pVTZ in base sets, being represented by an arrow in dark blue color, with their respective values in Debye. This also presents the orientation axes x, y and z and the distribution of electric charges through the molecule.
In the set of bases used the CC-pTZV and 6-311**(3df, 3pd) present the same characteristic for the distribution of charges to the polar end with Carbon atom (negative charge) bound to the -SiH3 radical and the two Lithium atoms. It is seen that Δδ =0.680 a.u. of CC-pTZV and Δδ =1.366 a.u. of 6-311 (3df, 3pd), this latter has a twice greater Δδ, Figure 2, although the dipole moments CC-pTZV are 3.69% larger, (Table 1). The main chain (backbone of the molecule) for the CC-pTZV base set has a small negative charge displacement for the Carbon atoms from the Hydrogen atoms attached to them. Therefore, with positive charge the Hydrogen atoms connected to the Carbon of the central chain. For the set of bases 6-311**(3df, 3pd) the carbon atoms of the main chain are presented with very small distribution of negative charge, coming from the Hydrogen linked to these neutrals, Figure 2. At the other polar end for the base set 6-311**(3df, 3pd) the cyclic chain shows the characteristics as the Beryllium atom with strong charge displacement positive, these charges shift to the Carbon atoms attached to it, Figure 2. The cyclic chain with a strong negative charge, displaced from the Beryllium atom. The two carbon atoms bonded in double bonds, present a slight positive charge, with their neutral Hydrogen, Figure 2. The Selenium atom connected to two Carbon atoms of the cyclic chain presents a slight negative charge, originating from the Carbon atom connected to the main chain with a slight positive charge, and the other Carbon atom connected to the cyclic chain presents a neutral charge, Figure 2. The magnitude of the electric dipole moment || total obtained was p = 4.7936 Debye for 6-311**(3df, 3pd), (Table 1). Figures 3 & 4 represent the normalized infrared spectrum for the base set RHF / 6-311G ** (3df, 3pd) and CC-pVTZ for Absorbance and Transmittance. Figures 5 represent the normalized infrared spectrum for the base set RHF/6-311G** (3df, 3pd and CC-pVTZ for absorbance, making a comparison between the two sets of base. The infrared spectrum for Standard RHF/CC-pVTZ shows peaks in transmittance, at wavelengths 1,125.44cm-1, 1,940.70cm- 1, 2,094.82cm-1, 2,178.43cm-1, 2,613.99cm-1 and transmittance 433.399km/mol, 399.425km/mol, 361.825km/mol, 378.993km/ mol, 433.774km/mol, respectively, Figure 3 and Table 2. The infrared spectrum for Standard RHF/6-311G**(3df,3pd) shows peaks in transmittance, at wavelengths 1,114.83cm-1, 1,936.81cm- 1, 2,081.49cm-1, 2,163.23 cm-1, 2,595.24cm-1 and transmittance 434.556km/mol, 394.430 km/mol, 345.287 km/mol, 375.381 km/ mol, 409.232 km/mol, respectively, Figure 4 and Table 3. It presents “fingerprint” between the intervals (680cm-1 and 1,500cm-1) and (3,250cm-1 and 3,500cm-1), Figures 3-5.
Conclusion
Calculations obtained in the ab initio RHF method, on the set of bases used, indicate that the simulated molecule, C13H20BeLi2SeSi, is acceptable by quantum chemistry. Its structure has polarity at its ends, having the characteristic polar-apolar-polar. The 6-311G (3df, 3pd) set of basis exhibits the characteristic of the central chain, with a small density of negative charges, near the ends of the Carbons of this. In the CC-pVTZ base set, the charge density in relation to 6-311G (3df, 3pd) is 50% lower. It is characterized infrared spectrum of the molecule C13H20BeLi2SeSi, for absorbance and transmittance, in Hartree method in the set of bases CC-pVTZ and 6-311G (3df, 3pd). The infrared spectrum for Standard RHF/ CC-pVTZ shows peaks in transmittance, at wavelengths 1,125.44cm- 1, 1,940.70cm-1, 2,094.82cm-1, 2,178.43cm-1, 2,613.99cm-1 and transmittance 433.399 km/mol, 399.425km/mol, 361.825km/ mol, 378.993km/mol, 433.774km/mol, respectively. The infrared spectrum for Standard RHF/6-311G**(3df,3pd) [7,30,60,71,72] shows peaks in transmittance, at wavelengths 1,114.83cm- 1, 1,936.81cm-1, 2,081.49cm-1, 2,163.23cm-1, 2,595.24cm-1 and transmittance 434.556km/mol, 394.430km/mol, 345.287km/ mol, 375.381km/mol, 409.232km/mol, respectively. It presents “fingerprint” between the intervals (680cm-1 and 1,500cm-1) and (3,250cm-1 and 3,500cm-1). The dipole moments CC-pTZV are 3.69% bigger than 6-311G (3df, 3pd). As the bio-inorganic molecule C13H20BeLi2SeSi is the basis for a new creation of a biomembrane, later calculations that challenge the current concepts of biomembrane should advance to such a purpose.
Acknowledgement
To the doctors: Prof. Ph.D. Tolga Yarman, Okan University, Akfirat, Istanbul, Turkey & Savronik, Organize Sanayi Bolgesi, Eskisehir, Turkey, and Prof. Ph.D. Ozan Yarman, Istanbul University, Rihtim Nr:1, 81300 Kadikoy, Istanbul, Turkey, for their valuable contributions to the work.
References
- Gobato R, Heidari A, Mitra A (2018) The Creation of C13H20BeLi2SeSi. The Proposal of a Bio-Inorganic Molecule, Using Ab Initio Methods for The Genesis of a Nano Membrane. Arc Org Inorg Chem Sci 3(4): 112-115.
- Levine IN (2003) Quantum Chemistry. Pearson Education (Singapore) (5th edn.); Delhi, India.
- Szabo A, Ostlund NS (1989) Modern Quantum Chemistry. Dover Publications, New York, USA.
- Ohno K, Esfarjani K, Kawazoe Y (1999) Computational Material Science. Springer-Verlag, Berlin.
- Wolfram K, Hothausen MC (2001) Introduction to DFT for Chemists (2nd edn.); John Wiley & Sons, New York, USA.
- Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136: B864-B871.
- Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140: A1133.
- Thijssen JM (2001) Computational Physics. Cambridge University Press, Cambridge, USA.
- Perdew M, Ernzerhof, Burke K (1996) Rationale for mixing exact exchange with density functional approximations. J Chem Phys 105(22): 9982-9985.
- Dunning TH (1983) Gaussian basis sets for use in correlated molecular calculations, The atoms boron through neon and hydrogen. J Chem Phys 90: 1007-1023.
- Kendall RA, Dunning TH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave function. J Chem Phys 96: 6796-67806.
- Woon DE, Dunning (1993) Gaussian-basis sets for use in correlated molecular calculations. The atoms aluminum through argon. J Chem Phys 98: 1358-1371.
- Peterson KA, Woon DE, Dunning TH (1994) Benchmark calculations with correlated molecular wave functions. The classical barrier height of the H+H2 -¿ H2+H reaction. J Chem Phys 100: 7410-7415.
- Wilson AK, van Mourik T, Dunning TH (1996) Gaussian basis sets for use in Correlated Molecular Calculations. Sextuple zeta correlation consistent basis sets for boron through neon. J Mol Struct (Theochem) 388: 339-349.
- Gordon MS (1993) General atomic and molecular electronic structure system (GAMESS). J Comput Chem 14: 1347-1363.
- Polak E (1971) Computational Methods in Optimization. Elsevier, New York, USA.
- Dunning TH, Hay PJ (1997) Modern Theoretical Chemistry. Plenum, New York, USA.
- Eliav E (2013) Elementary introduction to Molecular Mechanics and Dynamics.
- Hehre WJ (2003) A Guide to Molecular Mechanics and Quantum Chemical Calculations. Wavefunction, Inc, Irvine, CA.
- Gordon MS, Schmidt MW (2005) Advances in electronic structure theory: GAMESS a decade later. Theory and Applications of Computational Chemistry: The first forty years pp. 1167-1189.
- Amsterdam RG Parr W, Yang (1989) Density Functional Theory.
- Schaefer A, Huber C, Ahlrichs R (1992) Fully optimized contracted Gaussian-basis sets for atoms Li to Kr. J Chem Phys 97: 2571-2577.
- Schaefer A, Huber C, Ahlrichs R (1994) Fully optimized contracted Gaussian-basis sets of triple zeta valence quality for atoms Li to Kr. J Chem Phys 100: 5829-5835.
- Gobato R, Fedrigo DFG, Gobato A (2014) Inorganic arrangement crystal beryllium, lithium, selenium and silicon. In XIX Semana da Física. Simpósio Comemorativo dos 40 anos do Curso de Física da Universidade Estadual de Londrina. BrazilUniversidade Estadual de Londrina (UEL).
- Gobato R (2018) Benzocaína, um estudo computacional. Master’s thesis, Universidade Estadual de Londrina.
- Gobato R (2017) Study of the molecular geometry of Caramboxin toxin found in star flower (Averrhoa carambola L.). Parana J Sci Edu 3(1): 1-9
- Gobato R, Fedrigo DFG, Gobato A (2015) Molecular electrostatic potential of the main monoterpenoids compounds found in oil Lemon Tahiti - (Citrus Latifolia Var Tahiti). Parana J Sci Edu 1(1): 1-10.
- Gobato R, Fedrigo DFG, Gobato A (2015) Allocryptopine, Berberine, Chelerythrine, Copsitine, Dihydrosanguinarine, Protopine and Sanguinarine. Molecular geometry of the main alkaloids found in the seeds of Argemone Mexicana Linn. Parana J Sci Edu 1(2): 7-16.
- Gobato R, Heidari A (2018) Infrared Spectrum and Sites of Action of Sanguinarine by Molecular Mechanics and ab initio Methods. International Journal of Atmospheric and Oceanic Sciences 2(1): 1-9.
- Gobato R, Fedrigo DFG, Gobato A (2015) Molecular geometry of alkaloids present in seeds of mexican prickly poppy. Cornell University Library. Quantitative Biology arXiv: 1507.05042.
- Gobato R, Fedrigo DFG, Gobato A (2018) Study of the molecular electrostatic potential of D-Pinitol an active hypoglycemic principle found in Spring flower Three Marys (Bougainvillea species) in the Mm+ method. Parana J Sci Educ 2(4): 1-9.
- Gobato R, Fedrigo DFG, Gobato A (2015) Avro: key component of Lockheed X-35. Parana J Sci Educ 1(2): 1-6.
- Gobato R, Fedrigo DFG, Gobato A (2016) LOT-G3: Plasma Lamp, Ozonator and CW Transmitter. Cienciae Natura 38(1): 453.
- Gobato R (2016) Matter and energy in a non-relativistic approach amongst the mustard seed and the faith. A metaphysical conclusion. Parana J Sci Educ 2(3): 1-14.
- Gobato R, Fedrigo DFG, Gobato A (2016) Harnessing the energy of ocean surface waves by Pelamis System. Parana J Sci Educ 2(2): 1-15.
- Gobato R, Fedrigo DFG, Gobato A (2016) Mathematics for input space probes in the atmosphere of Gliese 581d. Parana J Sci Educ 2(5): 6-13.
- Gobato R, Fedrigo DFG, Gobato A (2006) Study of tornadoes that have reached the state of Parana. Parana J Sci Educ 2(1): 1-27.
- Gobato R, Simões F (2017) Alternative Method of RGB Channel Spectroscopy Using a CCD Reader. Ciencia e Natura 39(2).
- Gobato R, Heidari A (2017) Calculations Using Quantum Chemistry for Inorganic Molecule Simulation BeLi2SeSi. Science Journal of Analytical Chemistry 5(5): 76-85.
- Gobato MRR, Gobato A, Heidari (2018) Planting of Jaboticaba Trees for Landscape Repair of Degraded Area. Landscape Architecture and Regional Planning 3(1): 1-9.
- Gobato R (2012) The Liotropic Indicatrix 114: 62.
- Gobato R, Heidari A (2018) Calculations Using Quantum Chemistry for Inorganic Molecule Simulation BeLi2SeSi. Science Journal of Analytical Chemistry 5(6): 76-85.
- Gobato MRR, Gobato R, Heidari A (2018) Planting of Jaboticaba Trees for Landscape Repair of Degraded Area. Landscape Architecture and Regional Planning 3(1): 1-9.
- Gobato R (2012) The Liotropic Indicatrix 114: 62.
- Gobato R, Heidari A (2018) Calculations Using Quantum Chemistry for Inorganic Molecule Simulation BeLi2SeSi. Science Journal of Analytical Chemistry 5(6): 76-85.
- Gobato MRR, Gobato R, Heidari A (2018) Planting of Jaboticaba Trees for Landscape Repair of Degraded Area. Landscape Architecture and Regional Planning 3(1): 1-9.
- Gobato MRR, Gobato R, Heidari A (2018) Infrared Spectrum and Sites of Action of Sanguinarine by Molecular Mechanics and ab initio Methods. International Journal of Atmospheric and Oceanic Sciences 2(1): 1-9.
- Gobato MRR, Gobato R, Heidari A (2018) Molecular Mechanics and Quantum Chemical Study on Sites of Action of Sanguinarine Using Vibrational Spectroscopy Based on Molecular Mechanics and Quantum Chemical Calculations. Malaysian Journal of Chemistry 20(1): 1-23.
- Gobato MRR, Gobato R, Heidari A (2018) A Novel Approach to Reduce Toxicities and to Improve Bioavailabilities of DNA/RNA of Human Cancer Cells-Containing Cocaine (Coke), Lysergide (Lysergic Acid Diethyl Amide or LSD), Δ⁹-Tetrahydrocannabinol (THC) [(-)-trans-Δ⁹- Tetrahydrocannabinol], Theobromine (Xantheose), Caffeine, Aspartame (APM) (NutraSweet) and Zidovudine (ZDV) [Azidothymidine (AZT)] as Anti-Cancer Nano Drugs by Coassembly of Dual Anti-Cancer Nano Drugs to Inhibit DNA/RNA of Human Cancer Cells Drug Resistance. Parana Journal of Science and Education 4(6): 1-17.
- Gobato R, Heidari A (2018) Ultraviolet Photoelectron Spectroscopy (UPS) and Ultraviolet-Visible (UV-Vis) Spectroscopy Comparative Study on Malignant and Benign Human Cancer Cells and Tissues with the Passage of Time under Synchrotron Radiation. Parana Journal of Science and Education 4(6): 18-33.
- Gobato R, Heidari A (2018) Using the Quantum Chemistry for Genesis of a Nano Biomembrane with a Combination of the Elements Be, Li, Se, Si, C and H. J Nanomed Res 7(4): 241-252.
- Gobato R, Heidari A (2014) Inorganic arrangement crystal beryllium, lithium, selenium and silicon. Universidade Estadual de Londrina (UEL), arXiv: 1508-00175.
- Gobato R (2017) Study of the molecular geometry of Caramboxin toxin found in star flower (Averrhoa carambola L.). Parana J Sci Educ 3(1): 1-9.
- Gobato R, Gobato A, Fedrigo DFG (2015) Molecular electrostatic potential of the main monoterpenoids compounds found in oil Lemon Tahiti - (Citrus Latifolia Var Tahiti). Parana J Sci Educ 1(1): 1-10.
- Gobato R, Fedrigo DFG, Gobato A (2015) Allocryptopine, Berberine, Chelerythrine, Copsitine, Dihydrosanguinarine, Protopine and Sanguinarine. Molecular geometry of the main alkaloids found in the seeds of Argemone Mexicana Linn. Parana J Sci Educ 1(2):7-16.
- Gobato R, Heidari A (2018) Infrared Spectrum and Sites of Action of Sanguinarine by Molecular Mechanics and ab initio Methods. International Journal of Atmospheric and Oceanic Sciences 2(1): 1-9.
- Gobato R, Fedrigo DFG, Gobato A (2015) Molecular geometry of alkaloids present in seeds of mexican prickly poppy. Quantitative Biology arXiv: 1507.05042.
- Gobato R, Fedrigo DFG, Gobato A (2016) Study of the molecular electrostatic potential of D-Pinitol an active hypoglycemic principle found in Spring flower Three Marys (Bougainvillea species) in the Mm+ method. Parana J Sci Educ 2(4): 1-9.
- Gobato R, Fedrigo DFG, Gobato A (2015) Avro: key component of Lockheed X-35. Parana J Sci Educ 1(2): 1-6.
- Agarwal SK, Roy S, Pramanick P, Mitra P, Gobato R, et al. (2018) Marsilea quadrifolia: A floral species with unique medicinal properties. Parana J Sci Educ 4(5): 15-20.
- Mitra A, Zaman S, Gobato R (2018) Indian Sundarban Mangroves: A potential Carbon Scrubbing System. Parana J Sci Educ 4(4): 7-29.
- Yarman O, Gobato R, Yarman T, Arik M (2018) A new Physical constant from the ratio of the reciprocal of the “Rydberg constant” to the Planck length. Parana J Sci Educ 4(3): 42-51.
- Gobato R, Simões F (2017) Alternative Method of Spectroscopy of Alkali Metal RGB. Modern Chemistry 5(4): 70-74.
- Fedrigo DFG, Gobato R, Gobato A (2015) Avrocar: a real flying saucer. Cornell University Library arXiv: 1507.
- Fedrigo DFG, Gobato R, Gobato A (2012) Micellar shape anisotropy and optical indicatrix in reentrant isotropic-nematic phase transitions. The Journal of Chemical Physics 137: 204905.
- McDouall JJW (2013) Computational Quantum Chemistry. Molecular Structure and Properties in Silico. Royal Society of Chemistry p. 1-62.
- Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys Chem Chem Phys 7(8): 3297-3298.
- Creative Commons (CC BY 4.0) https://creativecommons.org/licenses/ by/4.0/. List of Intel Core i3 microprocessors. https://en.wikipedia.org/ wiki/List_of_Intel_Core_i3_microprocessors.
- Ivy Bridge.
- Dennington R, Keith T, Millam J, Gaussview (2009) Version 5.
- The Cambridge Crystallographic Data Centre (CCDC) Mercury - crystal structure visualisation, exploration and analysis made easy. Mercury 3.1 Development (Build RC5). The Cambridge Crystallographic Data Centre.
- Avogadro: an open-source molecular builder and visualization tool. Version 1.1.1.
- Marcus D, Hanwell DE, Curtis DC, Lonie TV, Zurek E, et al. (2012) Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4(1): 17.
- Creative Commons, (CC BY 4.0), https://creativecommons.org/licenses/ by/4.0/. Ubuntu (operating system). https://en.wikipedia.org/wiki/ Ubuntu_(operating_system).
- Origin Lab (2018) Evaluation Licence, Graphing & Analysis, ©OriginLab Corporation.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...