
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Preliminary Studies on the Effect of Functionalization on the Toxicity of Carbon Nanotubes Volume 5 - Issue 3
Seyed Yazdan Madani1,2* and Alexander Seifalian3
- 1School of Pharmacy, University of Nottingham Malaysia, Malaysia
- 2School of Pharmacy, University of Nottingham, University Park, UK
- 3Nanotechnology & Regenerative Medicine Commercialisation Centre, United Kingdom
Received:June 8, 2021 Published:July 29, 2021
*Corresponding author:Seyed Yazdan Madani, School of Pharmacy, University of Nottingham Malaysia, Jalan Broga, Malaysia
DOI: 10.32474/AOICS.2021.05.000215
Introduction
Carbon nanotubes (CNT) can have great potential in the field of medical science [1]. This potential includes the process of cancer treatment using drug delivery, thermal treatment of cancer and localization of cancer cells. [2,3] As well as the benefits about these materials, various negative points have also been presented with the CNTs. These have delayed their clinical usage for many years after their discovery. One main concern about nanoparticles [4] and in particular CNT is the issue that its exposure to the body could result in damage to the cells and their content [5]. For this reason, various research groups have focused on developing various methods to reduce the toxicity of nanoparticles and in particular carbon nanotubes [6]. Different factors such as length, purity, [7] dosages, production method and the functionalization techniques have been demonstrated to have a significant effect on the toxicity of the CNT. Regarding the length, it has been illustrated that CNTs with smaller sizes are generally less toxic. It has been suggested that large CNTs cannot be removed by the phagocytosis procedure [8]. Purity plays an important role in toxicity of CNT. Pristine CNT is not pure as various metals such as Co, Fe, Ni have been used as catalysts to promote the CNT’s growth during synthesis. [9] Following synthesis, residual metal is encapsulated within a layer of carbon. Various toxicology studies have shown that impurities are generally the cause of cell death through mitochondrial destruction. Functionalization is one of the most crucial aspects prior to using CNTs within cell studies. Functionalization is defined as the process of modification of the surface of the CNT via coating with new materials, which are not biologically hazardous [10]. Various studies indicate that following the successful functionalization of CNT, the toxicity of these materials significantly declines [2]. Different groups investigated the optimal concentration of the functionalization materials and the most favorable technique, which has been used to reduce the toxicity by a significant level [11].
Aims And Objectives
The aim of the present study is to investigate the effect of the functionalization and the length of the SWCNT on its toxicity. As it has been mentioned the pristine SWCNT is toxic, and it cannot be used for any cell work. Functionalization technique needs to be conducted before using these materials in any cell work. Using the correct length for minimizing the CNT’s toxicity is also essential.
The Objectives of the experiment are as follows
To understand about the effect of the functionalization of the SWCNT on its toxicity. To investigate the presence of the-COOH group and the Octa Ammonium-POSS and the combination of both on the toxicity of SWCNT. To investigate and statistically compare the effect of increasing the functionalization time interval on the toxicity of SWCNT. To investigate and statistically compare the effect of using different lengths of SWCNT on its toxicity. To understand the effect of SWCNT on the fluorescence of Alamar Blue and the Quant-it Pico green reagent
Material and Methods
Experimental agents
SWCNT (75% purity), with a diameter within the range of 0.7nm-1.3nm and a length of between 0.4μm to 1μm and SWCNT (75% purity) with a diameter within the range of 0.7-1.3nm and a length of between 1.5 to 2μm were purchased from Nanothinx (Greece). Also, SWNCT (75% purity) with the diameter of 2-10nm and length from 50-400nm were purchased from Nanothinx (Greece). 3-dimethylaminopropyl-N-ethylcarbodiimide hydrochloride (EDC) (99%), H2SO4 (95%), HNO3 (70%), 4% formaldehyde, N-hydroxysuccinimide (NHS), L-Glutamine-penicillin-streptomycin solution were purchased from Sigma Aldrich (Dorset, UK). Phosphate buffered saline (PBS), Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), trypsin-EDTA, AlamarBlue® reagent, Quant-iT™ Pico Green® DNA assay, To-Pro®-3 were purchased from in vitrogen™. Human colonic adenocarcinoma cell line (HT-29) and SW480, human breast cancer cell line (MCF-7) was purchased from Abcam® (Cambridge, UK). Octa Ammonium polyhedral oligomeric silsesquioxane (POSS) was purchased from Hybrid Plastics® (Hattiesburg, USA). HPA lectinfluorescein isothiocyanate (FITC) (20 μg/ml) was supplied by Westminster university.
Preparation of pristine SWCNT
10mg of Pristine SWCNT was measured. 10mls of water was added to 10mg of pristine SWCNT to make a 1mg/ml SWCNT mixture. The 1mg/ml pristine SWCNT mixture was then vortexed for 5min and sonicated for 1hr. The pristine SWCNT was then diluted to obtain solutions of 0.125mg/ml, 0.250mg/ml, 0.5mg/ml and 1mqg/ml concentration.
Preparation of carboxyl functionalized SWCNTs
Solid SWCNT (short) and SWCNT (long) in powder form were purchaqsed from sigma Aldrich. 10mg of each SWCNT was measured out and functionalized. For the full explanation of functionalization procedure please referee to the following paper: Functionalization of single walled carbon nanotubes and their binding to cancer cells Madani et al. [1,2] Both short and long SWCNT mixtures were then centrifuged for 30min at 2400xg. The supernatant was then discarded. PBS (5mls) was then added to the pellet and mixed. The mixture was then filtered with a vacuum filter flask and washed extensively in deionized water until a constant pH value within the range of 5-6 was attained. The resultant neutralized acid functionalized SWCNT left on the filter paper was then collected by putting the paper in a pre-weighted centrifuge tube and adding 1ml of deionized water. Upon the full removal of SWCNT from the paper the paper was then removed. The SWCNT mixture was then centrifuged for 30min at 2400xg. The supernatant was removed, and the tubes were then placed in the oven to dry. The tube was then weighed out to determine the total dry weight of functionalized SWCNT. The dry acid functionalized SWCNT powder was then dissolved in deionized water to produce a 1mg/ ml working solution. The above procedure was then conducted under conditions of 2hrs and 5hrs reflux time intervals for both SWCNT (short) and SWCNT (long). Following the formation of 1mg/ml SWCNT-COOH, the functionalized SWCNT was then diluted in differing volumes of water. (Table 1.1-Table 1.2) This allows the formation of SWCNT-COOH with (0.125mg/ml, 0.250mg/ml, 0.5mg/ml) concentrations.
Synthesis of pristine SWCNT and OctaAmmonium-POSS conjugate
0.125, 0.250, 0.5 and 1.00mg/ml OctaAmmonium-POSS was initially prepared as explained. 1ml of each concentration of OctaAmmonium-POSS was added to 1ml of short and long pristine SWCNT (1mg/ml). Please see Table 1.2 for the dilution strategy. The mixture was then subjected to vortex for 5min and sonicated for 1hr. This was then washed five times with water to remove nonadsorbed OctaAmmonium-POSS followed by vacuum drying at room temperature for 24hrs.
Synthesis of SWCNT-COOH and Octa Ammonium-POSS conjugate
The first step was to prepare (0.125mg/ml, 0.250mg/ml, 0.500mg/ml and 1mg/ml) of Octa Ammonium-POSS with –NH2 as the functional group. In this section, 1mg of Octa Ammonium-POSS was measured and dissolved in 1ml of NaOH aiming to convert the ammonium groups on the POSS molecule into amine groups. Conversion of the ammonium groups on the POSS molecule into amine groups provided 8 functional groups suitable for linkage to COOH groups on oxidized SWCNT. A 1mg/ml working solution was vortexed for 5min followed by 1hr of sonication until fully dissolved. The Octa Ammonium-POSS (1mg/ml) was then diluted in water accordingly to obtain Octa Ammonium-POSS in a solution of 0.125, 0.250, 0.500 and 1mg/ml concentration. (Table 1.2). In the next stage 1ml of short and long SWCNT-COOH (1mg/ml) was mixed with 5mg EDC and 5 mg NHS and stirred at room temperature for 2 hours, followed by ultrasonication for an additional 2 hours. In the next stage 1ml of each concentration of Octa Ammonium- POSS (0.125, 0.250, 0.500 and 1mg/ml) was added to 1ml of short SWCNT-COOH (1mg/ml) and long SWCNT (1mg/ml) solution as prepared in the previous stage. The mixture was then vortexed for 5min and sonicated for 1hr until fully dissolved. The mixtures were then washed at least five times with water to remove nonadsorbed Octa Ammonium-POSS followed by vacuum drying at room temperature for 24hrs.
Cell culture
Human breast cancer cell line (MCF7) and Human colon adenocarcinoma cell line (HT29) were used in this part. 96 well plates were used with each well containing 10,000 cells of HT29 cells and MCF7 cells. The cells were seeded for 24hrs. They were then incubated with different treatment groups for 24hrs, 48hrs and 72hrs. (Figures 1.3-1.10) The cell viability and the DNA concentration of each cell was then analysed using Alamar Blue and DNA assay after each time intervals. Treatment of cells Following synthesis of each treatment group, the treatment groups were initially vortexed for approximately 3min before adding to each well plate. 50μl of treatment solution was added (Growth medium in the case of the negative control) and incubated at 37°c with 5% CO2 for 24hrs, 48hrs and 72hrs.
Toxicity assay
Metabolic activity
Alamar Blue assay was utilised to measure the metabolic activity of the cells. In this experiment initially a 10% (v/v) dilution of stock Alamar Blue was prepared by diluting 4.5mls of Alamar Blue with 45mls of growth medium which was then stored at 4°c. Post preparation of diluted Alamar Blue, all culture medium and treatment groups were aspirated from the wells via the use of a multi-channel pipette, 50μl of diluted Alamar Blue (10%) was added into each well plate. The plates were then wrapped in aluminium foil and incubated for 4hrs at 37°c with 5% CO2. Following incubation 50μl from each well plate was transferred to a 96 well black micro plate. The black micro plate was then placed in the fluoroskan Ascent FL (Thermo labsystem) plate reader. Fluorescence was measured at excitation and emission wavelengths of 530 and 620 nm and recorded using Ascent software package. Wells containing medium and Alamar Blue without cells were used as blanks. The mean blank value was subtracted from each reading. This experiment was conducted independently three times therefore “n” is equal to 3.
DNA assay
Following the Alamar Blue assay all culture medium, treatment solution and Alamar Blue reagent were removed. 100μl of distilled water was then added to each well. The well plates were then freeze-thawed for three cycles. Each cycle involved freezing at -80°c for 30min followed by 30min of incubation at 37°c. 100μl of sample DNA (previously freeze-thawn in 100μl of distilled water per well) transferred from each well to their corresponding wells in a black 96 well plate. 100μl of aqueous working solution of the Quant-It Pico Green reagent was then added to every well, producing a total volume of 200μl per well. The well plates were then incubated at room temperature for 5min. Plates were then placed in the fluorooskan ascent machine to measure the fluorescence. The fluorescence value of the reagent blank was subtracted from each of the samples. The standard curve was then generated. All assay components were prepared as per Invitrogen™ protocol for QuantiT ™ Pico Green® DNA assay. Cell viability using a DNA assay was assessed on three consecutive days. Sample fluorescence was then measured using a fluorometer (wavelength of excitation 485 nm, emission 538 nm), and the data were statistically processed. This experiment was conducted independently three times therefore “n” is equal to 3.
Characterization study
FTIR
FTIR spectroscopy analysis was carried out to detect carboxylic acid groups (-COOH) and Octa Ammonium-POSS on both SWCNT. Full description is included in the following papers: Application of Octa Ammonium-POSS functionalized single walled carbon nanotubes for thermal treatment of cancer (Madani et al).
UV-VIS spectroscopy
UV-VIS spectrometer was used to indicate the changes in optical absorption following the functionalization of SWCNT with carboxylic acid and Octa Ammonium-POSS. Please referee to Application of Octa Ammonium-POSS functionalized single walled carbon nanotubes for thermal treatment of cancer
Morphological study
The pictures taken from TEM illustrate that in the case of pristine SWCNT; the tubes are interconnected and look clustered. Upon functionalization with acid the tubes appear separate from each other and a larger diameter of SWCNT is observed. Figure 1.1 shows that in the case of Octa Ammonium-POSS added in addition to SWCNT coated with carboxylic acid a thicker layer appears on the surface of the SWCNT. This thick black layer is due the presence of Octa Ammonium-POSS on the surface of the SWCNT.
Results
FTIR and UV-VIS spectroscopy
For a full description please refer to the following paper: Application of Octa Ammonium-POSS functionalized single walled carbon nanotubes for thermal treatment of cancer. Madani et al. [ 1,2]
Morphological study
The pictures taken from transmission electron microscopy (TEM) illustrate that in the case of pristine SWCNT; the tubes are interconnected and look clustered. Upon functionalization with acid the tubes appear separate from each other, and a thick black layer is observed on the surface of the SWCNT. Figure 1.1 shows that in the case of Octa Ammonium-POSS added in addition to SWCNT coated with carboxylic acid most of the SWCNT’ surface has become fully coated with the Octa Ammonium-POSS. However, with respect to the direct attachment of Octa Ammonium-POSS to the surface of the SWCNT, a break in clustered arrangement of the SWCNT can be observed. Some areas of the SWCNT have shown coating with Octa Ammonium-POSS.
Metabolic activity and DNA assay
Cell only
Results indicated that in both cell lines the cell’s metabolic activity increased over 72hrs. This pattern was shown to be greater in the case of HT29 cells in comparison to MCF7. The DNA assay also demonstrated a greater increase of DNA concentration in case of the HT29 in comparison to MCF7.
Octa Ammonium-POSS
Results have not shown any significant alteration of metabolic activity following the addition of all four concentrations of Octa Ammonium-POSS in both cell lines. This pattern was shown to be the same at all three-time intervals (24hrs, 48hrs and 72hrs). The DNA assay also represents no significant changes in DNA concentrations following the addition of different concentrations of Octa Ammonium-POSS to both cell lines at all three-time intervals.
Pristine SWCNT
Following the addition of all four concentrations of pristine SWCNT, the metabolic activity of both cell lines significantly declined in comparison to the cell only control. This pattern was the same at all three-time intervals. The graph produced indicated that by increasing the concentration of pristine SWCNT in both cell lines from 0.125mg/ml to 1.00mg/ml the cell’s metabolic activity declined. In the case of cell line HT29, with respect to the short SWCNT it was determined that following the addition of all four concentrations of SWCNT, the metabolic activity of the HT29 cell line significantly reduced in comparison to cell only (P<0.05). This pattern was observed at all three-time intervals. In the case of the MCF7 cell line, similar results were also obtained. The graphs determined that following the addition of all four concentrations of pristine short SWCNT to the MCF7 cell line the metabolic activity significantly reduced in comparison to cell only controls. The results show that as the concentration of short SWCNT increases from 0.125mg/ml to 1mg/ml the metabolism significantly declines (P<0.05). It has been demonstrated that in the case of MCF7 addition of pristine SWCNT decreases the cell’s metabolic activity with a greater magnitude at all their time intervals in comparison to the HT29 cell line. For example, with respect to the addition of 0.125mg/ml of pristine SWCNT to MCF7, results show that at 24hrs, 48hrs and 72hrs the metabolic activity decreased by approximately 64%, 72% and 78% respectively. However, in the case of HT29 following the addition of short pristine SWCNT (0.125mg/ml) the metabolic activity of the HT29 cell line decreased by approximately 57%, 55% and 50% at 24hrs, 48hrs and 72hrs respectively. About SWCNT displaying a longer length (1.5 to 2nm), in the case of both HT29 and MCF7 cell lines, results show that as the SWCNT’s concentration increases the cell’s metabolic activity decreases. In the case of the HT29 cell line it was determined that at all four concentrations of long SWCNT at all three-time intervals produced a significantly higher reduction in metabolic activity than in comparison to the exposure of short SWCNT to the HT29 cell line. P is determined to be less than 0.05 when the metabolic activity of the HT29 cell line was compared following the addition of each concentration of long SWCNT compared to short SWCNT. This was also observed in the case of MCF7. With respect to the DNA assay, results have also demonstrated a similar trend in metabolic activity. In the case of the HT29 cell line it was seen that upon the addition of the short SWCNT, the DNA concentration of the HT29 cell line significantly decreased. Results show following the addition of all four SWCNT’s concentrations, the DNA concentration significantly declined in comparison to cell only. This was illustrated at all three times intervals. It was illustrated that by increasing the concentration of pristine SWCNT the DNA concentrations significantly declined. In the case of long SWCNT, a similar trend as seen with the short SWCNT was observed at all four concentrations and at all three-time intervals (24hrs, 48hrs and 72hrs). The decrease in DNA concentrations following the addition of long SWCNT appeared to be significantly higher in comparison to the addition of short SWCNT. It was illustrated that in both cases at a higher concentration of SWCNT i.e. (0.5mg/ml and 1mg/ ml) by proceeding from 24hrs to 72hrs the DNA concentration does not increase with a magnitude as high as addition of lower concentration (i.e., 0.125mg and 0.250mg/ml). For example, following the addition of 0.125mg/ml of short pristine SWCNT to the HT29 cell line, it was seen that the DNA concentration from 24hrs to 48hrs increased by approximately 27% and from 48hrs to 72hrs increased by 32%. However, following the addition of 1mg/ ml of short pristine SWCNT to the cell line from 24hrs to 48hrs the DNA concentration increased by 19% and from 48hrs to 72hrs the DNA concentration increased by 24%. In the case of short SWCNT with respect to MCF7, results show that as the concentration of pristine SWCNT increases the DNA concentration decreases. The results show that the percentage decline in DNA concentration in the case of MCF7 following the addition of pristine SWCNT is higher than the DNA concentration decline seen in the HT29 cell line. This was observed at all three-time intervals. In the case of the MCF cell line, addition of short SWCNT illustrated a significant decrease in the DNA concentration. It was illustrated that the decrease in DNA concentration following the addition of 0.5mg/ml and 1.0mg/ml short SWCNT was at the higher end in most cases in comparison to 0.125mg/ml and 0.250mg/ml. In the case of long SWCNT, addition of all four concentrations to the MCF7 cell line has shown a significant decrease in DNA concentration. Again, as with the previous experiments, it was illustrated that as the concentration increases the DNA concentration decreases.
Functionalized SWCNT
The graphs produced portray that as the exposure time of acid to short SWCNT (functionalization) increased from 0.5hrs to 5hrs the metabolic activity of HT29 also increased in comparison to pristine SWCNT. It was observed that following incubation of short SWCNT with the cells, which were functionalized for a period of 2hrs and 5hrs led to a significant increase in metabolic activity (P<0.05). This trend was observed at all three-time intervals (24hrs, 48hrs and 72hrs). Results show no significant increase in metabolic activity of the HT29 cell line following incubation of all four concentrations of short SWCNT functionalized for 0.5hrs in comparison to pristine SWCNT. Results demonstrate a p value higher than 0.05 when the metabolic activity of HT29 cells following the addition of pristine SWCNT and SWCNT functionalized for 0.5hrs were compared. This result was observed to be consistent at all concentrations of SWCNT. This pattern appeared to be same at all three-time intervals (24hrs, 48hrs and 72hrs). With respect to long SWCNT results show that following the incubation of all four concentrations of SWCNT, which had been functionalized for 5hrs to the cells the metabolic activity of HT29 cell line significantly increased in comparison to incubation with pristine SWCNT. This was also the case at all threetime intervals. In the case of MCF7, with respect to both short and long SWCNT following 0.5hrs and 2hrs of functionalization the metabolic activity of the cells increased but this increase was only significant post 5hrs of functionalization. With respect to the short SWCNT this trend observed for all three-time intervals. With respect to long SWCNT a significant increase of DNA concentration was observed following a 5hrs functionalization at 24hrs interval. No further significant changes of metabolic activity were reported for other functionalization periods. With respect to the DNA assay, results also illustrated that functionalization had a direct effect on increasing the DNA concentration. According to our results, increasing the functionalization timing from 0.5hrs to 5hrs led to the increase in DNA concentration in both cell lines. In the case of the HT29 cell line, increasing the functionalization time of short SWCNT resulted in an increase of DNA concentration. This result showed that at all three-time intervals (24hrs, 48hrs and 72hrs) the concentration of DNA significantly increased following functionalization for as short a time as 0.5hrs. With respect to long SWCNT a similar result was obtained. In this experiment it was also indicated that by increasing the functionalization from 0.5hrs to 5.0hrs the DNA concentration significantly increased in comparison to pristine long SWCNT. This pattern appeared to be at all three-time intervals. The graphs show that the increase in DNA concentration at all functionalization timings in the case of long SWCNT is lower than the short SWCNT. This pattern was the same at all three-time intervals (24hrs, 48hrs and 72hrs). With respect to the MCF7 cell line similar results have been obtained. However, in the case of incubation of short SWCNT, it was illustrated that only following the 2hrs and 5hrs of functionalization of SWCNT and its incubation with the cells the DNA concentration significantly increase in comparison to pristine SWCNT. This result was only illustrated at 48hrs and 72hrs. At 24hrs no change in metabolism was detected following any acid treatment period. In the case of functionalization of long SWCNT, results illustrated no changes in DNA concentration following any period of functionalization in comparison to SWCNT-COOH. This pattern appeared to be the same for all four concentrations.
Pristine SWNCT (1mg/ml) + Octa Ammonium-POSS
Results have indicated that with respect to the incubation of pristine SWCNT (1mg/ml) following their conjugation with 0.250mg/ml, 0.5mg/ml and 1mg/ml concentration of Octa Ammonium-POSS with the HT29 cell line the metabolic activity of the cells significantly increased in comparison to pristine SWCNT (1mg/ml) at all three-time intervals. In the case of a 0.125mg/ ml concentration of POSS, results indicated that at all three-time intervals (24hrs, 48hrs and 72hrs) the cell’s metabolic activity did not significantly increase following the conjugation of POSS to the short SWCNT in comparison to pristine SWCNT. With regards to long SWCNT, results indicated that increasing the POSS’s concentration would result in increasing the metabolic activity of HT29 cell line. In the case of long SWCNT at 24hrs, it was shown that only at 0.5mg/ml and 1.00mg/ml POSS the metabolic activity of cells exposed to long SWCNT significantly increase. Results show that no change in concentration of POSS affects the metabolic activity at 48hrs and 72hrs. With respect to the MCF7, results have also shown a significant increase in the cell’s metabolic activity following the conjugation of Octa Ammonium-POSS concentrations to short SWCNT (1mg/ml) in comparison to SWCNT (1mg/ml) only. This was only observed following the addition of 0.5mg and 1mg of POSS at a 24hr time interval. No significant changes were observed following the conjugation of 0.125mg/ml and 0.250mg/ml Octa Ammonium-POSS. With respect to incubation of long SWCNT conjugated with Octa Ammonium-POSS, the result is an increase in metabolic activity. This increase is present only at 1mg/ml Octa Ammonium-POSS at 24hrs. A DNA assay has also demonstrated similar results to an Alamar Blue test. In this assay, results show that in the case of short SWCNT with respect to HT29 cell line, the DNA concentration significantly increases following the conjugation of 0.5mg/ml and 1mg/ml Octa Ammonium-POSS to SWCNT at all three-time intervals. With respect to long SWCNT, only 1mg/ml POSS at all three times appeared to increase the DNA concentration significantly. In the case of the MCF7 conjugation of 1mg/ml Octa Ammonium-POSS to short SWCNT increased the concentration of DNA significantly at 24hrs. No change was observed in the case of other concentrations at other time intervals. In case of the long SWCNT no concentrations of POSS’s elicited an increase in DNA concentration.
SWCNT-COOH (1mg/ml) + Octa Ammonium-POSS
In the case of HT29 cancer cells by increasing the concentration of Octa Ammonium-POSS the metabolic activity of the cell also increased. In the case of HT29 cells, results show that conjugation of all four concentrations (0.125mg/ml, 0.250mg/ml, 0.500mg/ ml and 1mg/ml) of Octa Ammonium-POSS to short SWCNT-COOH (1mg/ml) resulted in a significant increase in metabolic activity in comparison to short SWCNT-COOH (1mg/ml). This pattern appeared to be the consistent across all three-time intervals. With respect to long SWCNT-COOH, it was demonstrated that apart from the conjugation of 0.125mg/ml Octa Ammonium-POSS to SWCNT-COOH, the remaining three concentrations demonstrated an increase in metabolic activity of HT29 at all three-time intervals. In the case of the MCF7 cell line it has been determined that the conjugation of Octa Ammonium-POSS to short SWCNT-COOH at 0.250 mg/ml; 0.5mg/ml and 1.0mg/ml will result in a significant increase in metabolic activity of MCF7 in comparison to the 0.125mg/ml POSS concentration. This trend was repeated at all three-time intervals. Long SWCNT, using Octa Ammonium-POSS in conjugation with SWCNT-COOH (1mg/ml) also demonstrated an increase in metabolic activity. Results showed identical data obtained as seen with short SWCNT. In this case again, excluding the 0.125mg/ml concentration, (Figures 1.1-1.15) results show that the addition of the other POSS concentrations at all three-time intervals resulted in a significant increase in metabolic activity. A DNA assay was also conducted in all cases. With respect to HT29 cells results showed that conjugation of all four concentrations of Octa Ammonium-POSS to short SWCNT significantly increased the metabolic activity of the HT29 at all three-time intervals. With respect to conjugation of POSS to long SWCNT-COOH, the trend was similar in the case of the long SWCNT, however no significant increase of DNA concentration was observed following conjugation with 0.125mg/ml Octa Ammonium-POSS. In the case of MCF7 cells, the DNA assay indicated similar results as the HT29 cancer cell line. Results show that following the addition of all POSS concentrations to both the short and long SWCNT-COOH, DNA concentration significantly increases. It was demonstrated that this increase in the case of long SWCNT-COOH occurs with lower magnitude than seen in the short SWCNT-COOH. To summaries, graphs produced show the metabolic activity of HT29 and MCF7 cell lines at 24hrs, 48hrs and 72hrs time interval. An effect of different concentrations of pristine SWCNT with both short and long length was also observed on the metabolic activity of these two cell lines. This was followed by an observation into the effect of increasing the functionalization time on the metabolic activity of the cell lines. The effect of conjugation of Octa Ammonium-POSS both covalently and non-covalently on the metabolic activity of the cell lines were also observed. The DNA concentration of both HT29 and MCF7 cell lines were investigated at 24hrs, 48 hours and 72 hrs. The graph clearly demonstrates the effect of different concentrations of short and long pristine SWCNT on the concentration of DNA in both cell lines. The effect of different functionalization periods on DNA concentration was also observed. This was followed by investigating the effect of conjugation of Octa Ammonium-POSS both directly and indirectly on both cell lines.
Figure 1.1: TEM images of SWCNT with different functionalization groups. (A)Pristine SWCNT (B) OctaAmmonium-POSS (C) SWCNT-COOH+Ocata-Ammonium-POSS (D) SWCNT-COOH (E) SWCNT-OctaAmmonium-POSS
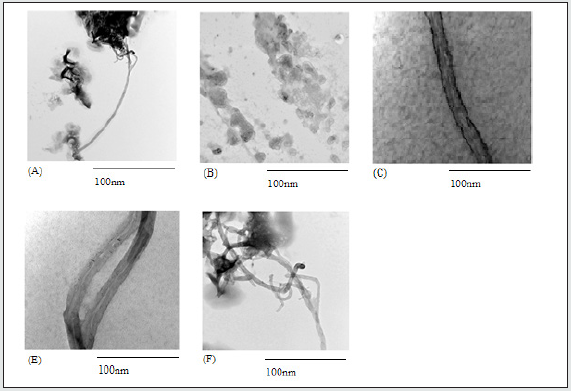
Figure 1.2: POSS incubation with the cells did not result in any significant change of emission in comparison to the cell only control (P>0.05). Increasing the functionalization time (2hrs, 5hrs) of SWCNT resulted in greater fluorescence emission in comparison to pristine SWNCT (P<0.05). Addition of all POSS concentrations to SWCNT (1mg/ml) except the 0.125mg/ml resulted in a significant increase in emission when compared to pristine SWCNT. Conjugation of all POSS concentrations to SWCNT-COOH (1mg/ml) resulted in a significant increase in emission compared with SWCNT-COOH (1mg/ml) (P<0.05).
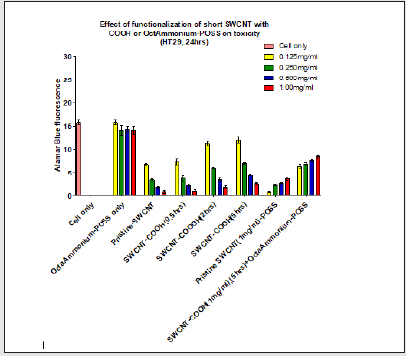
Figure 1.3: POSS incubation with the cells did not result in any significant change in emission in comparison to cell only (P>0.05). Increasing the functionalization time (2hrs, 5hrs) of SWCNT resulted in greater fluorescence emission when compared with pristine SWNCT (P<0.05). Addition of all POSS concentrations to SWCNT (1mg/ml) except 0.125mg/ml resulted in a significant increase in emission when compared with pristine SWCNT. Conjugation of all POSS concentrations to SWCNTCOOH (1mg/ml) resulted in a significant change in emission in comparison to SWCNT-COOH (1mg/ml) (P<0.05)
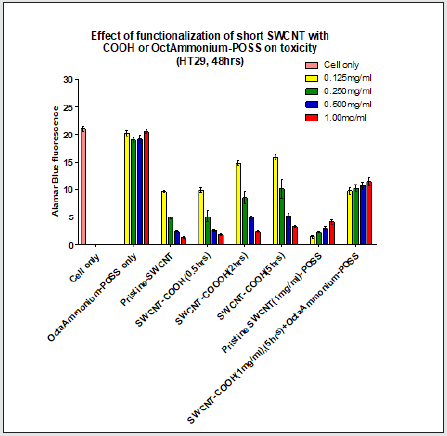
Figure 1.4: POSS incubation with the cells did not result in any significant change in emission in comparison to cell only (P>0.05). Increasing the functionalization time (2hrs, 5hrs) of SWCNT resulted in greater fluorescence emission in comparison to pristine SWNCT (P<0.05). Addition of all POSS concentrations to SWCNT (1mg/ml) excluding 0.125mg/ml resulted in a significant increase in emission in comparison to pristine SWCNT. Conjugation of all POSS concentrations to SWCNT-COOH (1mg/ml) resulted in a significant change in emission in comparison to SWCNT-COOH (1mg/ml) (P<0.05).
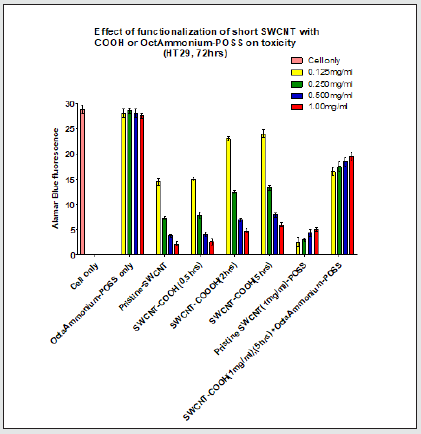
Figure 1.5: POSS incubation with the cells did not result in any significant change in emission when compared to cell only (P>0.05). Increasing the functionalization time (5hrs only) of SWCNT resulted in greater fluorescence emission in comparison to pristine SWNCT (P<0.05). Addition of POSS (0.500 and 1mg/ml) concentrations to SWCNT (1mg/ml) resulted in a significant increase in emission in comparison to pristine SWCNT. Conjugation of all POSS concentrations (except 0.125mg/ ml) to SWCNT-COOH (1mg/ml) resulted in a significant difference in emission when compared with SWCNT-COOH (1mg/ ml) (P<0.05).

Figure 1.6: POSS incubation with the cells did not result in any significant change in emission when compared to cell only controls (P>0.05). Increasing the functionalization time (5hrs only) of SWCNT resulted in greater fluorescence emission in comparison to pristine SWNCT (P<0.05). Addition of none of the POSS concentrations to SWCNT (1mg/ml) resulted in a significant increase in emission when compared with pristine SWCNT. Conjugation of all POSS concentrations (except 0.125mg/ml) to SWCNT-COOH (1mg/ml) resulted in a significant change in emission in comparison to SWCNT-COOH (1mg/ml) (P<0.05).

Figure 1.7: POSS incubation with the cells did not result in any significant change in emission in comparison to cell only controls (P>0.05). Increasing the functionalization time (5hrs only) of SWCNT resulted in greater fluorescence emission in comparison to pristine SWNCT (P<0.05). Addition of none of the POSS concentrations to SWCNT (1mg/ml) resulted in a significant increase in emission in comparison to pristine SWCNT. Conjugation of all POSS concentrations (except 0.125mg/ml) to SWCNT-COOH (1mg/ml) resulted in a significant change in emission compared with SWCNT-COOH (1mg/ml) (P<0.05).

Figure 1.8: POSS incubation with the cells did not result in any significant change in emission in comparison to cell only controls (P>0.05). Increasing the functionalization time (5hrs only) of SWCNT resulted in greater fluorescence emission in comparison to pristine SWNCT (P<0.05). Addition of 0.5 and 1mg/ml of POSS concentrations to SWCNT (1mg/ml) resulted in a significant increase in emission when compared with pristine SWCNT. Conjugation of all POSS concentrations (except 0.125mg/ml) to SWCNT-COOH (1mg/ml) resulted in a significant difference in emission when compared with SWCNTCOOH (1mg/ml) (P<0.05).
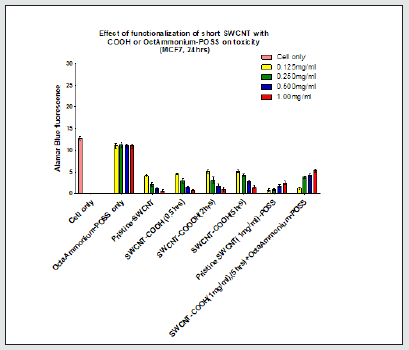
Figure 1.9: POSS incubation with the cells did not result in any significant change in emission in comparison to cell only controls (P>0.05). Increasing the functionalization time (5hrs only) of SWCNT resulted in greater fluorescence emission in comparison to pristine SWNCT (P<0.05). Conjugation of none of the POSS concentrations to SWCNT (1mg/ml) resulted in a significant increase in emission when compared with pristine SWCNT. Conjugation of all POSS concentrations (except 0.125mg/ml) to SWCNT-COOH (1mg/ml) resulted in a significant change in emission in comparison to SWCNT-COOH (1mg/ml) (P<0.05).

Figure 1.10: POSS incubation with the cells did not result in any significant change in emission in comparison to cell only controls (P>0.05). Increasing the functionalization time (5hrs only) of SWCNT resulted in greater fluorescence emission in comparison to pristine SWNCT (P<0.05). Conjugation of none of the POSS concentrations to SWCNT (1mg/ml) resulted in a significant increase in emission in comparison to pristine SWCNT. Conjugation of all POSS concentrations (except 0.125mg/ ml) to SWCNT-COOH (1mg/ml) resulted in a significant change in emission when compared with SWCNT-COOH (1mg/ml) (P<0.05).

Figure 1.11: POSS incubation with the cells did not result in any significant change in emission in comparison to cell only controls (P>0.05). Increasing the functionalization time of SWCNT did not display any significant change in fluorescence emission when compared to pristine SWNCT (P>0.05). Conjugation of none of the POSS concentrations to SWCNT (1mg/ml) resulted in a significant increase of emission in comparison to pristine SWCNT. Conjugation of all POSS concentrations (except 0.125mg/ml) to SWCNT-COOH (1mg/ml) resulted in significant increase in emission when compared with SWCNT-COOH (1mg/ml) (P<0.05).

Figure 1.12: POSS incubation with the cells did not result in any significant change in emission in comparison to cell only controls (P>0.05). Increasing the functionalization time of SWCNT did not show any significant change in fluorescence emission in comparison to pristine SWNCT (P>0.05). Conjugation of none of the POSS’s concentrations to SWCNT (1mg/ ml) resulted in a significant increase in emission in comparison to pristine SWCNT. Conjugation of all POSS’s concentrations (except 0.125mg/ml) to SWCNT-COOH (1mg/ml) resulted in a significant increase in emission when compared to SWCNTCOOH (1mg/ml) (P<0.05).

Figure 1.13: POSS incubation with the cells did not result in any significant change in its DNA concentration when compared to cell only controls (P>0.05). Increasing the functionalization time of SWCNT led to a significant increase in DNA concentration in comparison to pristine SWNCT (P<0.05). Conjugation of 0.5mg/ml and 1mg/ml of POSS to SWCNT (1mg/ml) resulted in a significant increase in DNA concentration when compared to pristine SWCNT. Conjugation of all POSS concentrations to SWCNT-COOH (1mg/ml) resulted in a significant increase in DNA concentration in comparison to SWCNT-COOH (1mg/ ml) (P<0.05).

Figure 1.14: POSS incubation with the cells did not result in any significant change in its DNA concentration when compared to cell only controls (P>0.05). Increasing the functionalization time of SWCNT led to a significant increase in DNA concentration in comparison to pristine SWNCT (P<0.05). Conjugation of 0.5mg/ml and 1mg/ml of POSS to SWCNT (1mg/ml) resulted in a significant increase in DNA concentration in comparison to pristine SWCNT. Conjugation of all POSS concentrations to SWCNT-COOH (1mg/ml) resulted in a significant increase in DNA concentration in comparison to SWCNT-COOH (1mg/ ml) (P<0.05).

Figure 1.15: POSS incubation with the cells did not result in any significant change in its DNA concentration when compared to cell only controls (P>0.05). Increasing the functionalization time of SWCNT showed a significant increase in DNA concentration in comparison to pristine SWNCT (P<0.05). Conjugation of 0.5mg/ml and 1mg/ml of POSS to SWCNT (1mg/ml) resulted in a significant increase in DNA concentration in comparison to pristine SWCNT. Conjugation of all POSS concentrations to SWCNT-COOH (1mg/ml) resulted in a significant increase in DNA concentration when compared to SWCNT-COOH (1mg/ ml) (P<0.05).

Discussion
As it has been explained previously the main aim of this experiment was to investigate the influence of addition of different treatment groups on two cancer cell lines. It was aimed to investigate whether increasing the length of SWCNTs had any influence on the toxicity of the SWCNT. In addition, the experiment was aiming to investigate the optimal functionalization technique for minimizing the toxicity of the SWCNT. Following the treatment of SWCNT with acid, various tests were conducted to ascertain successful attachment of the functional group to the surface of the SWCNT. These tests included FTIR, UV-VIS spectrometer and TEM. With respect to FTIR and UV-VIS, a comprehensive discussion is included in the following publication: Application of Octa Ammonium- POSS functionalized single walled carbon nanotubes for thermal treatment of cancer Madani et al. [1,2]. In both cases of SWCNTCOOH and SWCNT-COOH conjugated with Octa Ammonium-POSS the graph shows the presence of a peak at 270nm. However, the peak for SWCNT-COOH conjugated with Octa Ammonium-POSS appears to be at its lower area. The presence of POSS is illustrated as a peak at SWCNT-COOH conjugated with POSS is in its lower level in comparison to SWCNT-COOH. The presence of POSS decreases the peak absorbance level. If the POSS was not present a peak in the same area as the SWCNT-COOH only would have been observed. The change in peak towards a lower level means that POSS is present in addition to carboxylic acid. TEM images also illustrate successful functionalization (Figures 1.16-1.23).
Figure 1.16: POSS incubation with the cells did not result in any significant change in its DNA concentration when compared to cell only controls (P>0.05). Increasing the functionalization time of SWCNT led to a significant increase in DNA concentration in comparison to pristine SWNCT (P<0.05). Conjugation of 1mg/ml of POSS only to SWCNT (1mg/ml) resulted in a significant increase in DNA concentration in comparison to pristine SWCNT. Conjugation of all POSS concentrations except 0.125mg/ ml to SWCNT-COOH (1mg/ml) resulted in a significant increase in DNA concentration when compared to SWCNT-COOH (1mg/ml) (P<0.05).

Figure 1.17: POSS incubation with the cells did not result in any significant change in its DNA concentration when compared to cell only controls (P>0.05). Increasing the functionalization time of SWCNT led to a significant increase in DNA concentration in comparison to pristine SWNCT (P<0.05). Conjugation of 1mg/ml of POSS only to SWCNT (1mg/ml) resulted in a significant increase in DNA concentration in comparison to pristine SWCNT. Conjugation of all POSS concentrations except 0.125mg/ ml to SWCNT-COOH (1mg/ml) resulted in a significant increase in DNA concentration when compared to SWCNT-COOH (1mg/ml) (P<0.05).

Figure 1.18: POSS incubation with the cells did not result in any significant change in its DNA concentration when compared to the cell only control (P>0.05). Increasing the functionalization time of SWCNT showed a significant increase in DNA concentration in comparison to pristine SWNCT (P<0.05). Conjugation of 1mg/ml of POSS only to SWCNT (1mg/ml) resulted in a significant increase in DNA concentration when compared to pristine SWCNT. Conjugation of all POSS concentrations except 0.125mg/ml to SWCNT-COOH (1mg/ml) resulted in a significant increase in DNA concentration in comparison to SWCNT-COOH (1mg/ml) (P<0.05).

Figure 1.19: POSS incubation with the cells did not result in any significant change in its DNA concentration when compared to cell only controls (P>0.05). Increasing the functionalization time of SWCNT showed a non-significant increase in DNA concentration in comparison to pristine SWNCT (P>0.05). Conjugation of only 1mg/ml POSS to SWCNT (1mg/ml) resulted in significant increase in DNA concentration in comparison to pristine SWCNT. Conjugation of all POSS concentrations to SWCNT-COOH (1mg/ml) resulted in a significant increase in DNA concentration in comparison to SWCNT-COOH (1mg/ ml) (P<0.05).

Figure 1.20: POSS incubation with the cells did not result in any significant change in its DNA concentration compared to cell only controls (P>0.05). Increasing the functionalization time (2hrs and 5hrs) of SWCNT led to a significant increase in DNA concentration in comparison to pristine SWNCT (P<0.05). Conjugation of POSS concentrations to the pristine SWCNT did not show any significant increase in DNA concentration in comparison to pristine SWCNT. P was shown to be greater than 0.05 at all concentrations. Conjugation of all POSS concentrations to SWCNT-COOH (1mg/ml) resulted in a significant increase in DNA concentration in comparison to SWCNT-COOH (1mg/ml) (P<0.05).

Figure 1.21: POSS incubation with the cells did not result in any significant change in its DNA concentration when compared to cell only controls (P>0.05). Increasing the functionalization time (2hrs and 5hrs) of SWCNT showed a significant increase in DNA concentration in comparison to pristine SWNCT (P<0.05). Conjugation of POSS to the pristine SWCNT did not result in any significant change of DNA concentration in comparison to pristine SWCNT. Conjugation of all POSS concentrations to the SWCNT-COOH resulted in a significant increase in DNA concentration in comparison to SWCNT-COOH as P was shown to be smaller than 0.05 in all cases.

Figure 1.22: POSS incubation with the cells did not result in any significant change in its DNA concentration compared to cell only controls (P>0.05). Increasing the functionalization time of SWCNT did not show any significant increase in DNA concentration in comparison to pristine SWNCT (P>0.05). Conjugation of POSS to the pristine SWCNT did not result in significant changes in DNA concentration in comparison to pristine SWCNT. Conjugation of all POSS concentrations to the SWCNT-COOH resulted in a significant increase in DNA concentration in comparison to SWCNT-COOH P<0.05.

Figure 1.23: POSS incubation with the cells did not result in any significant change in its DNA concentration when compared to cell only controls (P>0.05). Increasing the functionalization time of SWCNT did not show a significant increase in DNA concentration in comparison to pristine SWNCT (P>0.05). Conjugation of POSS to the pristine SWCNT did not result in a significant change in DNA concentration in comparison to pristine concentration. Conjugation of all POSS concentrations to the SWCNT-COOH resulted in a significant increase in DNA concentration in comparison to SWCNT-COOH (P<0.05).

The TEM image of pristine SWCNT appeared as clustered tubes. Upon the treatment of SWCNT with acid the image clearly shows less clustered tubes with a black layer formed on the surface of the tubes. It is assumed this layer is due to the carboxylic acid. Further tests as explained above were also conducted to prove this hypothesis. Following the addition of Octa Ammonium-POSS to the SWCNT-COOH, TEM images illustrate the presence of black dots and in addition less clustered SWCNT. The black dots present are due to the presence of Octa Ammonium-POSS. Following successful characterization, the HT29 and MCF7 cell lines were treated with different functionalized SWCNT. Generally, with respect to both HT29 and MCF7 cell lines, results have illustrated both an increase in cell metabolism and DNA concentration over a 72-hr. period. It was seen that this increase is greater in the case of HT29 in comparison to MCF7 which clearly determines greater metabolic activity and cell proliferation of the HT29 cell line in comparison to MCF7. In both cases from 48hrs to 72hrs the DNA concentration and metabolic activity increases more than when compared between 24hrs and 48hrs. This shows that the major cell division events of both cells are happening after the second day. The experiment also aimed to investigate the effect of Octa Ammonium-POSS as a functionalization material to reduce the toxicity of the SWCNT.
Initially the effect of various concentrations of Octa Ammonium-POSS on the cell’s metabolism and any change in DNA concentration was observed. The effect of exposure of POSS to the cells and its effect on the cell’s metabolism and DNA concentration were investigated in the next stage of the experiment. Results show that by increasing the concentration of POSS no significant alteration occurred at a cellular metabolic level and the DNA concentration. This indicates that the POSS itself is biologically safe and has no toxic effect to the cells, without causing any interruption to the cell’s division. For this reason, it was concluded that Octa Ammonium-POSS can be used as an agent added to the SWCNT’s surface to increase the dispersibility of the SWCNT and reduce the SWCNT’s toxicity. Functionalization of SWCNT molecules with Octa Ammonium-POSS can substantially enhance the solubility and processability of the nanocomposite. Evidence of biocompatibility and amphiphilic properties of the POSS [12-15] nanocomposite has already prompted researchers to patent this molecule for use at the vascular interface in devices such as stents. POSS is short for polyhedral oligomeric silsesquioxane. This material has been used by wide range of researchers to reduce the toxicity of their materials. Following the addition of different functional groups to POSS this molecule will gain different properties [17]. One of the POSS molecules is called POSS-PCU. This molecule has been used by different groups of the researchers to reduce the toxicity of QDs [18]. A great reduction of QDs toxicity has been reported. POSS-PCU have also recently been used in the development of trachea in humans [19]. Based on the above findings it was decided to investigate the application of the POSS molecule on the reduction of the SWNCT’s toxicity [20-35]. In addition, the aim was to conjugate the functional group through the covalent bond [13] on the surface of the SWCNT rather than using a weak bond such as non-covalent or Vander walls bond. [22]. It has been indicated by different researchers that the attachment of the non-covalent bond would not be suitable as the functional group will be likely to be dissociated from the SWNCT’s surface [23].
The Octa Ammonium is another type of POSS molecule, [24] which consists of eight NH2 groups. The presents of eight –NH2 group allows the Octa Ammonium-POSS to be conjugated through the covalent bonds to the –COOH group that is located on the surface of the SWCNT [34]. Due to the above reason the Octa Ammonium-POSS was decided to be used for its further conjugation to the SWCNT-COOH. POSS would increase SWCNT dispersion in biological systems, due to its amphiphilic nature. POSS is known as a material resistant to degradation and has anti-calcification effects. It demonstrates superior biocompatibility [35] and is currently being assessed for use in medical implants. Prior to considering the effect of functionalization on the toxicity of the SWCNT, the effect of addition of pristine SWCNT on the cell’s metabolism and DNA concentration were assessed in line with whether adjusting the length would affect the toxicity of the SWCNT were monitored. It was determined that in general, addition of all four concentrations of pristine SWCNT significantly reduced the metabolism of both cell lines. The results produced show that regardless of the size of the SWCNT, by increasing the pristine SWCNT’s concentration from 0.125mg/ml to 1.0mg/ml the cell’s metabolic activity and DNA concentrations significantly decreased in both cell lines. This could have been due to different factors. The main one is that a larger concentration means there will be more SWCNT that can reach the nucleus following their exposure to the cancer cell therefore further interrupting the cells division. This trend was also observed with the MCF7 cell line. However, SWCNT are observed to decrease the cell’s metabolism of MCF7 with a higher magnitude than in the case of HT29 cells. This was the case with both short and long SWCNT. This could have been due to less cell division taking place .in the case of MCF7 in comparison to HT29 cells leading to a greater ratio of SWCNT to MCF7 cells in comparison to HT29. As a result, greater accumulation of SWCNT would occur in the case of MCF7 in comparison to HT29. Increasing the size of the SWCNT is one factor which has shown to increase its toxicity [27]. Generally, it is known that once a particle hits the cell membrane the particles will undergo phagocytosis into the cell [20]. However round nanoparticles are seen to be engulphed and internalized more efficiently than the rod shape [25] nanoparticles such as SWCNT. Various research groups have also shown that smaller nanoparticles are engulphed more significantly greater by the cell membrane than larger nanoparticle [26].
Large rod shape nanoparticles such as SWCNT could not be processed via phagocytosis as easily as small SWCNT [28]. It was also demonstrated by different researchers that large pristine nanoparticle could generally result in the rupture of the cell membrane once the engulfing process begins. Our results also follow the finding of different research groups, with respect to the effect of size on SWCNT’s toxicity. In the case of both HT29 and M.CF7 cells following the addition of various concentrations of large SWCNT, the cell’s metabolic activity and DNA concentration significantly decline in comparison to short SWCNT. This trend was observed over all three-time intervals. For example, with respect to MCF7 in the case of long SWCNT, DNA concentration results show that 0.250mg/ml, 0.5mg/ml and 1mg/ml appeared to decrease the DNA concentration significantly more than 0.125mg/ml. However, in the case of short SWCNT, the dose of 0.5mg/ml and 1.00mg/ml of SWCNT appeared more toxic than the 0.125mg and 0.25mg/ml, this is due to long SWCNT leading to easier cell destruction. In the case of short SWCNT a dose as small as 0.250mg/ml could destroy the cells whereas in the case of short SWCNT, the starting dose needed to be as high as 0.5mg/ml to dramatically reduce the DNA concentration. The experiment is now aiming to investigate whether functionalization would influence the reduction of SWCNT’s toxicity. Generally pristine SWCNT are not biocompatible [14]. The pristine SWCNT are clustered and networked materials. Functionalization is a process in which the SWCNT is treated with different chemical materials such as with a combination of HNO3 and H2SO4, Octa Ammonium-POSS and PEG [15]. This will result in the dissociation of Van der walls forces between the nanotubes. As a result, the tubes will be less clustered and lower toxic [27]. In addition, coating the surface of the SWCNT will increase the hydrophilicity of the SWCNT, during this process the sharp end of the SWCNT which normally results in the cell’s rupture will be disappeared. In our experiment it was demonstrated that generally the longer the SWCNT was in the treatment with the HNO3; H2SO4, the higher the DNA concentration and metabolic activity of the cells were observed. This is since the functionalization process will result in dissociation of the Van der walls forces between the tubes and as a result the SWCNT will appear less clustered. The longer functionalization period means the more SWCNT will become dissociated and released from the cluster arrangements. This breaks down of cluster arrangement would give a higher chance for better engulfment process of the SWCNT. Longer functionalization period of SWCNT could also result in the loss of any sharp ends present on the SWCNT. The sharps end could normally result in the rupture of the cell membrane. Also, many impurities, which are normally used as catalyst agents during the SWCNT synthesis, can also remove following the successful functionalization process. The other factors that functionalization would generally result in reducing the SWCNT toxicity is the diameter expansion of SWCNT following its functionalization [30]. Once the SWCNT is functionalized it will become thicker leading to a lower chance of SWCNT to be entered to the nucleus, which means less chance to interrupt the DNA’s normal function. Functionalization will result in opening the networked and clustered arrangement of the SWCNT. It changes their properties, making them hydrophilic. This hydrophilicity improvement will result in SWCNT’s better interaction with the cell membrane, which allows the SWCNT to be used as tolls for other applications such as drug [31] delivery and thermal treatment of cancer [32]. It has been reported that the functionalization of long SWCNT has also resulted in reduction of the SWCNT’s toxicity but with a lower magnitude than the short SWCNT. This could be since the longer SWCNTs results in further interruption of annulment in comparison to short SWCNTs. In the case of the HT29 cell line, it was shown that shorter SWCNTs need a lower treatment period with acid (minimum 2hrs) than the longer SWCNT (minimum 5hrs) to observe an improvement on the HT29 metabolic activity. The fact that the smaller size requires lower treatment period with acid in comparison to longer SWCNT for its toxicity to be reduced significantly, is one way of establishing that the smaller SWCNTs are less toxic than the longer variety. Long SWCNT requires a longer treatment time with acid to become less clustered than the short SWCNTs. In the case of the long SWCNT, there will be more interaction between the tubes than the short SWCNT and as a result longer treatment with acid is required. A short functionalization period will result in coating of some of the SWCNT. Those that are not coated would normally rupture the cell membrane with their sharp ends. The chance of rupture with the short SWCNT is lower than with long SWCNTs. In terms of a comparison between the two cell lines, with regards to the MCF7 cell line, it was observed that in the case of the short SWCNT, following functionalization for as long as 5hrs the metabolic activity of the cell line significantly improved. In the case of HT29, functionalization of short SWCNT for as little as 2hrs provided a significant increase in the metabolic activity of the HT29 cell line. This could be due to various reasons. One of which, being that MCF cell division is slower than that of the HT29 cell line and so there are less cells available. This means that in the case of MCF7, as there is a higher ratio of SWCNT to cell, even a small number of nonfunctionalized SWCNTs will result in a massive reduction in the cell’s normal activity. The other reason could be the uptake mechanism and the rate of uptake into MCF7 and HT29. 2hrs period of functionalization process of SWCNT will result in lowering the SWCNT clustered arrangement and formation of a thick layer of carboxylic acid on the SWCNT’s surface. In the case of MCF7 the cell’s metabolic activity decreases to the same level as pristine SWCNT following the addition of SWCNT functionalized for as long as 2hrs. This shows that with respect to the MCF7 the functionalized SWCNT can still diffuse through the cell membrane and reach to the nucleus. However, in the case of HT29 following a 2hrs functionalization of SWCNT the metabolic activity significantly increases in comparison to pristine SWCNT [33-35]. This means less SWCNT can diffuse into the HT29 cell line following a 2hrs functionalization. This could be due to the smaller pores of HT29 cell membrane or its slower uptake mechanism in comparison to MCF7. As mentioned earlier this experiment was designed to investigate the best functionalization technique to reduce toxicity of the SWCNT. Results from treating the SWCNT with acid clearly illustrate that in most cases of both short and long SWCNT, the longer the SWCNT stays in contact with the acid the greater the metabolic activity and DNA concentration of the cell observed in the case of both short and long SWCNT. This increase has been observed to be significant in most cases, however there are different scenarios in which a non-significant increase can be observed. The aim of the experiment is now to investigate the effect of Octa Ammonium-POSS as an additional material for reducing the SWCNT toxicity. The experiment was to initially investigate the effect of Octa Ammonium-POSS, non-covalently conjugated to the surface of the SWCNT. A set pattern cannot be observed with respect to the increase of cell’s metabolic activity and the DNA concentrations; however, in most cases the conjugation of Octa Ammonium-POSS has demonstrated an increase in cell metabolic activity and DNA concentration in both cell lines. The main reasons that the conjugation of Octa Ammonium-POSS is not as effective as treating the SWCNT with acid, which results in conjugation of SWCNT with carboxylic acid group could be due to different factors. One of which could be due to the weak non-covalent bonding formed between the SWCNT ‘s surface and the Octa Ammonium-POSS. This could result in easy dissociation of the Octa Ammonium-POSS from the SWCNT’s surface. The other could be due to non-uniform distribution of Octa Ammonium-POSS on the SWCNT’s surface. With respect to conjugating the Octa Ammonium-POSS onto the SWCNT already functionalized with acid, at almost all Octa Ammonium-POSS concentrations it was seen that following its conjugation to the SWCNT-COOH the cell’s metabolism and DNA concentration significantly improve. This could be since the Octa Ammonium-POSS was uniformly distributed. The presence of carboxylic acid on the SWCNT’s surface could provide a suitable platform for the Octa Ammonium-POSS to attach to a wider area of the SWCNT’s surface. Also, the covalent bond formed between the Octa Ammonium-POSS and the SWCNT-COOH surface is much stronger than non-covalent attachment of the Octa Ammonium- POSS. As a result, there will be less chance of Octa Ammonium-POSS disassociating from the SWCNT’s surface.
References
- Madani SY, Naderi N, Dissanayake O, Tan A, Seifalian AM, et al. (2011) A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int J Nanomedicine 6: 2963-2679.
- Madani SY, Tan A, Dwek M, Seifalian AM (2012) Functionalization of single-walled carbon nanotubes and their binding to cancer cells. Int J Nanomedicine 7: 905-914.
- Saleemi MA, Kogn YL, Yong PVC, Wong EH (2020) An overview of recent development in therapeutic drug carrier system using carbon nanotubes. Journal of Drug Delivery Science and Technology 59: 101855.
- Bull E, Madani SY, Sheth R, Seifalian A, Green M, et al. (2014) Stem cell tracking using iron oxide nanoparticles. Int J Nanomedicine 9: 1641-1653.
- Hassan A, Saeed A, Afzal S, Shahid M, Amin I, et al. (2020) Applications and hazards associated with carbon nanotubes in biomedical sciences. Inorganic and Nano-Metal Chemistry 50(9): 741-752.
- Zandwijk NC, Frank A (2020) Potential toxicities of carbon nanotubes: time for a reminder. Journal of Expert review of respiratory medicine 14(4): 339-340.
- Yan H, Xue Z, Xie J, Dong Y, Ma Z, et al. (2019) Toxicity of Carbon Nanotubes as Anti-Tumor Drug Carriers. Int J Nanomedicine 14: 10179-10194.
- Francis AP, Devasena T (2018) Toxicity of carbon nanotubes: A review. Toxicology and Industrial Health 34(3): 200-210.
- Yao D, Zhang Y, Williams PT, Yang H, Chena H (2018) Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Applied Catalysis B: Environmental 221: 584-597.
- Wojtera K, Walczak M, Pietrzak L, Fraczyk J, Szymanski L, et al. (2020) Synthesis of functionalized carbon nanotubes for fluorescent biosensors. Nanotechnology Reviews 9(1): 1237-1244.
- Dyachkova TP, Rukhov A, Tkachev A, Tugolukov E (2018) Functionalization of Carbon Nanotubes: Methods, Mechanisms and Technological Realization. Advanced Materials & Technologies 2: 18-41.
- Huang L, Tan J, Li W, Zhou L, Liu Z, et al. (2019) Functional polyhedral oligomeric silsesquioxane reinforced poly (lactic acid) nanocomposites for biomedical applications. Journal of the Mechanical Behavior of Biomedical Materials 90: 604-614.
- Zare H, Ahmadi S, Ghasemi A, Ghanbari M, Rabiee N, et al. (2021) Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int J Nanomedicine 16: 1681-1706.
- Aoki K, Saito N (2020) Biocompatibility and Carcinogenicity of Carbon Nanotubes as Biomaterials. Nanomaterials 10(2): pp. 264.
- Manasraha A, Laoui T, Zaidi S, Atieh M (2017) Effect of PEG functionalized carbon nanotubes on the enhancement of thermal and physical properties of nanofluids. Experimental Thermal and Fluid Science 84: 231-241.
- Zhao X, Ma R, Yang M, Yang H, Jin P, et al. (2017) Fabrication of POSS-coated CdTe quantum dots sensitized solar cells with enhanced photovoltaic properties. Journal of Alloys and Compounds 726(5): 593-600.
- Ghanbari H, Marashi S, Rafiei Y, Chaloupka K, Seifalia A, et al. (2011) Biomedical Application of Polyhedral Oligomeric Silsesquioxane Nanoparticles. Organic chemistry pp. 363-399.
- Schneider J, Dudka T, Xiong Y, Wang Z, Gaponik N, et al. (2018) Aqueous-Based Cadmium Telluride Quantum Dot/Polyurethane/Polyhedral Oligomeric Silsesquioxane Composites for Color Enhancement in Display Backlights. The Journal of physical chemistry 122(25): 13391-13398.
- Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, et al. (2011) Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet 378(9808): 1997-2004.
- Mohajeri M, Behnam B, Sahebkar A (2018) Biomedical applications of carbon nanomaterials: Drug and gene delivery potentials. Cellular Physiology 234(1): 298-314.
- Rahmani B, Tzamtzis S, Sheridan R, Mullen M, Yap J, et al. (2017) “In Vitro Hydrodynamic Assessment. of a New Transcatheter Heart Valve Concept (the TRISKELE). J Cardiovasc Transl Res 10(2): 104-115.
- Khan F, Mubarak N, Khalid M, Walvekar R, Abdullah E, et al. (2021) Functionalized multi-walled carbon nanotubes and hydroxyapatite nanorods reinforced with polypropylene for biomedical application. Scientific Reports 11: 843.
- Zhou Y, Fang Y, Ramasamy R (2019) Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 19(2): 392.
- Brakmane G, Madani SY, Seifalian A (2013) Cancer Antibody Enhanced Real Time Imaging Cell Probes- a Novel theranostic Tool using Polymer Linked Carbon Nanotubes and Quantum Dots. Anticancer Agents Med Chem 13(5): 821-832.
- Behzadi S, Serpooshanb V, Tao W, Hamaly M, Alkawareek M, et al. (2017) Cellular Uptake of Nanoparticles: Journey Inside the Cell. Chemical Society Reviews 46(14): 4218-4244.
- Sabourian P, Yazdani G, Ashraf S, Frounchi M, Mashayekhan S, et al. (2020) Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. International Journal of molecular science 21(21): 8019.
- Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, et al. (2012) Toxicity of nanomaterials. Chem Soc Rev 41(6): 2323-2343.
- Gustafson H, Holt-Casper D, Grainger D, Ghandehari H (2015) Nanoparticle Uptake: The Phagocyte Problem. Nano Today 10(4): 487-510.
- Tan A, Madani SY, Rajadas J, Pastorin G, Seifalian A, et al. (2012) Synergistic photothermal ablative effects of functionalizing carbon nanotubes with a POSS-PCU nanocomposite polymer. Journal of Nanobiotechnology 10: 34.
- Madani SY, Mandel A, Seifalian AM (2013) A concise review of carbon nanotube's toxicology. Nano Review 4: 21521.
- Naderi N, Madani SY, Mosahebi A, Seifalian A (2015) Octa-ammonium POSS-conjugated single-walled carbon nanotubes as vehicles for targeted delivery of paclitaxel. Nano Review 6: 28297.
- Naderi N, Madani SY, Ferguson E, Mosahebi A, Seifalian A (2013) Carbon nanotubes in the diagnosis and treatment of malignant melanoma. Anticancer Agents Med Chem 13(1): 171-185.
- Madani SY, Shabani F, Dwek M, Seifalian A (2013) Conjugation of quantum dots on carbon nanotubes for medical diagnosis and treatment. Int J Nanomedicine 8: 941-950.
- Madani SY, Tan A, Naderi N, Seifalian A (2012) Application of OctaAmmonium-POSS functionalized single walled carbon nanotubes for thermal treatment of cancer. Journal of Nanosci Nanotechnol 12(12): 9018-9028.
- Ghanbari H, Cousins BG, Seifalian AM (2011) A nanocage for nanomedicine: polyhedral oligomeric silsesquioxane (POSS). Macromol Rapid Commun 32(14): 1032-1046.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...







