Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Prediction of Physico-Chemical Properties for Polycyclic Aromatic Hydrocarbons Based on Electronic Characteristics of Molecules Volume 4 - Issue 2
Mikhail Yu Dolomatov1,2*, Nataliya H Paymurzina1 and Ella A Kovaleva1
- 1Ufa State Petroleum Technological University, Russia
- 2Institute of Physics and Technology of Bashkir State University, Russia
Received: August 08, 2019; Published: August 29, 2019
*Corresponding author: Mikhail Yu Dolomatov, Ufa State Petroleum Technological University, Ufa, Bashkortostan Republic, Russia
DOI: 10.32474/AOICS.2019.04.000185
Abstract
QSPR models have been developed to predict of polycyclic aromatic hydrocarbons (PAHs) based on quantum chemical and integral spectroscopic descriptors. The first ionization potentials calculated from the energies of the highest occupied molecular orbital (HOMO), relative autocorrelation empirical parameters and the total number of electrons of non-ionized molecules were used as quantum chemical descriptors. Ionization potentials, electron affinities, boiling points, molecular masses, saturation vapor pressure of PAHs were studied as physical-chemical properties. Ionization potentials and electron affinities (IPs and EAs) are calculated by the use of density functional theory (DFT). The predictive power of resulting model is demonstrated by testing it on unseen data that were not used during model generation. The obtained models make it possible to estimate physical and chemical properties with sufficient accuracy for practical applications.
Keywords: PAHs; QSPR; Integral spectroscopic descriptors; Ionization potential; Electron affinity; Boiling point; Saturation vapor pressure
Introduction
In organic chemistry and chemical Informatics known QSPR models (Quantitative Structure-Property Relationship) allowing to predict the various properties of polycyclic aromatic hydrocarbons (PAHs). In work [1] the authors were dependencies of physical and chemical properties, such as molar mass, normal boiling point, density, refractive index at 20 °C on ionization potentials (IPs) established. In [2] the authors were the dependence of IPs and electron affinities (EAs) of PAHs characteristics on the relative integral spectroscopic descriptor and the relative autocorrelation empirical parameter μ developed. The study [3] show the use of the integral oscillator strength of the optical absorption spectrum and the total number of electrons (protons) of all atoms in the composition of non-ionized molecules as descriptors for predicting the IPs of nitrogen-and oxygen-containing compounds.
The aim of this study was to evaluate the ability to predict the physical and chemical properties of PAHs using descriptors such as IP and μ.
Materials and Methods
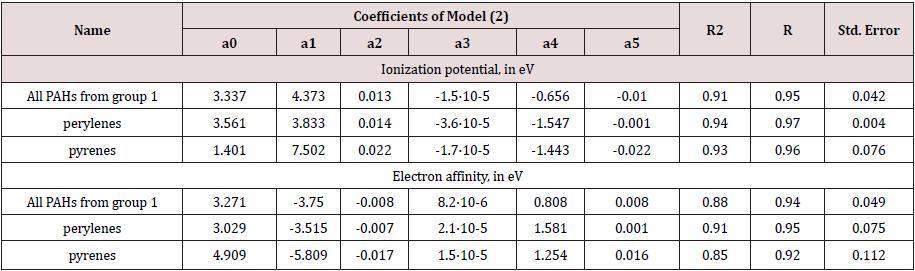
In this paper, hydrocarbons containing from one to five linearly annelated rings (19 compounds), perylenes (18 compounds), pyrenes (14 compounds) (group 1), as well as PAHs, which are widely distributed in hydrocarbon fractions of oils, coal-tar and fuel combustion products, namely Naphthalene, Acenaphthalene, Fluorene, Phenanthrene, Anthracene, Fluoranthene, Pyrene, Chrysene, Benz(a)anthracene, 2-Methylnaphthalene, Biphenyl (group 2).
To predict the physical and chemical properties of PAHs, we were QSPR models based on quantum chemical and integrated spectroscopic descriptors developed. The first ionization potentials is equal to the negative energies of the highest occupied molecular orbital (HOMO), relative autocorrelation empirical parameters and the total number of electrons of the non-ionized molecules were used as quantum chemical descriptors. As properties we have considered: IP, EA, boiling point, molecular weight and saturated steam pressure. Relative empirical autocorrelation parameters μ were calculated from the spectra of PAH molecules experimentally obtained and taken from the databases.
It is known that the autocorrelation function determines the degree of dependence between the different states of the molecule. The correlated electronic states means that all electronic states affect the energy of the highest occupied and lowest occupied molecular orbitals and, consequently, the IP and EA of molecules.
According to research [3], the relative empirical autocorrelation parameter μ equal to the ratio of the spectrum energy in the UV region to the energy of the entire electronic spectrum. In the semi-logarithmic coordinate system μ-parameter, which can be determined mathematically, characterizes the ratio of magnitudes of energies absorption from an electromagnetic field.
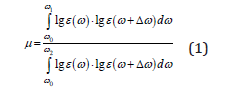
where numerator of fraction - integral autocorrelation function (IACF) in the UV spectral region, denominator – IACF in the UV-Vis spectral region, Δω – small increment of the argument (the analysis step of 1015 Hz), lgε(ω), lgε(ω +Δω) – molar absorption coefficients at a certain frequencies. IPs and EAs are calculated by the use of density functional theory (DFT).
In this paper, we propose QSPR models (2) and (3) that are nonlinear two-parameter regression dependencies. The coefficients for these dependencies are obtained by solving the equations using least squares method and presented in Tables 1 & 2.
To predict the electron donating properties of PAHs, the model has the form
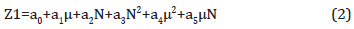
where Z1 are electron donating characteristics (IPs или EAs); μ is relative empirical autocorrelation parameter; N is the total number of electrons in molecule; а0, а1, а2, а3, а4, а5 are empirical coefficients correspond to a certain class of PAHs, eV.
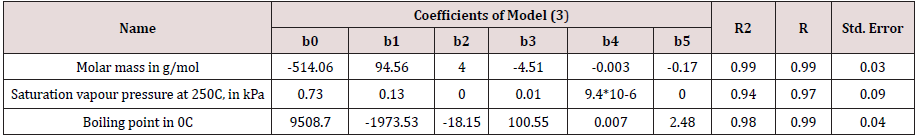
For prediction of the physical and chemical properties (molecular weight, boiling point, saturated steam pressure [4]) the model looks like
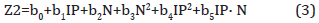
where Z2 is physical-chemical property; IP is ionization potential, eV; N is the total number of electrons in molecule; b0, b1, b2, b3, b4, b5 are empirical coefficients correspond to a certain class of PAHs.
In Tables 1 & 2 present the main results of statistical processing of calculated and experimental data. The ranges of values of the coefficients of determination (R2) for the electron donating ability of PAHs are (0.93, 0.97) and (0.92, 0.95) for IPs and EAs, respectively. For other physical and chemical properties the correlation coefficients R2 take the values between 0.97 and 0.99. To assess reliability of the correlation coefficient, its standard error was calculated.
The predictive power of resulting model is demonstrated by testing it on unseen data that were not used during model generation. For them the average relative errors were estimated: for the electron-donating ability of PAHs is 1.11% (for IP) and 0.86% (for EA); at about 3.1% for boiling point s; not more than 0.51% for molar mass. According to the saturated vapor pressure, the error is more significant, which is apparently due to the difficulties of determining this value.
Conclusion
Dependencies were the links between physical and chemical properties, spectroscopic relative autocorrelation parameter μ, IP, EA and the total number of electrons in PAH molecules established. These dependencies make it possible to predict the electron donating ability, molecular weight, boiling point with an error of not more than 3.1%. The research results can be practically used in petrochemistry, carbon chemistry, organic chemistry, for prediction of physical and chemical properties PAH molecules.
References
- Dezortsev SV, Manzullina LI, Netsvetaeva KI, Petrov AM (2014) Connection of physical- chemical properties with the first ionization potential in homologous series benzene-penthacene. Bashkir chemical journal 21(4): 33-39.
- Mikhail Y Dolomatov, Natalia H Paymurzina, Ella A Kovaleva (2018) Evaluation of donor-acceptor properties of polycyclic hydrocarbon molecules by the integral autocorrelation characteristics of the optical spectra. Butlerov Communications 53(2): 28-37.
- Mikhail Yu Dolomatov, Kamil F Latypov, Ella A Kovaleva (2019) Prediction of vertical ionization potentials for organic compounds by integral characteristics of optical spectra and the number of protons in molecules. Butlerov Communications 58(6): 62-72.
- Tsymbaliuk KK, Denga Iu M, Antonovich VP (2013) Determination of polycyclic aromatic hydrocarbons (PAHs) in the environment (Review). Methods and objects of chemical analysis 8(2): 50-62.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...