Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Review Article(ISSN: 2637-4609) 
Photocatalysis with Xanthene Dyes in Liquid Homogeneous Solutions Volume 4 - Issue 5
Igor V Khudyakov*
- Department of Chemistry, Columbia University, USA
Received: December 21, 2020; Published: January 19, 2021
*Corresponding author:Igor V Khudyakov, Department of Chemistry, Columbia University, New York, USA
DOI: 10.32474/AOICS.2021.04.000199
Abstract
Xanthene dyes (XD) are widely used a photocatalysts in many important organic chemical reactions in the liquid phase. Several such reactions are listed in this brief article. XD react in the excited triplet state XD* of a dye. The reasons for utilizing XD as the redox photocatalysts are explained. A problem is outlined, whether XD act as the photocatalysts or as the reagents partially consumed during a synthesis.
Keywords:Photocatalysis; Xanthene dyes; Redox reactions; Oxidation and reduction cycles
Introduction
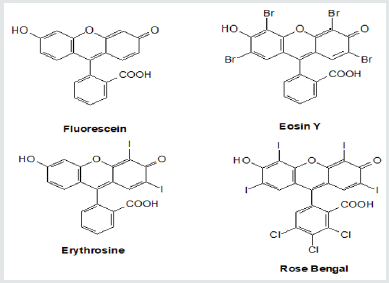
Photocatalysis is a rapidly developing area of photochemistry, catalysis, material science. The appearance of a new Journal of Photocatalysis testifies to this fact. Photocatalysis is homo- or heterogeneous catalysis by molecules [radicals] in the electronically excited singlet or triplet states [excited doublet or quartet states]. There are several recent review articles devoted to photocatalysis [1-3].This brief review article is devoted to photocatalysis with xanthene dyes (XD) in homogenous liquid solutions. These and some other dyes proved to be effective photocatalysists of diverse liquid-phase chemical reactions, see below. A mechanism of action of XD is our main focus. The structures of XD are presented below. See scheme 1. Scheme 1 does not exhaust all the known XD. XD is used also as mono- or di-substituted, either sodium carboxylates or sodium carboxylates and sodium alkoxides. Some XD structures have a methyl or an ethyl substituent. It is necessary to refer to the original publications for details.
Photoreactions of Xanthene Dyes
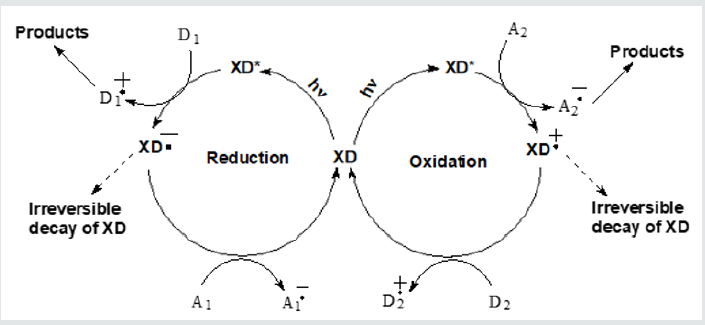
Absorption of visible light leads to the formation of an excited singlet state of 1XD* with a subsequent intersystem crossing into a reactive triplet state 3XD*. We will note parenthetically that XD (Scheme 1) is the classical example of an internal heavy atom effect: introduction of halogen atoms into the Fluorescein moiety leads to an increase of quantum yield of a triplet state. XD reacts in the excited triplet state [4]. We will denote that state as XD*. The use of XD as photocatalysts is based on the change of redox properties of XD under photoexcitation. XD become “more powerful” oxidants and “more powerful” reductants in the excited state compared to the same properties in the ground state. XD* easily oxidize donors (D) in the solution like amines and XD* are oxidized by acceptors (A) like nitrobenzene. Such reactions do not proceed with notphoto excited XD. Table 1 below presents approximate values of the redox potentials of XD and their derivatives for a semi-quantitative comparison of potentials.
Table 1: Approximate Values of Redox Potentials of XD, XD* and Their Radical-Cations and Radical-Anionsa.

aPotentials vs. saturated calomel electrode (SCE). Solvent – acetonitrile. Data of ref. [2,3,5,6]. See the original publications for the precise data. The principle of action of XD as photocatalysts is presented in Scheme 2. XD*produces charged (Scheme 2) or neutral radicals of a substrate. These radicals participate in further reactions leading to the organic products of interest. A1(2) stands for an electron acceptor, D1(2) stands for an electron/hydrogen donor. We present elementary reactions of XD* as a single electron transfer (SET) [5]. A2-.(D1+.) can react with H+ (can release H+) and form a neutral radical, see Scheme 2. Alternatively, the reaction of XD* may be H- abstraction with a charge-transfer transition state. D1+.and A2- (Scheme 2) form the intermediate radical products. These intermediate products eventually lead to the product of interest after two or more reactions with available reagents. One of the intermediate products of XD* reactions can serve as A1 or D2, see Scheme 2. A1 has often considered dioxygen is the air-saturated solutions, see e.g. [3]. We will mention some of such important for organic chemistry reactions photo catalyzed by XD: bromination, hydroxylatioin, arylation of heteroarenes, cyclization of thioamides, arylation of bezothiophenes, borylation of aryl diazonium salts, dehydrogenative coupling and cross-coupling, coupling of substituted tetrahydro- isoquinoline with nitromethane, reduction of nitrobenzene in the presence of amine, desulfurization, the formation of iminium ions, photopolymerization in the presence of amine and photoinduced electron/energy transfer−reversible addition−fragmentation chain transfer (PET-RAFT) polymerization [2,3,5, 7-12]. See for details the cited literature and references cited therein.
Scheme 2: Generalized presentation of a reductive and of an oxidative cycle with the participation of xanthene dyes (XD). Two circles represent two different reactions.
Photocatalysis or photoinduced reactions?
The original publications on photocatalysis with XD usually present oxidative or reductive cycles like it is depicted in Scheme 2. Reactions leading to Products (Scheme 2) are also identified [2,3,5,7-12]. It is stated that reactions with XD are photocatalysis. Strictly speaking, a photocatalyst should not be consumed in the course of a photocatalytic reaction. However, some publications indicate consumption of XD in the course of its action as a “photocatalyst” [8,11]. In the cases of consumption of XD one can talk on the “visible light-mediated” photoreactions [11] or light promoted reactions [10], or the photoinduced reactions. New products obtained with the help of XD* are mostly interesting for organic chemistry and not a fate of XD. It would be interesting to know the results of a simple experiment: take Vis spectra of solutions of XD prior to and after the ending of a reaction [13]. (Essential changes of [XD], which are probably due to bleaching, can be noticed by a naked eye in a glass vessel.) We presented with dashed lines possible and even probable irreversible decay of XD in the course of its action, see Scheme 2. Usually irreversible decay of XD is not reflected at a nice circle in publications.
A concentration of XD in such photocatalytic reactions is different. XD use in a concentration of 1.10-5 M [8,11], 1-2 mol% [3,5]. Sometimes higher [XD] is desirable up to 5 ml% [10]. (Unfortunately, we have different units of concentration.) Initial [O2] in the air-saturated solutions is ~10-3 M [6]. It is not obvious that A1= O2 effectively regenerates XD during the whole process (Scheme 2).
The matter is not only of using strict terminology. (Photo)chemistry of dyes is a valuable area of chemistry, and side transformations of XD maybe of importance. Some publications pay attention to quenching of XD* by dioxygen in the air-saturated solutions with a formation of singlet dioxygen in the course of photocatalysis [3]. 1O2 is not an inert species and it can participate in different reactions [3]. In general, it is not surprising that the yield of the final product of interest catalyzed by XD is less than 100% like it often happens in the dark synthesis.
Conclusions
It is possible nominally call XD participating in reactions cited above as photocatalysts. XD is relatively inexpensive. They succeeded in many processes expensive photocatalysts - complexes of “heavy atoms” (Ir, Ru) [2]. XD are excited with visible light, and the modern LED light sources can be utilized. XD as photocatalysts meet the demand for green chemistry.
References
- Kabra K, Chaudhary R, Sawhney RL (2004) Treatment of Hazardous Organic and Inorganic Compounds through Aqueous-Phase Photocatalysis: A Review. Ind Eng Chem Res 43(24): 7683-7696.
- Wang L, Huang W, Li R, Gehrig D, Blom PWM et al. (2016) Structural Design Principle of Small-Molecule Organic Semiconductors for Metal-Free, Visible-Light-Promoted Photocatalysis. Angew Chem Int Ed Engl 55(33): 9783 -9787.
- Corrigan N, Shanmugam S, Xu J, Boyer C (2016) Photocatalysis in organic and polymer synthesis. Chem Soc Rev 45: 6165-6212.
- Hari DP, Kӧnig B (2014) Synthetic applications of eosin Y inphotoredox catalysis. Chem Commun 28; 50(51): 6688-6699.
- Klimtchuk ES, Irinyi G, Khudyakov IV, Margulis LA, Kuzmin VA (1989) Effect of Magnetic Field on Radical Yields During the Photoreduction of Xanthene Dyes in Viscous Media. J Chem Soc Faraday Trans I 85: 4119-4128.
- Davies J, Booth SG, Essafi S, Dryfe RAW Leonori D (2015) Visible-Light-Mediated Generation of Nitrogen-Centered Radicals:Metal-Free Hydroimination and Iminohydroxylation Cyclization Reactions. Angew Chem Int Ed 54: 14017-14021.
- Montalti M, Credi A, Gandolfi MT (2006) Handbook of Photochemistry. CRC Boca Raton.
- Xie LY, Liu YS, Ding HR, Gong SF, Tan JX et al. (2020) C(sp2)-H/O-H cross-dehydrogenative coupling ofquinoxalin-2(1H)-ones with alcohols under visible-light photo redox catalysis. Chin J Catalysis 41: 1168-1173.
- Yang XJ, Chen B, Zheng LQ, Wu LZ, Tung CO (2014) Highly efficient and selective photocatalytic hydrogenation of functionalized nitrobenzene’s. Green Chem 16(3): 1082-1086.
- Wu C, Corrigan N, Lim CH, JungK, Zhu J (2019) Guiding the Design of Organic Photocatalyst for PET-RAFT Polymerization: Halogenated Xanthene Dyes. Macromol 52: 236-248.
- Xie LY, Chen YL, Qin L, Wen Y, Xie JW (2019) Visible-light-promoted direct C-H/S-H cross-coupling of quinoxalin-2(1H)-ones with thiols leading to 3-sulfenylated quinoxalin-2(1H)-ones in air. Org Chem Front 6: 3950-3955.
- Yoon J, Jung YJ, Yoon JB, Damodar K, Kim HH (2019) The heavy-atom effect on xanthene dyes for photopolymerization by visible light. Polym Chem 10(42): 5737-5742.
- Pigot C, Noirbent G, Brunel D, Dumur Fv (2020) Recent Advances on Push-pull Organic Dyes as Visible Light Photo initiators of Polymerization. Europ Polym J 133: 109797.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...