Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Phytochemical Screening and Proximate Analysis of Garlic (Allium Sativum) Volume 4 - Issue 2
Igor Barchiy1*, Anatolii Fedorchuk2, Valeriya Tovt1, Michal Piasecki3 and Anatolii Potapchuk1
- 1Department of chemistry, Uzhhorod National University, Ukraine
- 2Department of Inorganic and Organic Chemistry, Lviv National University of Veterinary Medicine and Biotechnologies, Ukraine
- 3Institute of Physics, J Dlugosz University, Poland
Received: May 23, 2019; Published: May 30, 2019
*Corresponding author: Igor Barchiy, Department of chemistry, Uzhhorod National University, Pidgirna St., 46, 88000 Uzhgorod, Ukraine
DOI: 10.32474/AOICS.2019.04.000182
Abstract
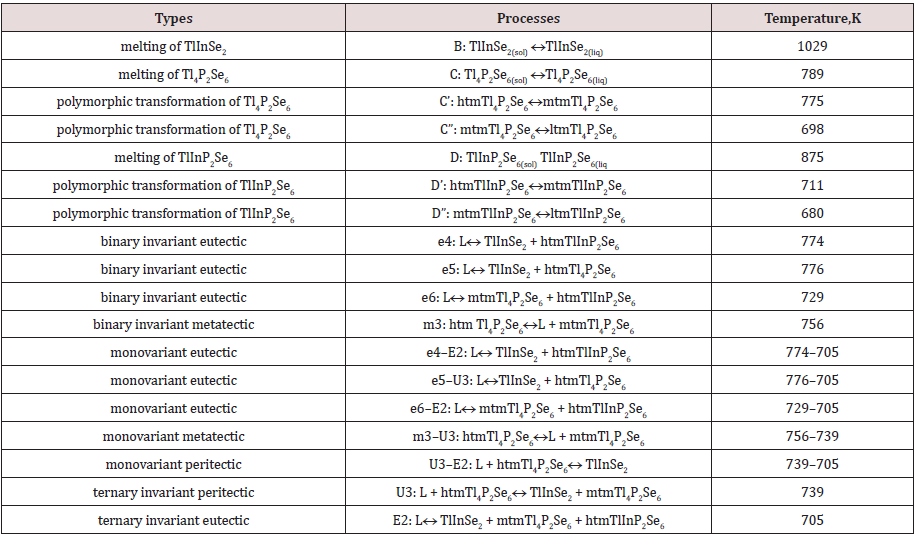
For the first time the phase equilibrium of the Tl4P2Se6–TlInP2Se6 binary and TlInSe2–TlInP2Se6–Tl4P2Se6 ternary systems were investigated by the methods of physical-chemical analysis (DTA, XRD, MSA) and mathematical design in multi-component system. It has been established that the Tl4P2Se6–TlInP2Se6 system belongs to the eutectic type and characterized by the formation of boundary solid solutions on the basis of complex compounds. The space phase diagrams, projection of liquidus surface of the TlInSe2–TlInP2Se6– Tl4P2Se6 system were constructed. Ternary system characterized by two invariant processes – eutectic (L ↔ mtmTl4P2Se6 + TlInSe2 + htmTlInP2Se6,705K) and peritectic (L + hhtmTl4P2Se6 ↔ TlInSe2 + htmTlInP2Se6,739K). On the basis of complex selenophosphate compounds takes place metatectic, eutectoid (Tl4P2Se6) and two peritectoid (TlInP2Se6) processes, respectively. Specifics of crystal structure of complex compounds were given from the position of the second coordination surrounded of atoms in cationic and anionic sublattice.
Keywords: Selenophosphate; Phase diagram; Liquidus projection; Solid solution; Crystal structure
Introduction
Creation of new materials with a complex of predicted properties is the most important task of the modern inorganic science. The solution of these problems is based on the obtaining of new substances by changing of the composition by iso- and heterovalent substitution of the structures, the formation of solid solutions, composite eutectic (peritectic) phase. The study of the physical-chemical interaction of multi-component systems allows us to determine the phase formation patterns according to the composition and temperature, to determine the boundary concentration of solid solutions, to find coordinates of invariant transformations, to select the rational composition and obtain technological mode of growth qualitative single crystals, to consider the laws of “composition – crystalline structure – properties”.
Perspective compounds that are widely used in production of working elements for semiconductor IR and laser technology, thermal generation, solar power, are materials based on complex chalcogenide compounds [1-4]. Special attention is paid to compounds of the M2P2Se6 type [5-12]. Modification of the composition of M2P2Se6 type compounds by isovalent substitutions of the chalcogen S→Se, which form the sublattice of the anionic group [P2X6]4–, as well as Sn2+→Pb2+, heterovalent substitutions 2Sn2+→4M1+ (M1 – K+, Na+, Rb+, Tl+, Ag+, Cu+), 2Sn2+→M1++M23+ (M2 – In3+, Sb3+, Bi3+, Fe3+) leads to the formation of new composition with different structure of cationic sublattice, which is accompanied by a change in crystal-chemical parameters [13].
Study of physical-chemical interaction in the Tl2Se–In2Se3– “P2Se4” system showed that intermediate complex selenides which melts congruently In2Se3 (655К) [14], TlInSe2 (1023 К) [14], Tl4P2Se6 (758К) [15-17], In4(P2Se6)3 (880К) [16], TlInP2Se6 (875К) [16,18] form five quasi-binary eutectic type sections with formation of limited solid solution. Quasi-binary sections divided initial Tl2Se– In2Se3–“P2Se4” ternary system on secondary quasiternary systems [13], one of them is TlInSe2–Tl4P2Se6–TlInP2Se6.
Materials and Methods
TlInSe2, Tl4P2Se6, TlInP2Se6 ternary compounds were prepared by the single-temperature method from stoichiometric amounts of the initial Tl2Se binary compound and elementary In, P, Se in evacuated quartz containers. Synthesis of compounds was carried out with high-purity elements (more than 99.99wt.%). The highest synthesis temperatures were for TlInSe2 – 1073К, Tl4P2Se6 – 893К, TlInP2Se6 – 853К, respectively. Speed of heating and cooling were 25-30K per hour. Maximum temperature for synthesis of binary and ternary alloys was 1073K. After thermal treatment at highest temperature (823K) for 48h the samples were slowly cooled (250K per hour) down to 573K and homogenized at this temperature for 336h. Subsequently the ampoules were quenched into cold water.
The phase equilibria in the ternary system were investigated by the differential thermal (DTA, a chromel-alumel thermocouple, with an accuracy of ±5K), X-ray powder diffraction (DRON-3- 13 diffractometer, Cu Kα radiation, Ni filter), microstructure (MSA, metallographic microscope Lomo Metam R1) analyses in combination with the simplex method of mathematical modeling of phase equilibria in multi-component systems [19,20]. Crystal structure calculation was carried out with program WinCSD [21].
Results and Discussion
Tl4P2Se6–TlInP2Se6 quasibinary system
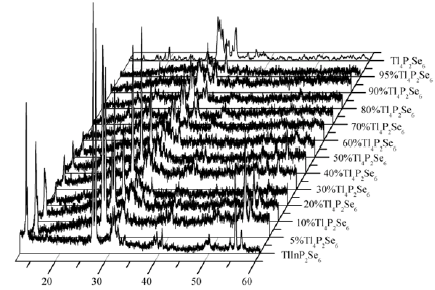
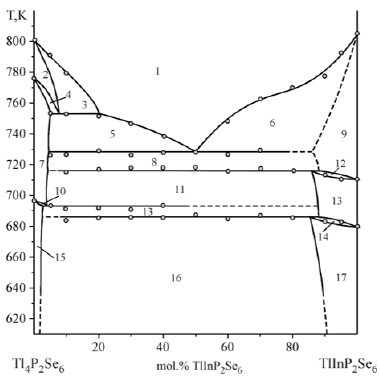
Results of X-ray investigation and phase diagram of the Tl4P2Se6–TlInP2Se6 system are presented in Figures 1 & 2. In this system are formed γ–, γ’– γ”– solid solutions based on low-, middle-, high-temperature polymorphic modifications (ltm-, mtm-, htm-) of TlInP2Se6 and ε–, ε’–, ε’– solid solutions based on low-, middle-, hightemperature polymorphic modifications of Tl4P2Se6, respectively. Tl4P2Se6–TlInP2Se6 system belongs to the Rozeboom V type and is characterized by the invariant eutectic processes L ↔ mtm Tl4P2Se6 + htmTlInP2Se6. The coordinates of the eutectic point correspond to 50mol.% TlInP2Se6 at 729К. The metatectic process htmTl4P2Se6 ↔ L + mtmTl4P2Se6 and eutectictoid process based on the polymorphic transformation of the ternary compound Tl4P2Se6 are observed at 750К and 695K, respectively. Two peritectictoid processes htmTlInP2Se6 ↔ mtmTlInP2Se6 and mtmTlInP2Se6 + ltmTl4P2Se6 ↔ ltmTlInP2Se6 based on the polymorphic transformation of TlInP2Se6 takes place at 715К and 685K. At 573K the existence of the solid solutions range of low-temperature polymorphic modification of TlInP2Se6 is less than 7mol.%, for ltmTl4P2Se6 – do not exceed than 5mol.% [22,23].
Figure 2: Phase diagram of the Tl4P2Se6–TlInP2Se6 system.
1–L, 2–htmTl4P2Se6, 3–L+htmTl4P2Se6, 4–htmTl4P2Se6+mtmTl4P2Se6, 5–L+mtm Tl4P2Se6, 6–L+htmTlInP2Se6, 7–mtm Tl4P2Se6, 8–mtm Tl4P2Se6+htmTlInP2Se6, 9–htmTlInP2Se6, 10–mtm Tl4P2Se6+ltm Tl4P2Se6, 11–mtm Tl4P2Se6+mtmTlInP2Se6, 12–htmTlInP2Se6+mtmTlInP2Se6, 13–mtmTlInP2Se6, 14–mtmTlInP2Se6+ltmTlInP2Se6, 15–ltm Tl4P2Se6, 16–ltm Tl4P2Se6+ltmTlInP2Se6, 17–ltmTlInP2Se6
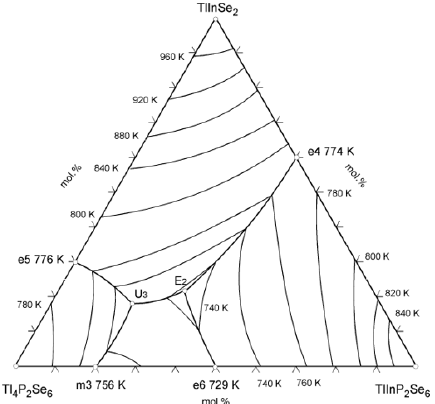
TlInSe2–TlInP2Se6–Tl4P2Se6 quasiternary system
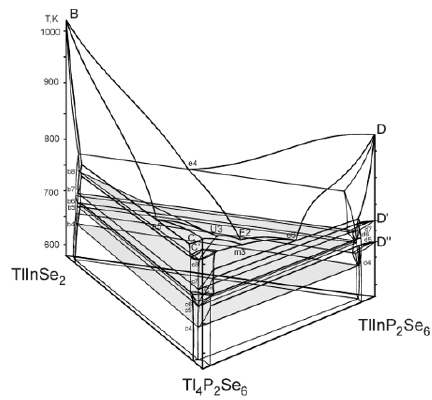
A perspective view and the projection of the liquidus surface of the TlInSe2–TlInP2Se6–Tl4P2Se6 system are shown in Figures 3 & 4, respectively. The points B, C and D, which are located on the edges of triangular prism, represent the melting temperature of the ternary selenophosfates TlInSe2 (1029K), Tl4P2Se6 (789K), TlInP2Se6 (875K). The points C’, C”, D’ and D” represent the temperature of polymorphic transformation of TlInP2Se6 and TlInP2Se6 compounds. The liquidus of the ternary system consists of four primary crystallization areas: TlInSe2(B)–e4–E2–U3–e5– TlInSe2(B) (β phase), Tl4P2Se6(C)–e5–U3–e6–m3–Tl4P2Se6(C) (ε” phase), TlInP2Se6(D)–e4–E2– TlInP2Se6(D) (γ” phase) and m3– U3–E2–e6–m3 (ε’ phase). The fields of primary crystallization are divided by monovariant lines e4–E2 (process L↔ TlInSe2 + htmTlInP2Se6), e5–U3 (process L↔ TlInSe2 + htmTl4P2Se6), m3– U3 (process htmTl4P2Se6 ↔ L + mtmTl4P2Se6), e6–E2 (process L↔ mtmTl4P2Se6 + htmTl4P2Se6), U3–E2 (process L↔ TlInSe2 + mtmTl4P2Se6) which cross at the ternary invariant peritectic points U3 (17mol.% TlInSe2, 62mol.% Tl4P2Se6, 21mol.% TlInP2Se6, 739К) and ternary invariant eutectic points Е2 (22mol.% TlInSe2, 46mol.% Tl4P2Se6, 32mol.% TlInP2Se6, 705К). In the subliquidus part two surfaces depict the monovariant metatectic process at 739K (b8–U3–c8–b8) based on the polymorphic transformation between the middle- and high-temperature modifications of the ternary compound Tl4P2Se6 (739K) and invariant eutectic process at 705K (b7–c7–d7–b7). In subsolidus part three surfaces describe the invariant eutectoid process at 675K (b5–c5–d5–b5) based on the polymorphic transformation mtmTl4P2Se6 ↔ ltmTl4P2Se6, invariant peritectoid process at 685K (b6–c6–d6–b6) based on the polymorphic transformation htmTlInP2Se6 ↔ mtmTlInP2Se6 and invariant peritectoid process at 642K (b4–c4–d4–b4) based on the polymorphic transformation mtmTlInP2Se6 ↔ ltmTlInP2Se6. At temperature below for 705K all alloys are in solid state phase. New complex compounds were not observed in the ternary system. The types and temperature of the processes in the TlInSe2–TlInP2Se6– Tl4P2Se6 quasiternary system are shown in Table 1.
Crystal-chemistry of the TlInSe2, TlInP2Se6 and Tl4P2Se6 compounds
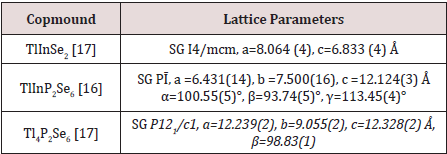
Crystal-structure studies of TlInSe2, TlInP2Se6 and Tl4P2Se6 complex chalcogenides were carried out by a powder method, refinement of the structural parameters – by the Rietveld method. The lattice parameters of initial TlInSe2, TlInP2Se6 and Tl4P2Se6 compounds are shown in Table 2.
The second coordination surrounding (SCS) of the anions atoms indicates the diversity of anionic sublattices in the compounds of the system. In the structure of the TlInSe2 compound individual selenium atoms form anionic sublattice. SCS of atoms Se formed defective (-1) hexagonal analogue of the cubo-octahedron (Figure 5). The defect in anionic sublattice indicates covalent type of the cation – anion bonds.
Figure 5: Second coordination surrounding (SCS) of anionic atoms in the structure of TlInSe2, TlInP2Se6 and Tl4P2Se6 compounds.
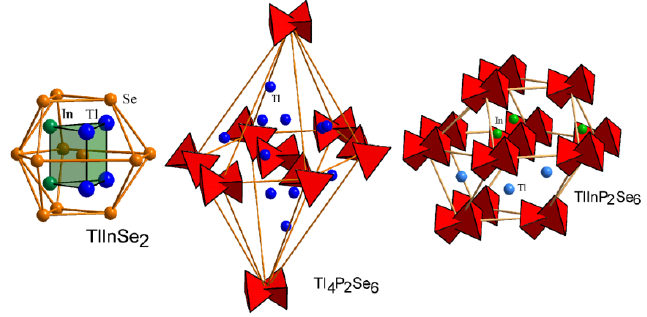
The crystal analysis of Tl4P2Se6 and TlInP2Se6 complex compounds showed that in the structure of both compounds can be isolated anionic group of atoms [P2Se6]4– in the form of two fused tetrahedrons (occupied the nodes of the anionic sublattice). In the structure of the TlInP2Se6 compound atoms In are displaced in the tetrahedral cavity and located on the verge of tetrahedral and octahedral cavities, atoms Tl are displaced into octahedral cavities. Atoms of cations are absent in the gap between layers of anionic groups [P2Se6]4–. SCS in the form of a hexagonal analogue of the cubooctahedron for TlInP2Se6 compound indicate that the anions are dense packing in this compound. With an increase in the number of cations atoms per anionic group in Tl4P2Se6 compound atoms Tl are displaced into tetrahedral cavities and evenly distributed in space. SCS in the form of a hexagonal bipiramide for Tl4P2Se6 compound indicates the primitive packing of anion atoms.
Conclusion
Differential thermal, X-ray phase, microstructure analyses and mathematical modeling of phase equilibria in the multi-component systems were used to construct the of the Tl4P2Se6–TlInP2Se6 binary system, perspective view and liquidus surface projection of the TlInSe2–TlInP2Se6–Tl4P2Se6 ternary system. The character of the monovariant processes, the temperatures and coordinate of the invariant processes in the ternary system were determined. In this system there exists ternary invariant peritectic process (U3: 17mol.% TlInSe2, 62mol.% Tl4P2Se6, 21mol.% TlInP2Se6, 739К) and ternary invariant eutectic process (Е2: 22mol.% TlInSe2, 46mol.% Tl4P2Se6, 32mol.% TlInP2Se6, 705К). Also, in the system take places monovariant metatectic process mtmTl4P2Se6 ↔ htmTl4P2Se6 (739K), invariant eutectoid process mtmTl4P2Se6 ↔ ltmTl4P2Se6 (675K), invariant peritectoid process htmTlInP2Se6 ↔ mtmTlInP2Se6 (685K) and invariant peritectoid process mtmTlInP2Se6 ↔ ltmTlInP2Se6 (642K). The existence of solid solutions of the ternary compounds TlInSe2, TlInP2Se6, Tl4P2Se6 was established. In the ternary system, the components of which are compounds with different types of anionic sublattices, it was necessary to wait for the formation of new phases with a layered structure: separate atoms of selenium – paired tetrahedrons. But the results of physical-chemical investigations showed that the new complex compounds were not formed in the ternary system.
References
- Kanadzidis MG (2001) The Role of Solid State Chemistry in the Discovery of New Thermoelectric Materials. In: Terry M Tritt (Edt.), Semiconductors and Semimetals. Academ Press, San Diego, San Francisco, New York, USA, 69: 51-100.
- McGuire MA, Reynolds TK, Di Salvo FJ (2005) Exploring Thallium Compounds as Thermoelectric Materials: Seventeen New Thallium Chalcogenides. Chemistry of Materials 17(11): 2875-2884.
- Barchij IE, Yu Sabov M, El-Naggar AM, Al Zayed NS, Albassam AA, et al. (2016) Tl4SnS3, Tl4SnSe3 and Tl4SnTe3 crystals as novel IR induced optoelectronic materials. Journal of Materials Science: Materials in Electronics 27(4): 3901-3905.
- Reshak AH, Alahmed ZA, Barchij IE, Yu Sabov M, Plucinski KJ, et al. (2015) The influence of replacing Se by Te on electronic structure and optical properties of Tl4PbX3 (X=Se or Te): Experimental and Theoretical investigations. RSC Advances 5: 1-9.
- Kanatzidis MG (1997) New directions in synthetic solid state chemistry: chalcophosphate salt fluxes for discovery of new multinary solids. Solid State and Materials Science 2(2): 139-149.
- Chung I, Karst AL, Weliky DP, Kanatzidis MG (2006) [P6Se12]4–: A Phosphorus-Rich Selenophosphate with Low-Valent P Centers. Inorganic Chemistry 45(7): 2785-2787.
- Israel R, De Gelder R, Smits JMM, Beurskens PT, Eijt SWH, et al. (1998) Crystal structures of di-tin-hexa(seleno)hypodiphosphate, Sn2P2Se6, in the ferroelectric and paraelectric phase. Zeitschrift für Kristallographie 213(1): 34-41.
- Galdamez A, Manriquez V, Kasaneva J, Avila RE (2003) Synthesis, characterization and electrical properties of quaternary selenodiphosphates: AMP2Se6 with A = Cu, Ag and M = Bi, Sb. Materials Research Bulletin 38(6): 10631072.
- Gave MA, Bilc D, Mahanti SD, Breshears JD, Kanatzidis MG (2005) On the lamellar compounds CuBiP2Se6, AgBiP2Se6 and AgBiP2S6. antiferroelectric phase transitions due to cooperative Cu+ and Bi3+ ion motion. Inorganic Chemistry 44(15): 5293-5303.
- Cajipe VB, Ravez J, Maisonneuve V, Simon A, Payen C, et al. (1996) Cupper ordering in lamellar CuMP2S6 (M=Cr, In) Transition to an antiferielectric or ferielectric phase. Ferroelectrics 185(1): 135-138.
- Pfeiff R, Kniep R (1992) Quaternary selenodiphosphates(IV): M(I)M(III)[P2Se6], (M(I)= Cu, Ag; M(III)= Cr, Al, Ga, In). Journal of Alloys and Compounds 186(1): 111-133.
- Bourdon X, Maisonneuve V, Cajipe VB, Payen C, Ravez J, et al. (1999) Cupper sublattice ordering in layered CuMP2S6 (M=Cr, In). Journal of Alloys and Compounds 283: 122-127.
- Potoriy MV, Milyan PM (2016) Regularities and peculiarities of interaction in Me–P–S(Se) systems (Ме – Cu, Ag, Zn, Cd, In, Tl, Sn, Pb, Sb, Bi). Ukranian Chemistry Journal 82(2): 71-78.
- Tovt VO, Barchii IE, Piasetsky M, Kityk IV, Fedorchuk AO, et al. (2016) Triangulation of the Tl2Se–In2Se3–“P2Se4” quasiternary system. Scientific Bulletin of the Uzhgorod University (Series Chemystry) 2(36): 14-17.
- Mucha I (2012) Phase diagram for the quasi-binary thallium(I) selenide–indium(III) selenide system. Thermochimica Acta 550: 1-4.
- Voroshilov YV, Gebesh VY, Potorii MV (1991) Phase equilibria in the system In-P-Se and crystal structure of β-In4(P2Se6)3. Inorganic Materials 27: 2141-2144.
- Tovt V, Barchiy I, Piasecki M, Kityk I, Fedorchuk A (2017) Investigations of TlInP2Se6–In4(P2Se6)3 system and optical properties of complex compounds. Hungarian Journal of Industry and Chemistry 3: 146-152.
- Tovt VO, BarchIy IE, Fedorchuk AO, Piasecki M, Kitik IV, et al. (2017) Interaction in the TlInSe2–Tl4P2Se6 Scientific Bulletin of the Uzhgorod University (Series Chemystry) 1(37): 55-58.
- Barchii IE, Tovt VA, Piasecki M, Fedorchuk AA, Solomon AM, et al. (2018) Physicochemical Interaction in the TlInSe2–TlInP2Se6 Russian Journal of Inorganic Chemistry 63(4): 537-542.
- Barchij IE (2001) Mathematical design of phase equilibria in the Tl2S–Tl2Se–Tl5Se2I quasiternary system. Ukranian Chemistry Journal 67(11): 18-23.
- Barchiy IE, Tatzkar AR, Fedorchuk AO, Plucinski K (2016) Phase diagrams of novel Tl4SnSe4-TlSbSe2-Tl2SnSe3 quasi-ternary system following DTA and X-ray diffraction. Journal of Alloys and Compounds 671: 109-113.
- Akselrud L, Yu Grin (2014) WinCSD: software package for crystallographic calculations (Ver.4). Journal of Applied Crystallography 47: 803-805.
- Fedorchuk AO, Parasyuk OV, Kityk IV (2013) Second anion coordination for wurtzite and sphalerite chalcogenide derivatives as a tool for the description of anion sub-lattice. Materials Chemistry and Physics 139(1): 92-99.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...