Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Entiline E A New Limonoid Isolated from Root Bark of Antrocaryon Klaineanum Pierre (Anacardiaceae) Volume 5 - Issue 2
Yannick Fouokeng1*, Ahri Bernie Djamen Mbeunkeu1, Stevine Claudiale Popwo Tameye1, Sandrine Mewaba Goulefack1, Hartmut Laatsch2 and Anatole Guy Blaise Azebaze1
- 1Department of Chemistry, University of Douala, Faculty of Sciences, France
- 2Institute for Organic and Biomolecular Chemistry, University of Göttingen, Germany, France
Received:April 1, 2021; Published:May 25, 2021
*Corresponding author:Yannick Fouokeng, Department of Chemistry, University of Douala, Faculty of Sciences, France
DOI: 10.32474/AOICS.2021.05.000207
Abstract
One new pentacyclic triterpenoids, entiline E [1] together with ten known compounds were isolated from the methanol extract from the root barks of Antrocaryon klaineanum, a Cameroonian medicinal plant. The structures of all compounds were determined by comprehensive analyses of their 1D and 2D NMR, mass spectra (EI and ESI) data and comparison with previously known analogues.
Keywords:Antrocaryon Klaineanum; Anacardiaceae; Entiline E
Introduction
Antrocaryon klaineanum (Anacardiaceae) is an evergreen tree that usually grows up to 35 m high. It occurs in central Africa and is used in traditional medicine to treat chlamydia infections, wounds, back pain, liver diseases and female sterility [1-3]. Recent phytochemical investigations on bark of this specie revealed steroids, limonoids and phenolic compounds [4-6]. In this report, we describe the isolation and structural elucidation of one new limonoid entiline E [5] together with ten know compounds.
Experimental
General
The optical rotations were measured with a Perking-Elmer polarimeter, model 241, at the sodium D line (λ = 589 nm). Melting points were determined on a Melter FP61 melting point apparatus. The IR spectrum was recorded on a FT/IR-4100 Jasco spectrometer. UV/Vis’s spectra were obtained on a Jasco V-650 spectrophotometer. The NMR spectra were recorded on a Varian Inova-500 NMR spectrometer at 600 MHz (1H) or 150 MHz (13C), respectively. Chemical shifts are given in δ values (ppm), and coupling constants are reported in [Hz]. HRESI mass spectra were obtained on a micrOTOF (Bruker) mass spectrometer. Open column chromatography was performed with silica gel (70-230 mesh). Thin-layer chromatography (TLC) was carried out on precoated silica gel 60 F254 plates (Merck), and the TLC spots were viewed at 254 nm and visualized by heating the plates at 80 0C for 10 minutes after spraying with 50% aqueous sulfuric acid.
Plant Material
The root bark of Antrocaryon klaineanum were collected at Mount Elounden, in the Center Region of Cameroon in November 2014 and identified by Mr Victor NANA, botanist at the National Herbarium of Cameroon (Yaounde), where a voucher specimen (N° 21247SRF/CAM) was deposited.
Extraction and Isolation
The air-dried and powdered root bark of A. klaineanum (7.5 Kg) was submitted twice to extraction with methanol at room temperature for 48 h. After evaporation with a rotary evaporator under reduced pressure at 40°C, 435.7 g of crude extract were obtained and were fractionated using vacuum chromatography into fractions A [hexane/ethyl acetate (1:0 and 1:1), 20.40 g], B [ethyl acetate, 170.35 g] and C [methanol, 180.25 g]. 20.1 g of fraction A was subjected to silica gel column chromatography and eluted with a gradient system of n-hexane/EtOAc (1:0 to 0:1); 185 sub-fractions (100 mL each) were collected and pooled based on their TLC profile to yield seven sub-fractions. Sub-fractions 20-33 obtained by eluting the column with n-hexane/EtOAc (97.5:2.5) afforded 5 (17.2 mg). Sub-fractions 54-73 obtained by eluting the column with n-hexane/EtOAc (97.5:2.5) was further chromatographed by silica gel column chromatography and eluted with a gradient of n-hexane/EtOAc (0:1 to 97:3). 65 sub-fractions (25 mL each) were collected and pooled based on their TLC profile leading in the order of their Rf values to give 2 (70.4 mg) and 3 (370.1 mg) respectively. Sub-fractions 74-113 (6.9 g) obtained by eluting the column with n-hexane/EtOAc (95:5) were further chromatographed by silica gel column chromatography and eluted with a gradient of n-hexane/ EtOAc (1:0 to 95:5) 75 sub-fractions (10 mL each) were collected and pooled on the basis of their TLC profile leading in the order of their Rf values to give 4 (2155.6 mg), 1 (2.0 mg), 7 (1.3 mg) and 12 (10.1 mg) respectively. Sub-fractions 114-133 obtained with n-hexane/EtOAc (92.5: 7.5) yielded 6 (10.8 mg), while n-hexane/ EtOAc (85:15) (134-160 sub-fractions) gave 9 (176.01 mg). 160.7 g of fraction B was subjected to silica gel column chromatography and eluted with a gradient system of n-hexane/EtOAc (7:3 to 0:1); 266 sub-fractions (100 mL each) were collected and pooled based on their TLC profile to yield seven sub-fractions. Sub-fractions 11- 25 obtained by eluting the column with n-hexane/EtOAc (7: 3) was further chromatographed by silica gel column chromatography and eluted with a gradient of n-hexane/EtOAc (9:1 to 85:15). 45 subfractions (10 mL each) were collected and pooled based on their TLC profile leading in the order of their Rf values to give again 3 (70.4 mg) and 4 (370.1 mg) with 57.51 mg of 11. Sub-fractions 26-80 obtained by eluting the column with n-hexane/EtOAc (6: 4) afforded 3 (70.4 mg) and 9 (15.9 mg). Sub-fractions 184- 237 obtained by eluting the column with n-hexane/EtOAc (4: 6) afforded 10 (160.01 mg). The fraction C is under examination.
Results and Discussion
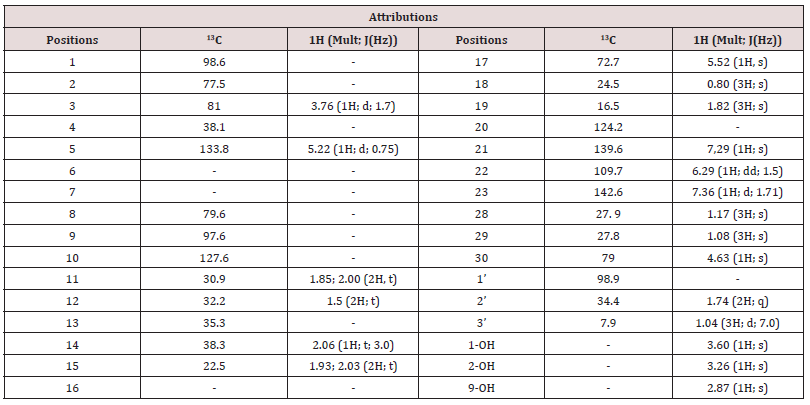
The n-hexane/ethyl 1:1 fraction A and pure acetate fraction B obtained by chromatography of the crude A. klaineanum extract was further separated by a combination of column chromatography on silica gel and preparative thin layer chromatography, resulting in the isolation and characterization of eleven compounds. On the basis of detailed spectroscopic analyses and comparison with reported data, the structures of ten of them (Figure 1) were determined as entilin B (2)[7], entilin C (2)[8], antrocarine A (4), antrocarine C (6) [5], oleanoic acid (7)[9], 7α,20(S)-dihydroxy-4,24(28)-ergostadien- 3-one (9)[10], β-sitosterolglucosid (10)[11], antrocarine F (11) [5], β-sitosterol and stigmasterol (12) [11]. Compound 1 was isolated as a white powder, which gave a positive test for limonoids with the ehrlich reagent (green) and had the molecular formula C26H34O8Na, as established by ESI-HRMS (m/z 497. 2146 [M+Na] +) regarding the ten degrees of unsaturation. The IR spectrum of 5 showed typical vibrations bands of hydroxyl group at 3499.2 and 3413.39 cm-1, at 2924.52 and 2852.00 cm-1 an elongation band of C-H bond and at 1714.41 cm-1, a large band characteristic of conjugated double bond. Analysis of the 1H-NMR data (Table 1) of compound 1, indicated resonances of aromatic protons at δH 6.29 (1H, d, J = 1.5 Hz), δH 7.29 (1H, s) and δH 7.36 (1H, d, J = 1.71) characteristic of furanic cycle β -substituted; a singlet at δH 5.52 (1H, s), two oxymetines protons at δH 5.22 (1H, d, 0.75 Hz) and δH 3.76 (1H, d, 1.7Hz), three hydroxyls protons at δH 3.60, 3.26 and 2.87, and four methyl’s groups at δH 1.81 (3H). These findings agree with the furan limonoid skeleton especially entiline’s type [7,12]. We also observed in the strong fields a methylene signal at δH 1.74 (2H, m) and an aliphatic methyl which appears in triplet at δH 1.04 ppm (3H, t).
This base skeleton is confirmed on its 13C NMR spectrum which reveals the presence of 26 carbons atoms including: Four olefinic carbon characteristics at δC 142.6 (C-23); 139.7 (C-21); 124.6 (C-20) and 109.7 (C-22) of the furanic cyle as well as those of a double-bond tri-substituted at δC 132.5 (C-5) and 128.6 (C-10). The C-17 carbon signal of furolimonoids is observed at δC 72.52. We also observe the presence of six other sp3 carbon atoms linked to oxygen including, three ketals at δC 98.9 (C-1 ‘); 98.6 (C-1) and C 97.6 (C-9), a quaternary at δC 77.5 (C-2) and oxymethyl at δC 79.0 (C-30) and δC 81.0 (C-3). Also, five methyl signals are observed, thus the gem dimethyl at δC 27.9 (C-28) and δC 27.8 (C-29) as well as those δC 24.5 (C-18) and 16.5 (C -19). We finally observed another aliphatic methyl at δC 7.9 (C-3’). The presence of ethyl group was confirmed in COSY spectrum where we observed the correlations between protons H-22 and H-23, H-11 and H-12, H-3 and H-14, another correlation between aliphatic methyl protons (H-3’) with methylene (H-2’) was observed. In other to determine the position of ethyl group, 2D experiments were used. The HSQC spectrum showed that the carbon C-1’ is quaternary and suggested that it would be the seat of junction. This is confirmed on the HMBC spectrum in which we observed the correlations of H-3’ (δH 1.04) with C-2’ ( δC 34.4) and C-1’ ( δC 98.9) in 2J and 3J respectively, with H-2 ‘ ( δH 1.92) and C-1’ ( δC 98.9) and C-15 ( δC 22.5). To determine the relative configuration around the C-1’ carbon, we used the NOESY spectrum on which we observed the correlation of protons of methylene H-3’ with proton H-14 in alpha. There are also other couplings with proton H-2’ and H-15 in alpha. This makes it possible to assign the configuration S to the carbon C-1’. All these data compared to those of the literature confirm that the compound 1 is a new tetranortriterpenoide described here for the first time that we have named trivially entiline E.
Acknowledgement
We thank Dr. H. Frauendorf and Dr. M. John for MS and NMR measurements, respectively. The author thanks the DFG (German Research Foundation) for funding (grant DI 921/6.1).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Kemeuze VA, Nkongmeneck B (2011) Antrocaryon klaineanum Pierre. In: Lemmens, RHMJ, Louppe D. Oteng-Amoako AA (Editors). Prota 7(2): Timbers/Bois d’œuvre 2. [CD-Rom] PROTA, Wageningen, Netherlands.
- Betti JL (2002) Medicinal plants sold in Yaounde markets Cameroon Afr. Study Monogrm 23(2): 47-64.
- Betti JL (2004) An ethnobotanical study of medicinal plants among the Baka Pygmies in the Dja biosphere reserve Cameroon Afr Study Monogr 25(1):1-27.
- Fongang ALM, Laure Nguemfo E, Djouatsa Nangue Y, Bogning Zangueu C, Fouokeng Y, et al. (2017) Antinociceptive and anti-inflammatory effects of the methanolic stem bark extract of Antrocaryon klaineanum Pierre (Anacardiaceae) in mice and rat J. Ethnopharmacol. 203:11-19.
- Douanla PD, Tabopda TK, Tchinda AT Cieckiewicz E, Frederich M, Boyom FF, et al. (2015) antiplasmodial ergostane steroids from the stem bark of Antrocaryon klaineanum Phytochemistry. 117:521-526.
- Fouokeng Y, Feumo Feusso HM, Mbosso Teinkela JE, Siwe Noundou X, Wintjens R, et al. (2019) In vitro antimalarial antitrypanosomal and HIV-1 integrase inhibitory activities of two Cameroonian medicinal plants: Antrocaryon klaineanum (Anacardiaceae) and Diospyros conocarpa (Ebenaceae), South African J Bot 122: 510-517.
- Tchouankeu JC, Tsamo E, Sondengam BL, Connolly JD, Rycroft DS, et al. (1990) A and B two novel heptanortriterpenoid derivatives from Entandrophragma utile (Meliaceae): Structural elucidation using 2D NMR long-range δC/δH correlation experiments, Tetrahedron Lett. 31: 4505-4508.
- Daniewsky WM, Gumułka M, Pankowska E, Szafranski BEF, Jacobsson U, et al. (1994) a Novel Heptanortriterpenoid from Entandrophragma utile (Meliaceae) Pol J Chem 68: 499-502.
- Ayatollahi AM, Ghanadian M, Afsharypour S, Abdella OM, M. Mirzai M, et al. (2011) Pentacyclic Triterpenes in Euphorbia microsciadia with Their T-cell Proliferation Activity, Iran. J Pharm Res IJPR 10(2): 287-294.
- Tchouankeu JC, Nyasse B, Tsamo E, Connolly JD (1996) 7R,20(S)-Dihydroxy-4,24(28)-ergostadièn-3-one from Entandrophragma utile. J Nat Prod 59: 958959.
- Yili A, Mutalipu, Aisa HA, Isaev MI (2009) Betulinic acid and sterols from Astragalus altaicus, Chem Nat Compd 45: 592-594.
- Arigoni D, DBarton DHR, Corey EJ, Jeger O, Caglioti L (1960) The constitution of limonin, Experientia. 16: 41-49.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...