
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Opinion(ISSN: 2637-4609) 
Do Doubly Charged Monatomicanions Exist in Aqueous Solutions? Volume 2 - Issue 2
*Yizhak Marcus
- Institute of Chemistry, The Hebrew University of Jerusalem, Israel
Received: March 13, 2018; Published: March 21, 2018
*Corresponding author: Yizhak Marcus, Institute of Chemistry, The Hebrew University of Jerusalem, Israel
DOI: 10.32474/AOICS.2018.02.000134
Introduction
The chalcogenide atoms (X = oxygen, sulfur, selenium, tellurium, polonium) form doubly charged monatomic anions, X2-, which exist in crystalline salts. The question arises whether, once such salts are dissolved in water, such species persist, or do they hydrolyze completely to form the hydrochalcogenide anion:
X2- + H2ODHX-+ OH - (1)
It is clear that in the case of oxygen this reaction proceeds to the right to completion: O2- + H2O → 2OH-. Recently it was shown that also in the case of sulfur the anion S2- does not exist in aqueous solutions [1]. In attempts to push reaction (1) backwards by the addition of massive quantities (high concentrations) of hydroxyl ions, the necessarily added cations (say, Na+)associate with the sulfide anion, yielding the ion pairs (NaS(H2O)p-), but not S(H2O) n2-. On the other hand, spectroscopic evidence shows that the corresponding addition of CsOH does not yieldthe hydrated sulfide dianions either. It was concluded that the doubly charged hydrated sulfide anions S(H2O)n2- do not exist.
It is therefore of interest to examine whether the reported evidence related to the existence of hydrated selenide Se(H2O) n2- and telluride Te(H2O)n2- anions in aqueous solutions can be reinterpreted in terms that negate their existence.
The evidence concerning the aqueous selenide and telluride dianions pertains to calculations of the second dissociation constant of hydrogen selenide and telluride or the dissociation constant of the aqueous hydroselenide and hydrotelluride anions, K2:
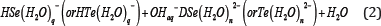
The existence of the dianions Se2- and Te2- in aqueous solutions was assumed a priori in earlier publications as a matter of course. In the polarographic study of the anodic oxidation of H2X [2] it was stated that no experimental value of K2 (for dissociation of HSe- and HTe) was available, so a value was assumed (10-14 and 10-11, respectively) in order to fit the half-wave potentials up to pH = 13.9 (1 M NaOH), but no fit was tried with the value K2 = 0. For the telluride anion there was the added complication that the anodic oxidationresulted in the intermediate formation of elemental tellurium that yields theditelluride anion Te22-, which is then further oxidized. In fact, the polarography of the ditelluridedianion was studied in [3] in aqueous 0.01, 0.1 and 1.0 M NaOH (translated to pH = 12, 13, and 14) on the assumption that the disproportionation Te22-DTe + Te2- takes place, but, again, the nature of the reduced telluride species was not established. What was established was that the polarographic electrode reaction corresponded to a two electron change: H2TeDTe + 2H ++ 2e“ .
In a subsequent examination of the Se(-II) case [4], the change of pH of 0.2 M aqueous KOH on addition of H2Se was interpreted as the formation of HSe- + Se2-, the latter constituting 8% of the total dissolved selenium. Conversion of pH values obtained with a glass electrode in mildly alkaline solutions to actual concentrations of the hydroxide anion is problematic, however, and avoidance of the assumption of Se2- in solution could yield the same change in pH as that measured. The solubility of Na2Se measured in [4] in aqueous NaOH solutions in the presence of high concentrations of Na+ ions (4.31 m, but their source was not specified), was interpreted by inclusion of Se2- species, but the formation of the NaSe- ion pairs was ignored. The complication of the formation of Se22- by partial oxidation was mentioned in this study.
An ultraviolet spectroscopy (charge transfer to solvent spectra) study of the selenide species [5] again assumed the presence of the aqueous Se2- anions in 0.12 to 11.6 m NaOH assigning to it and to HSe- anions specific absorption maxima. The wavelengths of these depended, however, on the ionic strength of the solutions, and shifts had to be taken into account. The possibility that NaSe- ion pairs do occur but Se2- species do not in the solutions wan not taken into account. In a later paper [6] it was stated that the second dissociation step:
HSe-DH ++ Se2-(3)
is completely shifted to the left at pH = 12.3, hence Se2- does not play any role and fully reduced selenium appears only as the monoprotonated HSe-. However, the polyselenidedianion Se32- is a species that has to be taken into account.
The most recent study was that of the charge transfer to solvent ultraviolet spectra of telluride anions. The spectrum in 0.52M NaOH was assigned to the Te2- species, whereas in a NH3/NH4+ buffer at pH > 5 and ionic strength of 0.01 M the observed spectrum was assigned to HTe-. However, the possibility that NaTe- was formed in the 0.52 M NaOH solution and was responsible to the shift by the higher ionic strength of the NaOH solution in the direction of lower energies was not considered. Thus, there was no compelling reason to assign the spectrum in the NaOH solution to the dianion Te2- species, and the assignment was due to the a priori assumption that such a species should exist in alkaline aqueous solutions [7]. Nothing appears to have been published regarding the polonium Po (-II) or polonide anion.
Conclusion
In conclusion, there is no compelling reason to interpret the experimental results concerning the chalcogenide X(-II) species as ultimately forming the X(H2O)n2- species by dissociation of the well- established HX(H2O)q- in highly alkaline solutions.
References
- P M May, D Batka, G Hefter, E Konigsberger, D Rowland (2018) Goodbye to S2- I aqueous solution. Chem Commun Ahead of print.
- J J Lingane, L W Niedrach (1948) Polarography of selenium and tellurium I The -2 states. J Am Chem Soc 70: 4115-4120.
- A J Panson (1963) Polarography of the ditelluride anion. J Phys Chem 67(10): 2177-2180.
- R H Wood (1958) The second ionization constant of hydrogen selenide. J Am Chem Soc 80(7): 1559-1562.
- D E Levy, R J Myers (1993) Spectroscopic determination of the second dissociation constant of H2Se and the activity coefficients and spectral shifts of the ions. J Phys Chem 94: 7842-7847.
- A Goldbach, M L Saboungi, J A Johnson, A Cook, D Meisel (2000) Oxidation of aqueous polyseledide solutions. A mechanistic pulse radiolysis study J Phys Chem A 104: 4011-4016.
- R J Myers (2007) Second dissociation constant of H2Te and the absorption spectra of HTe-, Te2-, and Te22- in aqueous solution. J Solution Chem 36(3): 395-403.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...














