Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Development and Qualification of an Assay Method for Deferoxamine Mesylate and p-SCN-DFO Volume 5 - Issue 3
Paul Jurek1*, Montgomery M Martensen1, A Eli De Lira1, and Garry E Kiefer1
- 1Macrocyclics, Inc. An Orano Med Company, 700 Klein Rd, Plano, TX 75074, United States
Received:June 4, 2021 Published:June 15, 2021
*Corresponding author:Paul Jurek, Director of Custom Product Development, Macrocyclics, Inc an Orano Med Company, 700 Klein Rd, Plano, TX 75074, United States
DOI: 10.32474/AOICS.2021.05.000211
Abstract
A simple, colorimetric assay method has been developed for deferoxamine mesylate and a related bifunctional chelating agent, p-SCN-DFO. A classic back-titration is performed where an excess amount of a standardized zirconium solution is added to the chelating agent solution. The excess zirconium is then titrated with standard EDTA. A qualification study was undertaken to evaluate the integrity of the method. Accuracy, specificity, and precision were evaluated and found to be excellent. The method is traced back to an analytical primary standard thus avoiding the need to create and use compound-specific reference standards.
Keywords:Zirconium; assay; p-SCN-DFO; titration; deferoxamine
Introduction
An assay method is a typical quality control test for both raw materials and products in the pharmaceutical industry. It measures the amount of the target substance present in a material. Tracing an assay test to a primary standard lends a high degree of accuracy to the analysis. The reason is primary standards are extremely pure, stable, and have no waters of hydration. Over the years the American Chemical Society has defined several reagents as primary standards. Potassium dichromate and sodium oxalate are primary standards for redox titrations. Tris(hydroxymethyl)amino methane (TRIS) and Potassium hydrogen phthalate are acid-base primary standards. Calcium carbonate is a primary standard for chelometric titrations.
Assay tests can also be traced to established reference standards. The United States Pharmacopeia (USP) [1] provides reference standards (RS) for official articles in the USP or National Formulary (NF). An RS is typically specified in a monograph for use in the Assay test or an Identification test. An RS is considered 100.0% pure for quantitative applications unless a calculation value for an adjustment is stated on the label. For example, if a specific HPLC purity test shows the purity of the RS is 98.1%, a multiplier of 0.981 must be incorporated into an assay test. USP RSs are available for purchase for legal metrology purposes. The USP states that per the International Vocabulary of Metrology, they are defined as reference standards [2].
There exists a United States Pharmacopeia (USP) monograph for Deferoxamine Mesylate (DFO) [3]. The drug substance is used to treat sudden iron poisoning. It is also used to treat high levels of iron because of numerous blood transfusions. The USP monograph for DFO describes an assay test that is based on the use of a reference standard (Deferoxamine Mesylate RS). There is great value when scientists on different sides of the world can test a product to the same standard. However, since RSs typically lack the chemical properties of a primary standard, assay tests that can be traceable to a primary standard are inherently superior.
There is no USP monograph for the related bifunctional chelating agent, p-SCN-DFO. It does not fall under the “official article” definition by the USP [4] That is, it is not considered a drug product, drug substance, dietary ingredient, dietary supplement, excipient, compounded preparation, other ingredient, device, or part of a device. As a result, there is no USP reference standard. DFO and its derivatives (e.g., p-SCN-DFO) are used as raw materials for bioconjugations. They can be conjugated to a peptide or antibody. Both compounds are commonly considered raw materials, critical raw materials, or registered starting materials when used in conjugations. For quality assurance purposes, however, the regulations of a drug substance are commonly placed upon them. Regardless of how they are considered, having an assay test with an appropriate specification on the material is a good idea.
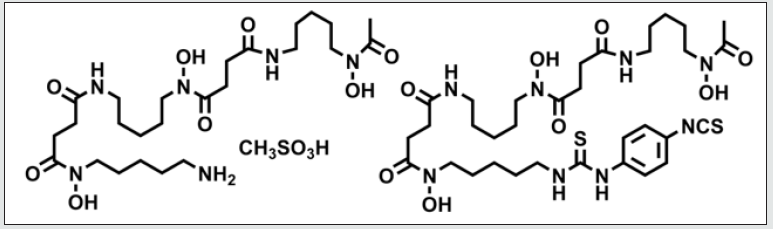
The primary goal of this work was to develop a simple, colorimetric titration method that is traceable to a primary standard that could be used as an assay test for both DFO and p-SCN-DFO. See (Figure 1) for the structures.
A second goal of this work was to incorporate the use of a standardized zirconium solution into the assay test. Both DFO and p-SCN-DFO can be attached to antibodies and peptides for PET imaging with Zr-89. [5-8] Use of standardized zirconium solution can then demonstrate the molecules’ ability to bind zirconium [9].
The last goal was to qualify the assay method. In the early stages of drug substance development, analytical methods must be based on sound science. When a higher degree of confidence in an analytical method is desired, the methods are qualified. Down the road, when the highest degree of confidence in methods is required, validation is undertaken. The ICH Guidance Document Q2(R1) contains strict requirements for what must go into validating an analytical method [10], the requirements for qualifying a method are not defined. In short, a qualified method is a subset of a validated method. It gives the user more confidence in the reliability of the analytical results [11]. Much debate can go into what subset is appropriate to carry out. Our decision and justification are discussed.
Material and Methods
The following reagents were ACS grade: ZrOCl2-8H2O, DMSO, EDTA disodium salt, xylenol orange, nitric acid, calcium carbonate, and hydroxy naphthol blue. Calcium carbonate is a chelometric standard with an assay of 99.95-100.05% on a dried basis. Deferoxamine mesylate was purchased from Millipore Sigma. The compound p-SCN-DFO is available as catalog number B-705 from Macrocyclics Inc. Deferoxamine mesylate RS was purchased from the USP and is catalog number 1166003. Class A volumetric flasks and pipets were used for critical volumes. The quantities used were chosen to optimize the number of significant figures while minimizing the quantity of materials needed for the test.
A single EDTA solution was prepared and used by all analysts. Any error associated with this solution preparation or standardization is imparted to all assay test results equally. The solution was standardized using the calcium carbonate chelometric standard after drying. Hydroxy naphthol blue was used as in indicator. A sharp color change from purple to blue denotes the endpoint. Strict instructions for carrying out a test can be found in the supporting information.
The zirconium assay method that was developed is a classic back titration. Excess standardized zirconium solution is present in a solution of DFO or p-SCN-DFO. The excess zirconium is titrated with a standardized EDTA solution. Xylenol orange is used as the indicator. A sharp color change from pinkish orange to yellow denotes the endpoint. Based on the volume of EDTA, the concentration of zirconium, and the concentration of EDTA, an apparent formula weight of the original solid DFO or p-SCN-DFO can be calculated. The ratio of the theoretical value to the calculated formula weight can then give an assay value. Strict instructions for carrying out a test can be found in supporting information.
Three zirconium solutions were prepared. Analyst one used zirconium solution number one to perform all his/her assay tests on DFO and p-SCN-DFO. Analyst two used zirconium solution two and so on. The titrator that was used was an SI Analytics TITRONIC Basic. The burette holds 20mL and can dose in 0.01mL increments. Since its purchase, the instrument has routinely been tested to meet a 0.5% RSD for dosing small and large volumes. Since the endpoint can be determined with the naked eye, the assay test is performed by manual dosing. However, the titration could be automated with the incorporation of a photo rode if desired. For a residue on ignition test, a crucible with cover was placed at 900°C for two hours to clean. Solid ZrOCl2-8H2O was dried to obtain an easily weighable solid that had a formula of approximately ZrOCl2- 4H2O based on the results obtained. This solid was weighed in the crucible after cooling. The solid was placed in the furnace for a total of 4 hours. Assuming the residue that remains is pure ZrO2, the percentage Zr in the original solid can be calculated.
Results and Discussion
Assay Method
Many colorimetric methods have been developed over the years for the determination of zirconium including those in the presence of interfering ions. [12-20] No method was found in the literature that worked for our application. Hundreds of titrations with varying conditions were carried out before settling on the optimal conditions. The method has many of the hallmarks of an ideal colorimetric titration. The titration can be performed quickly (i.e., minutes) so there is no long wait for equilibrium to be achieved. The method is colorimetric so there is no need for a UV/VIS instrument to determine an endpoint. The method is a classic complexometric method that uses EDTA as titrant, so no precipitation/filtration steps are required. The method uses commercial ACS reagents, so no expensive fine chemicals are needed. Lastly, solution preparations are simplified to dissolving solids in aqueous/DMSO solutions. No tricky heating or transfer steps that can add error are needed.
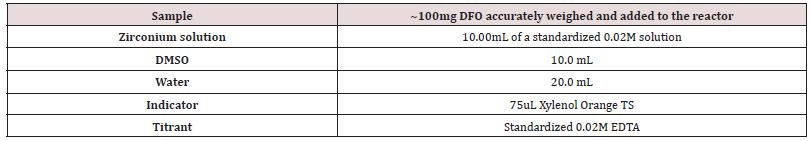
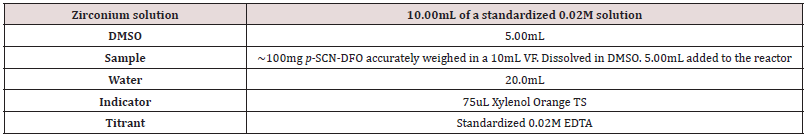
The final conditions developed for the DFO and p-SCN-DFO assay test are found in (Tables 1,2). Due to the limited solubility of p-SCN-DFO in water, its sample preparation differs slightly. Conditions in (Table 1,2) are considered the 100% level. The order of addition is critical to prevent the zirconium from turning into an unreactive form in solution. Thus, the solutions or solids must be added in order from top to bottom.
Preparation of Zirconium Solution
The preparation of solutions of many metal ions can easily be prepared from their chloride or nitrate salts. The preparation of zirconium solutions is not straightforward. Unlike some transition metals, zirconium does not form a simple aqua ion in solution. The aqueous speciation depends on several factors such as pH, concentration, and counterions. [21-25] This work does not attempt to identify what species exist in solution under the conditions employed. It was sufficient to demonstrate the conditions we used were repeatable when preparing solutions.
It was critical to prepare a zirconium solution that can easily be prepared and standardized. Originally, a NIST-traceable zirconium standard was tested. These standards are commonly used for ICP quantification of zirconium. Despite their preparation in acidic solutions, we could not come close to the zirconium value listed on the certificate of analysis. The solution preparation of this standard is not reliable for our purposes. Instead, we decided to prepare our own solution from ACS grade chemicals. The best solution preparation was made by dissolving ZrOCl2-8H2O in 1.0N nitric acid. ZrOCl2-8H2O was chosen over ZrO (NO3)2-xH2O because the latter is difficult to dissolve in acid solutions. It can be dissolved in a reasonable time only after heating. ZrOCl2-8H2O, on the other hand, dissolves readily in acidic solutions. Nitric acid was chosen over hydrochloric acid. When zirconium solutions are prepared in hydrochloric acid, the titration endpoint takes minutes. While it is a sharp endpoint, each drop of titrant near the end takes minutes to discern if the endpoint was reached. Since not every analyst has the same amount of patience, the nitric acid solution preparation was chosen instead. As a result, endpoints are both sharp and immediate. Regarding acid content, 1.0N acid was optimal although 2N HNO3 was also acceptable. When zirconium solutions were prepared in 0.1N HNO3 or less, or in >2N HNO3, the endpoint was unclear.
It was critical to verify all the zirconium in prepared solutions is in a form that can react with EDTA, DFO, and p-SCN-DFO. A residue on ignition (ROI) test was used as a separate method to verify the results of the titration. Three sources of ZrOCl2-8H2O were used to prepare and standardize three solutions of zirconium. The same sources of ZrOCl2-8H2O were tested for ROI. The % Zr was calculated using both methods. The results agreed to ≤ 0.8% absolute. This is a very good agreement for the comparison of two different methods. Consequently, we believe all the zirconium in solution is available to take part in the reaction.
Xylenol Orange Indicator
Many indicators can complex zirconium and then be displaced by the addition of EDTA. However, not all have this property under the highly acidic conditions. Based on literature reports, eriochrome cyanine R, arsenoazo III, and xylenol orange were the first indicators to evaluate. The former two did not give a distinct color change at the endpoint. Xylenol orange, however, gave a very sharp endpoint. The color change was from a pinkish-orange color near the endpoint to a pure yellow. ACS grade xylenol orange was used as purchased without any purification. It is known that commercial xylenol orange is a mixture of several related compounds. [26] Nonetheless, 0.1% solutions in water were prepared from two different lots and gave identical results.
The volume of indicator used will impact the sharpness of the endpoint. It was determined that 75-100μL of the 0.1% solution was ideal. Under the acidic solutions employed, the indicator is either sparingly soluble or forms a colloid. The use of DMSO increases the clarity of the color transition by increasing the solubility or preventing colloid formation.
Temperature
Various temperatures were tried. Typically, higher temperatures favor faster equilibriums. In our case, temperatures above 70°C were detrimental. Endpoints were difficult to discern. Temperatures 50-70°C led to protracted equilibria. Addition of EDTA titrant produced a fast color change to yellow that slowly turned back to pinkish orange. When temperatures in the 30-50°C range were used, the equilibrium was fast. Once the color changed to yellow, it did not revert to pinkish orange. Ultimately, 40°C was settled upon.
Method Qualification
Table 3: Tests used to qualify the DFO and p-SCN-DFO assay method. Validation tests are given as a reference.
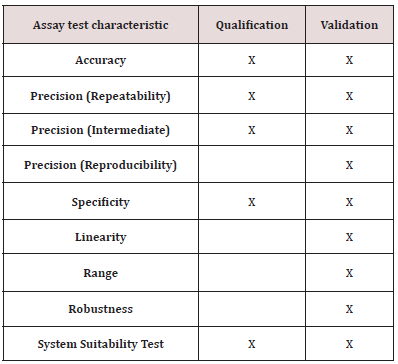
(Table 3) is derived from ICH Q2(R1). It lists characteristic tests performed to validate an assay method. Note that the list differs from those for validating an identification test or one for impurities. The table also lists the subset of tests used in this study to qualify the assay method. This qualification study focused on accuracy, specificity, and precision. The other aspects were not evaluated or minimally evaluated. Only one lab took part in the study, so Precision (Reproducibility) was not evaluated. The range was limited to three different concentration levels of DFO and p-SCNDFO. Many robustness aspects of the method were evaluated during development. For example, different lots of reagents were used. However, they were not formally tested as part of the qualification study.
Accuracy
The assay method has a high degree of accuracy built into the test. As mentioned in the introduction, the results of this test can be traced back to a primary standard. Calcium carbonate is used to standardize an EDTA solution. This EDTA solution is used to standardize a zirconium solution. Finally, both the zirconium and EDTA solutions are used to determine the assay value of DFO or p-SCN-DFO. No other reference standard is needed.
The fact that DFO and p-SCN-DFO are pure substances is a consideration when evaluating a test for accuracy. A drug product typically has excipients or other inert ingredients that can inhibit or mask a target analyte. Pure substances typically do not have this issue. That does not mean the accuracy of the analysis can be taken for granted. The sample preparation of p-SCN-DFO had an impact on the results. DMSO was required to both dissolve the product and keep it in solution so the test could be performed. Without DMSO, the accuracy as well as the precision were poor.
An ROI test was used to confirm the accuracy of the zirconium titration. When heated in a furnace at 900°C, zirconium salts quantitatively turn into ZrO2. Three different ZrOCl2-xH2O solids were used for the ROI test and to make solutions that could be standardized. The zirconium content between the two methods differed by at most 0.8%. This confirms the titration method is capable of accurately determining the concentration of zirconium in solution.
Precision (repeatability)
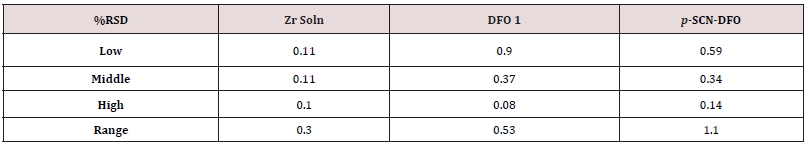
ICH Q2(R1) gives recommendations for repeatability. One way is to analyze in triplicate three concentrations that span the usable range of the assay test. To this end, one analyst analyzed one zirconium solution at the 50, 100, and 150% levels. One lot of DFO was analyzed at the 50, 100, and 150% levels. Finally, one lot of p-SCN-DFO was analyzed at the 70, 100, and 130% levels. See the results in (Table 4). The %RSD values throughout the range are all excellent at 1.1% or less. The repeatability for DFO and p-SCNDFO improved (lower %RSD) when larger amounts of the solid was used. In practice conditions representing the 100% level have been sufficient. Specifying final test conditions at the 150% level would improve the precision.
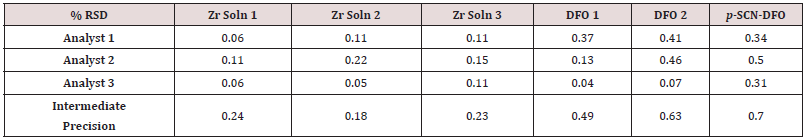
Intermediate precision
Since it is typical for more than one analyst to perform QC testing, intermediate precision is an important qualification test. In triplicate, three different analysts standardized three zirconium solutions, analyzed two lots of DFO, and analyzed one lot of p-SCNDFO. These precision tests were on different days at the 100% level. It did not seem meaningful to use different equipment as the equipment that was used had already gone through its own qualification. As mentioned before, one solution of EDTA was prepared and used by all analysts to remove any variability associated with its preparation. Intermediate precision was calculated by averaging all nine results from three analysts. See the results in (Table 5). While the endpoint of the titration method is subjective (a color change detected by the human eye), %RSD values for intermediate precision of < 1.0% were obtained in every case. Thus, the intermediate precision was excellent. This means the method was able to be understood and put into practice by different people and very similar results were obtained.
Specificity
Some analytical methods are more specific than others. That is, they can respond to a single analyte better than other methods. A high degree of specificity for a titration requires 100% complexation and a sharp endpoint. Under the conditions employed, DFO and p-SCN-DFO will react 100% with zirconium. Common impurities such as salts, residual solvents, and adsorbed water will not react with zirconium. One of the reasons this method works is DFO and p-SCN-DFO are highly specific chelating agents for zirconium. The formation constants are higher than those with EDTA – which is a critical aspect of this method. For this method to work properly, the stability constants with zirconium must be of the following order: NO3-/Cl-/H2O/DMSO < Xylenol Orange < EDTA < DFO/ p-SCN-DFO.
Thus, the specificity of this method is acceptable. A limitation of this method is that some related substances are also going to form a complex with zirconium under the test conditions. It is easy to imagine that putting a methyl group on the terminal primary amine of DFO will create a related substance that will still form a very stable complex with zirconium. However, not all related substances will bind zirconium under the conditions employed. If one or two of the hydroxamic acid moieties is not present in a related substance, it probably will not bind zirconium. A well-developed chromatographic purity test measures the related substances. These two tests can be used in conjunction to evaluate the quality of the products.
System Suitability Test
In our experience 0.02M solutions of EDTA are stable for at least six months. When prepared as described, solutions of zirconium are also stable at least six months. The system suitability test we employed the day of testing was to verify the concentration of the zirconium solution. If the zirconium concentration was within 0.5% of the established concentration, the system suitability test passed.
USP RS Analysis
Since it is available, the USP RS for Deferoxamine Mesylate was analyzed using the developed method. Due to having only a limited quantity, each analyst tested the material once. Averaging the singlet results from the three analysts produced an assay value of 99.6±0.3%. For this lot it was specified to multiply the amount of solid used by 0.996 for quantitative spectrophotometric applications. Our assay value corresponds exactly to the assigned value specified by the USP. For reference, the spectrophotometric application referenced above is a non-specific assay test described in the monograph for Deferoxamine Mesylate for Injection. [27] In this test, an iron complex is formed and then quantitated using a UV-VIS instrument at 485nm.
When this same lot of Deferoxamine Mesylate RS is used as a reference in an HPLC assay test, a factor is incorporated to account for the fact it is not considered 100% pure. This factor accounts for any residual solvent and related substances. The acceptance criteria are calculated on the anhydrous basis, so it understood that the adjustment factor does not consider associated water. The USP does not share the values determined for each component (i.e., residual solvent, water, related substances, etc.) that go into the adjustment factor. They consider those values proprietary. Analysis of the RS using our own developed method gave a purity value of 97.8%. The USP specifies an assigned value of 0.981 for quantitative HPLC applications. If residual solvents are negligible, our value corresponds nicely to the USP multiplier.
Conclusions
A fast and inexpensive assay method for DFO and p-SCNDFO has been developed. The method can be traced back to the quality of an analytical primary standard. Thus, there is no need to incorporate a product-specific reference standard into the test for either compound. The method includes the non-trivial preparation and standardization of a zirconium solution. This assay method was then qualified with both compounds. The method can easily be integrated into a quality system to test these compounds as raw materials or as drug substances. The method has its limitations. Primarily, it is a non-specific method such that some related compounds to DFO and p-SCN-DFO will react equally as well. However, any associated water or residual solvents are accounted for. The test forms an excellent counterpart to a well- developed HPLC purity method that accounts for related substances. The assay method was qualified for both compounds. Method qualifications are designed to evaluate the integrity of the method. Based on the results from the tests, this assay method is of sufficient quality such that it could easily be moved into a validation study.
References
- USP Mission and Preface; Current DocID: GUID-E09DC39F-1D28-4D90-8D76-0ED109FFB640_5_en-US; Currently Official as of 1-May-2020.
- USP <11> USP Reference Standards; Current DocID: GUID-041BE2C7-30A1-44FA-AC03-6F6997D2F251_3_en-US; Currently Official as of 1-Nov-2020.
- USP Monograph Deferoxamine Mesylate; Current DocID: GUID-2DA07CF4-1552-473A-BC94-B87C1B7C97B5_4_en-US; Currently Official as of 1-May-2020.
- USP General Notices and Requirements; Current DocID: GUID-6E790F63-0496-4C20-AF21-E7C283E3343E_6_en-US; Currently Official as of 1-Nov-2020.
- Heskamp S, Raavé R, Boerman O, Rijpkema M, Goncalves V, et al. (2017) 89Zr-Immuno-Positron Emission Tomography in Oncology: State-of-the-Art 89Zr Radiochemistry. Bioconj Chem 28(9): 2211-2223.
- Vosjan MJWD, Perk LR, Visser GWM, Budde M, Jurek P, et al. (2010) Conjugation and Radiolabeling of Monoclonal Antibodies with Zirconium-89 for PET Imaging using the Bifunctional Chelate p-Isothiocyanatobenzyldesferrioxamine. Nat Prot 5(4): 739-743.
- Dilworth JR, Pascu SI (2018) The Chemistry of PET Imaging with Zirconium-89. Chem Soc Rev 47(8): 2554-2571.
- Deri MA, Zeglis BM, Francesconi LC, Lewis JS (2013) PET Imaging with 89Zr: From Radiochemistry to the Clinic. Nucl Med Biol 40(1): 3-14.
- Savastano, M, Bazzicalupi C, Ferraro G, Fratini E, Gratteri P, et al. (2019) Tales of the Unexpected: The Case of Zirconium (IV) Complexes with Desferrioxamine. Molecules 24(11): 2098-2105.
- ICH Harmonized Tripartite Guideline (2005) Q2(R1) Validation of Analytical Procedures: Text and Methodology
- Ritter N, Advant SJ, Hennessey J, Simmerman H, McEntire J, et al. (2004) What is Test Method Qualification? Bioprocess International 1-11.
- Antepenko RJ (1982) Spectrophotometric Determination of Zirconium with Xylenol Orange. the US Department of Energy Office of Scientific and Technical Information 61(6): 311-313.
- Savvin SB (1961) Analytical Use of Arsenazo III: Determination of Thorium, Zirconium, Uranium, and Rare Earth Elements. Talanta 8(9): 673-685.
- Ross LE, Drabek VM, Larsen RP (1969) Colorimetric Determination of Zirconium with 1-(2-Pyridylazo)-2-Naphthol. Talanta 16(6): 748-750.
- Vasil’ev VP, Katrovtseva AV, Lytkin AI, Chernyavskaya NV (2004) Photometric Determination of Zirconium with Semimethylthymol Blue. J Anal Chem 59: 206-210.
- Sharma V, Nijhawan M, Malik AK, Rao ALJ (2001) 3-Hydroxy-2-(2’Thienyl)-4H-Chromon-4-one as a Spectophotometric Reagent for the Trace Determination of Zirconium in an Aqueous Phase. J Anal Chem 56: 830-832.
- Jain A, Prakash O, Kakkar LR (2010) Spectrophotometric Determination of Zirconium with 5,7-Dibromo-8-hydroxyquinoline in Presence of Thiocyanate. J Anal Chem 65(8): 820-824.
- Hirn CF, Lucchesi CA (1959) Determination of Zirconium in Zirconium Driers. Anal Chem 31: 1417-1418.
- Yuchi A, Hokari N, Wada H, Nakagawa G (1993) Semi-Xylenol Orange Complex of Zirconium (IV) as a Photometric Reagent System for Fluoride Based on Mixed Ligand Complex Formation. Analyst, 118(2): 219-222.
- Pechishcheva NV, Shunyaev KY, Melchakova OV (2018) Zirconium in Modern Analytical Chemistry. Rev Anal Chem 37(2): 1-26.
- Clearfield A, Vaughan PA (1956) The Crystal Structure of Zirconyl Chloride Octahydrate and Zirconyl Bromide Octahydrate. Acta Cryst 9(7): 555-558.
- Mak TCW (1968) Refinement of the Crystal Structure of Zirconyl Chloride Octahydrate. Canadian J Chem 46(22): 3491-3497.
- Devia DH, Sykes AG (1981) Aqueous Solution Chemistry of Zirconium (IV). 1. Kinetic Studies on Hydrogen Ion and General Acid (HX) Induced Dissociations of the tetrameric Ion [Zr4(OH)8(H2O)16]8+. Inorg Chem 20(3): 910-913.
- Kragten J, Parczewski A (1981) Photometric Complex-Formation Titration of Submicromolar Amounts of Zirconium. Talanta 28(3): 149-155.
- Noren B (1973) The Hydrolysis of Zr4+ and Hf4+. Acta. Chem. Scand. 27, 1369-1384.
- Nakayama H, Tachiyashiki S, Ishii M (1989) Preparation of High Purity Xylenol Orange and isolation of a New Metallochromic Dye by Cation Exchange Column Chromatography on SP-Sephadex. Analytical Sciences 5(5): 619-621.
- USP Monograph Deferoxamine Mesylate for Injection; Current Doc ID: GUID-6D2F10AC-EB2C-42CD-A4A3-B03041A53193_3_en-US; Currently Official as of 1-May-2018.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...