Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Characteristics and Evaluation of Leaching Behavior of Uranium Mineralization in Qash Amir Granite, South Eastern Desert, Egypt Volume 5 - Issue 1
Mohamed S Nagar*, Bayoumi MB and Walid M Morsy
- Nuclear Materials Authority, El Maadi, Egypt
Received: December 18, 2020; Published: January 28, 2021
*Corresponding author: Mohamed S Nagar, Nuclear Materials Authority, El Maadi, Egypt
DOI: 10.32474/AOICS.2021.05.000201
Abstract
In the Halaib area, South Eastern Desert, Egypt, numerous occurrences of uranium have been found. Uranium occurs as disseminated minerals (uraninite, uranophane, beta-uranophane) in G. Qash Amir muscovite monzogranite. The muscovite monzogranite (G. Qash Amir) is affected by deutric alteration and characterized by gradational contact with two-mica monzogranite, peraluminous in nature with visible primary and secondary uranium minerals, beryl and columbite. Uranium dissolution efficiency of 81.0 % was obtained using acid agitation leaching without oxidant addition, while dissolution efficiency increased to about 92% when ORP was increased to about 475mV using MnO2 as an oxidizing agent in Qash Amir uranium mineralization. Column tests were performed to study the effect of the parameters on uranium leaching and acid consumption. After 40 days of columns leaching testes, uranium recovery of 74.2% was obtained at a flow rate of 10 l/m2/h and acid consumption was achieved by 26.2 kg per ton of ore. The addition of MnO2 as oxidant leads to a significant increase in the column leaching efficiency to 87% and decreasing acid consumption to 22kg per ton of ore in 35 days. The plot of 1-(1-x)1/3 vs. t is linear and the R squared values for particle diffusion control line is 0.98, therefore the shrinking-core model is verified.
Keywords:Column leaching; Uranium leaching efficiency; MnO2 oxidant; shrinking core model
Introduction
A deposit of uranium discovery by various exploration techniques is evaluated to determine the amount of uranium materials that are extractable at specified costs. Uranium quantities are the amounts of material (either in U or U3O8) that are estimated to be recoverable at stated costs. Uranium ore can be extracted through covenantal mining by open cut and under methods. In some cases, uranium is recovered as a by-product, for example of copper mining. Mined uranium ores normally are processed by grinding the ore materials to a uniform size and then treating the ore to extract the uranium by chemical leaching. The milling commonly yields dry powder-form material consisting of natural uranium, yellow cake, which is sold on the uranium market as U3O8. The Egyptian Eastern Desert has been described as one of the most intensively dyke-intruded the granitoid pluton-pierced segments of the continental crust [1,2]. The small epizonal late Pan-African alkali feldspar granite to monzogranite plutons of the Eastern Desert range in Rb-Sr age from 620 to 570 Ma [3,4]. These plutons are typically rounded to elliptical or teardropshaped, propose more than one tectonic setting for these granites (including both compressional and extensional) to account for trace element pattern differences. El-Nisr et al. stated that the younger granitoids at Gabal Qash Amir area are syenogranite compositions namely biotite- hornblende subsolvus syenogranite at Gabal El Sela showing I-type affinity (SGR I), and biotite-bearing hypersolvus syenogranite at Gabal Qash Amir with A-type affinity (SGR II). He also stated that, SGR I is characterized by low Rb/Sr ratios and high field strength element (HFSE) concentrations (e.g., Nb, Ta, Y), fractionated LREE with flat HREE, high LREE/HREE ratios, presence of a negative Nb-Ta anomalies and lack of negative Ba and Eu anomalies. On the other hand, SGR II is characterized by high Rb/Sr ratios and HFSE concentrations, slightly fractionated (SGR Ila) to unfractionated (SGR l1b) LREE with a positive slope for the HREE [4]. Hussein (1990) stated that Qash Amir granitic mass is traversed by sub horizontal fissures filled with quartz veins with very limited wolframite mineralization [5]. Assaf et al concluded that the younger granites of Gabal Qash Amir and Gabal El Sela were generated in extensional environment (within – plate regime) and equivalent to A-type granites [6,7]. El Gammal and Cherif , mentioned that Gabal Qash Amir area are example of tungsten vein deposits associated with alkaline granite [8]. Rashed, stated that Gabal Qash Amir is monzogranite and syenogranite composition of I- type granite [9]. Masoud. S. Masoud, stated that Qash Amer granites are per aluminous younger granites [10]. Although nuclear power is considered to be a non-renewable energy source, as there is a finite supply, it is sustainable due to its ability to provide energy without environmental detriment [11]. As the demand for sustainable energy resources continues to grow, nuclear power is being utilized by many countries and governments [12]. According to the World Nuclear Association around 13% of the world’s electricity is generated using nuclear energy and this is set to increase in the future as a number of countries have announced nuclear energy targets including China, the United States, Japan, Russia, and India [13].
A hydrometallurgical testing program to define the basic criteria, like uranium recovery and sulfuric acid consumption, for a commercial heap leach system design, to process the uranium mineralization. The process of the uranium particles leaching is very complex, and the leaching rate is related to the leaching time, ore grade, chemical composition, type, and concentration of the leaching reagent. Recently a lot of international works have been done on uranium heap leaching and some related articles were published. Chunlin Feng et al. concluded that leaching performance of hexavalent uranium was still good without adding oxidants in low-grade uranium ore leaching experiments [14]. Xiaobo Wang et al concluded that the leaching rate of uranium ore could be accelerated with adding a small amount of leaching oxidants by increasing uranium concentration [15]. The duration of leaching, including the ‘rest period’ between leaching cycles, is of interest for stop leaching mining operations as mine water is often used to wash down the stop [16-18]. Heap leach studies were carried out in Argentina, Trekkopje in Namibia, Canada, France, Spain, and Australia [19-24]. At present, commercial operations have utilized sulphuric acid leaching including those at Ranger in Australia, Rössing in Namibia, and Somaïr in Niger, while the first commercial use of alkaline leaching started to operation in 2012 at Areva’s Trekkopje mine in Namibia.
Egyptian Nuclear Materials Authority, (NMA) conducted comprehensive programs for exploration of U and other nuclear elements mineralization in Egypt. The discovered localities were subjected to extensive studies from the geological, mineralogical, geochemical, and even hydrometallurgical points of view. Different promising areas containing U mineralization were recorded; the most important of them are Gattar, El-Sela and Abu-Rusheid areas. Agitation and column percolation leaching tests of uranium mineralization were carried out to determine the optimum conditions for maximizing the recovery of uranium. There have been some studies on the leaching and recovery of uranium was carried out on Halaib area, South Estern Desert, Egypt. A preliminary study to recover uranium from El Sela mineralization through applying acid agitation leaching technique using sulfuric acid, then the uranium was adsorbed from the pregnant leach liquor by Amberlite IRA – 400 anion exchange resin [25]. Leaching process on technological scale sample for studying the mining ability, leaching characteristics and the recovering conditions, the results shown that El Sela U-ore material is easily mineable and easily recovering [26]. The extraction / stripping condition were successfully applied for extraction of uranium from samples collected from El Sela area, South Eastern Desert, Egypt [27].
Uranium was recovered from the sulphate leaching solutions of El-Sela mineralization in a pilot scale using Amberlite IR-A400 packed in fixed columns with flow rate of 2.3 l/min [28]. Finally, Mohamed S.Nagar et al. have successfully recovered uranium from El-Sela mineralization after column leaching operations with sulfuric acid [29]. Among the different rock units in the G. El Sella area, the two-mica monzogranite and muscovite monzogranite (G. Qash Amir) are the most favorable host rocks for uranium and thorium mineralization. The muscovite monzogranites (G. Qash Amir) are affected by deutric alteration and characterized by gradational contact with two-mica monzogranite (G. El-Sella), peraluminous in nature with visible primary and secondary uranium minerals, beryl, and columbite without shearing or channel ways for mobilization of uranium [30]. The goal of the present study was to investigate the leachability of uranium from G. Qash Amir muscovite monzogranite. using sulfuric acid with and without oxidant. Agitation and column leaching testing programs were carried out to achieve the basic criteria, like recovery, and sulfuric acid consumption, for a commercial heap leach system design, to process the uranium mineralization from Qash Amir area.Geologic setup
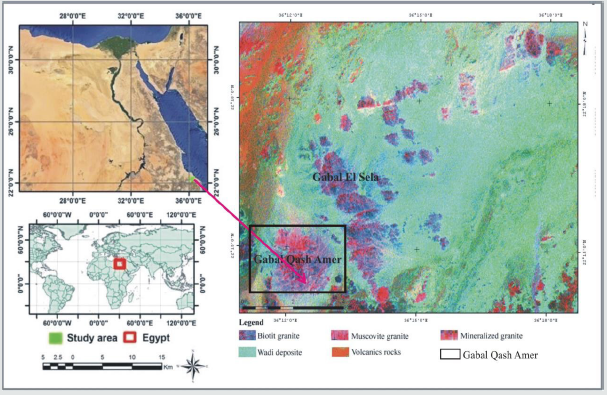
Gabal Qash Amir form the eastern extinction of Sul Hamid shear zone ( 682m. a.s.l) and it is located at about 28 km southwest of Abu Ramad city close to the Sudanese border at latitudes 22° 14’ 07’’– 22° 15’ 21’’ N and longitudes 36° 10’ 59’’ - 36° 14’ 24’’ E, (Figure 1). Qash Amir granites were comprise dismembered ophiolitic mélange structurally thrusted over and inter-slices with serpentinite talc carbonate basic to intermediate calc alkaline metavolcanics and metagabbro, mineralized rocks at study area and there features of alterations were appears on the land sate image, (Figure 1). It is oval body trending NW, occupies approximately 4 Km² and surrounded by vast sand sheet (Figure 2). This granite is strongly weathered, cavernous exfoliated and jointed muscovite monzogranite to syenogranite. These rocks are pale pink, leucocratic, coarse grained and composed essentially of quartz (25%-35%). K-feldspar, plagioclase, and muscovite ± biotite. These rocks are affected by N-S, E-W and NE-SW faults, joints and fractures filled with Mnmineralization. Some alteration processes such as hematization, kaolinitization, albitization and greisening tacks place at the peripheries, (Figure 3). At the southeastern part of the stock there are zones of albitized leaucogranite rich by dark green muscovite aggregates and manganese oxides. These zones cut by low dipping quartz- veins at the southern and eastern margin of the granite body and locally associated with wolframite ± sulfide. These rocks cut by basic and acid dykes, basic dykes cut the western part of G. Qash Amir and characterized by dark black in colour, fractured and low in relief than the surrounding country rocks. Their thickness ranges from 1to 3m at some places and extends about 150 m. Acidic dykes are cutting the Qash Amir granitic rocks with E-W direction, they are pale pink in colour, fine grained texture, highly relief and its thickness less than 0.5m and its nearly vertical.
Figure 2: Geological map of Gabal Qash Amer area, South eastern Desert, Egypt, after M. S. Masoud (2011).
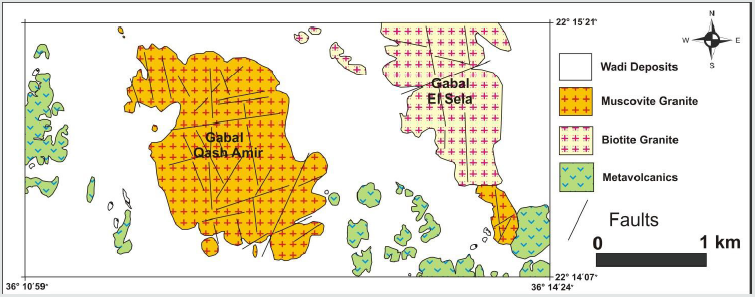
Figure 3: A, B, C, D, E and F, Field photographs shows different geological features at Qash Amer area, South Eastern Desert, Egypt

Material and Methods
Spectrometric Investigations
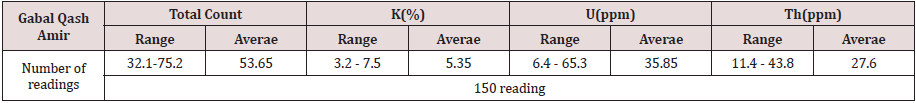
Regional field radiometric measurements using a portable four channel, gamma-ray spectrometer Model RS-230 was used on Gabal Qash Amir granites to identify their radiometric anomalies. The Spectrometric measurements were mad at different stations in study area and recorded 150 reading.
Uranium Recovery Study
Sample preparation and chemical analyses
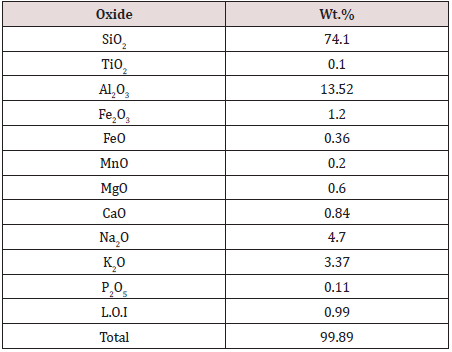
Samples from Qash Amir were received in a variety of forms, including unsorted bulk samples, large and small diameter. Approximately 250 kg uranium mineralization was taken for preparing representative sample. After homogenizing and splitting into smaller parts, about 150 kg were selected randomly and crushed to (-20 - + 10, -10 + 5, and -5 mm). Another part of the representative main sample is taken and milled to less than -100 mesh, which is sent for agitation leaching and chemical analysis. Table 1 shows result of chemical analyses. Based on the chemical composition of uranium ores (Table 1), the main composition is formed from silicates, and the ore rock solution leaching reagent meets the need of the acid leaching method, i.e., the low price of the H2SO4 and the convenient transportation, hence H2SO4 solution was used as the solution leaching reagent. For low uranium content colorimetric method using Arsenazo III was used [31], while the titration method of titanium trioxide was used to estimate the high uranium content [32].
Agitation leaching test
For agitation leaching tests, carefully quarried out of the uranium mineralization sample was ground to less than -100 mesh. 250g scale in a 1500 ml glass reactor with mechanical shaking adjusted at 200 rpm at the room temperature.(250C) provided with a pH-temperature and ORP electrode to determine the maximum solubilization. Agitation leaching tests were conducted for 48 to 72hours and using four various acid concentrations (30, 50, 70 and 100g/l) with and without MnO2 as oxidant. The ORP was determined to the slurry without addition of oxidant and then measured the quantity of MnO2 was added to obtain the suitable potential which give high recovery of uranium. Oxidation redox potential (ORP) was measured during the ore tests using Ag/AgCl with a platinum-based electrode.
Column testing
A program of column tests was performed to define the leaching parameters for the treatment of the Qash Amir samples. Small PVC columns (100 mm internal diameter and a height of 1500 mm) were used. Columns are operated for 40 days, during which, tests were performed to study the effect of the following parameters on uranium leaching, and acid consumption, ore particle size, application rate, lixiviant acid concentrations and consumption. Three leach columns were used to evaluate different particle sizes (-20 - + 10, -10 + 5, and -5 mm). Three leach solutions were prepared with different acid concentration to evaluate their effect on uranium recovery, acid consumption, and time. The solutions were applied to leaching column having 30, 70, and 100gpl acid strength. Both solutions were applied to the same flowrate. Three different flowrates were testing (one for every column) 10 l/m2/h, 15 l/m2/h, and 20 l/m2/h. The acid leaching process with the addition of the oxidizing agent was examined. In the present work different concentrations of MnO2were used as an oxidizing agent. The final residue obtained of column leaching was likewise determined for its remained uranium content. Percent extraction efficiency was calculated using the formula,
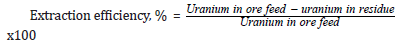
Result and Dissection
Characteristics of uranium mineralization
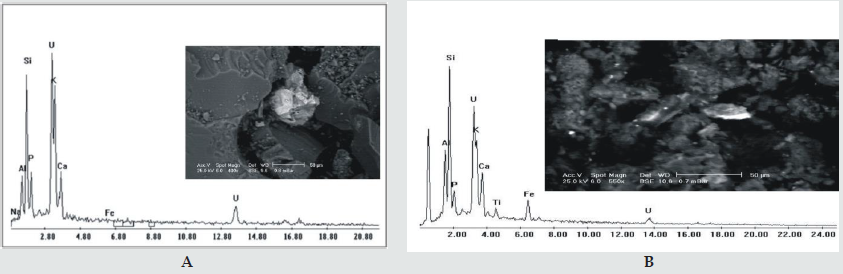
The uranium minerals present in the study area are mainly of the secondary type which reflects the alteration processes under oxidation conditions. The primary uranium phases (uraninite) were only recorded in G. Qash Amir by Rashed, [9]. However, the following are the secondary uranium phases encountered in the present work: A-Uranophane and beta-uranophane [Ca(UO2) (SiO3)2(OH).5H2O] which symbolizes the secondary uranium in the study area. It occurs either as disseminated clusters or as micro- fracture infilling and coating joint surface (Figure 4). Uranophane is characterized by its yellow and lemon-yellow shades in G. Qash Amir granitic rocks. The mineralization is associated with alteration features such as silicification, hematitization and fluoritization. El Agami, et al., (1999) stated that numerous occurrences of U, Mn-Fe and metalliferous quartz veins have been found in Halaib area [33]. They classified the mineralization of Halaib area into four categories on the basis of occurrence and lithological association as follows: Disseminated U-mineralization: The uranium mineralization occurs either as disseminated or as micro- fractures infill or coat joint surface. Assaf et al., identified U-minerals as uraninite (UO2), uranophane and beta-uranophane [6].
Figure 4: A and B show EDX analyses and back scattered electronic images showing uranophane minerals
Radiometric measurements for gabal qash amir granite
Gabal Qash Amir coarse grained granite shows variable contents of K, U, Th, and total count (Table 2). Total count ranges from 32.1 to 75.2 ppm with average 53.65ppm. K contents range from 3.2 to 7.5% with average 5.35%, U contents range from 6.4 to 65.3 ppm with average 35.85ppm and thorium contents varies from 11.4 to 43.8 ppm with average 27.6 ppm.
Radioelements plots
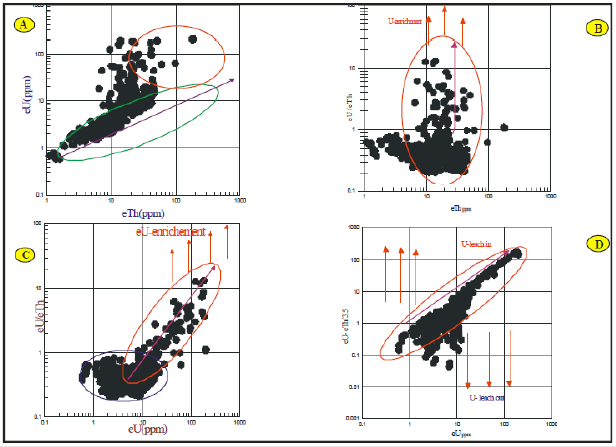
Uranium remained relatively immobile in the original rocks, the eTh/eU diagram of the investigated rock units of Gabal Qash Amer area (Figure 5a) reveals an enrichment of uranium contents, as a result of migration process of uranium minerals with water rains and washing and re precipitated at faults and joints. According to this model, the radioelement increases gradually during magmatic fractionation, but the ratio changes due to different alteration processes [34]. The eU/eTh versus eTh diagram (Figure 5b) indicates a reverse relationship, in which eU/eTh ratio decreases with increasing eTh for most plotted rock units, indicating the important role of hydrothermal solutions in redistribution of these elements and is proved that, there is a leaching of some of the uranium content from the altered rock units. On the other hand, the eU/eTh ratio versus eU diagram (Figure 5c) reveals a strong direct relationship which shows hydrothermal U enrichment. So, different alteration processes play an essential role in U mobilization, than that of the hypothetical uranium distribution (Figure 5d) and so the mobilization gives positive values, which in turn show that U of these different studied rock units is leaching in [35]. Lastly, from the analysis of the gamma-ray spectrometric data, which is revealed on binary diagrams of eTh vs. eU, eU vs. eU/eTh and eU against eUeTh/ 3.5, the studied rock units of Gabal Qash Amer area shows high enrichment by U and Th mineralization.
Figure 5: Radioactive elements plot for ground gamma-ray spectrometry measurements at Gabal Qash Amer area, South Eastern Desert, Egypt.
Uranium recovery results
In G. Qash Amir muscovite monzogranite uranium occurs as disseminated minerals (uraninite, uranophane, beta-uranophane). The hexavalent uranium is readily soluble in sulphoric acid, but tetravalent uranium must be oxidized to a hexavalent state for dissolution in diluted sulphoric acid [36]. Acid leaching of uranium mineralization requires the presences of Fe3+ regardless of the reagent used as an oxidant [37]. The ferric iron Fe3+oxidizes the tetravalent uranium to hexavalent soluble uranium, while the oxidant reagent oxidizes the ferrous iron to ferric, and the mechanisms are described by the following reactions with MnO2 as oxidant.
2Fe2+ + MnO2 + 4H+ 2Fe3+ + Mn2+ + 2H2O
UO2 +2Fe3+ UO22+ + 2Fe2+
The hexavalent uranium cation (UO22+) formed after the oxidation process complexes with SO4-2 in the lixivant of sulphoric acid at leaching process to produces uranyl sulphate and complex uranyl sulfate anions as follows:
UO22+ + SO42- = UO2SO4
UO2SO4+ SO42- = [UO2(SO4)2]2-
[UO2(SO4)2]2-+ SO42- = [UO2(SO4)3]4-
The maximum uranium recovery is obtained for most of uranium mineralization by maintaining the oxidation reduction potential (ORP) at 400-500 mV [38]. The leachability of uranium from G. Qash Amir, were investigated using sulfuric acid via agitation leaching experiments, bench scale leaching tests using small column also investigated after obtained optimum conditions.
Agitation leaching result
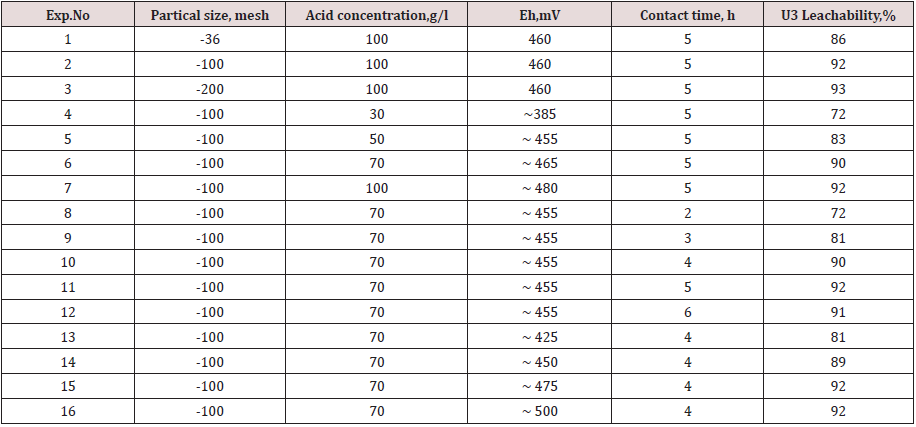
The optimum conditions that influence the leaching of uranium from uranium mineralization such as particle size, acid concentration, acid consuming, oxidant effect, and leaching time, were investigated. The results of these testes are shown in Table 3. From the reported data in Table 3 (experimental 1 to 3), it appears that the leaching efficiency of uranium slightly increases from 92 to 93% at 100g/l H2SO4 concentration, as the grain size decreases form -100 mesh to -200 mesh screen, after which any decrease in the grain size has no effect upon U-dissolution. Thus, for leaching uranium from Qash Amir granitic rock, -100 mesh size is suitable due to uranium occurs as disseminated minerals. It can be seen (experimental 4 to 7) that uranium leach extraction sharply increased from 72 to 90% with increasing acid concentration between 30-70g/l and more slowly between 70-100 g/l. In the same Table (experimental 8 to 12) shown the effect of contact time on uranium dissolution, at constant of other experimental factors. About 72% of uranium was dissolution after 2hrs of reaction and reach about 90% after 4hrs. The leachability of uranium as a function of Eh is given in Table 1 (experimental 13 to 16). As shown the redox potential was varied from 420 to 500mV the redox potential was adjusted to the required value using different concentration of MnO2 as oxidizing agent. The rate of leaching increased from 81 to 92 % with increase of MnO2 dosage from 0.2 to 0.75 g/l which produced redox potential (Eh) of 425 to 475 mV. However, further increase in MnO2 concentration did not result in any significant enhancement of leachabilities. From the above results, it's necessary to maintaining the ORP at 475-500mV to reach the maximum uranium leaching efficiency using 0.75g/l MnO2 as oxidizing agent.
Table 3: parameters and results of sulphoric acid leaching from G. Qash Amir uranium mineralization.
Column Leaching Tests (percolation leaching)
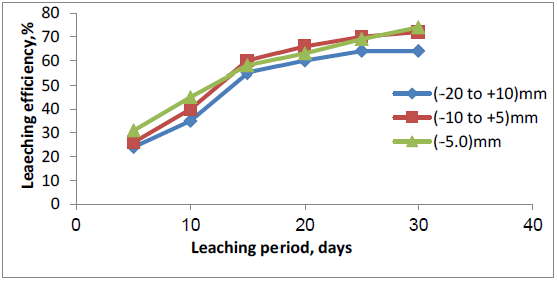
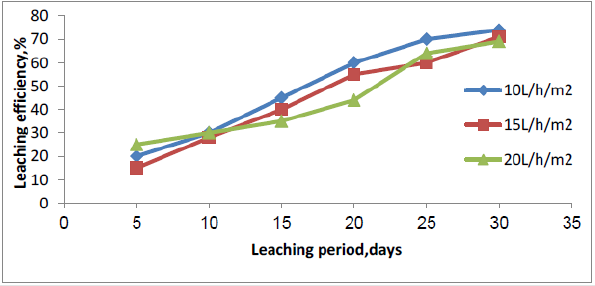
After reaching the ideal conditions of uranium leaching by agitation, next stage of dissolution experimental is carried out using columns leaching during which leach parameters such as, amount of sulfuric acid, particle size of the material, leach solution’s flowrate application, and leach solution’s acid strength, were evaluated using 12 column leach tests. The initial columns experimental were performed to determine the suitable particle size for heap leaching. Three particle sizes selected were (-20 - + 10, -10 + 5.0, and -5.0 mm). The results of column leaching are presented in Figure 6. The lowest acid consumption was obtained with the coarsest sample (22g/kg). It was concluded that the -10 + 5.0 mm ore particle size had acceptable uranium recovery (74%) and acid consumption (28g/kg) in 30 days leaching period. This particle size would be conducted for future heap leaching process. Reducing the particle size increases the contact surface between minerals and sulfuric acid which results the increase of uranium recovery. Three different flowrates were testing, 10 l/m2/h, 15 l/m2/h, and 20 l/m2/h (one for every column). All three leach columns were operated for a 40 days period. Monitoring the daily uranium concentration in PLS showed uranium concentration is low in column with high irrigation flow rate. In contrast, high uranium concentration in PLS obtained by column with low irrigation flow rate. As shown in Figure 7, leach column by 10 l/m2/h reached a recovery of 74% of soluble uranium and had a value of 26.2 g/kg ore for acid consumption, the uranium recovery for irrigation rate of 15 l/m2/h was about 72% of soluble uranium with 28 g/kg acid consumption, 69% uranium recovery and 31g/kg acid consumption were the results for irrigation rate of 20 l/m2/h. It can be concluded that at low irrigation flow rate, contact time is sufficient between minerals and sulfuric acid, then reaction is completed.
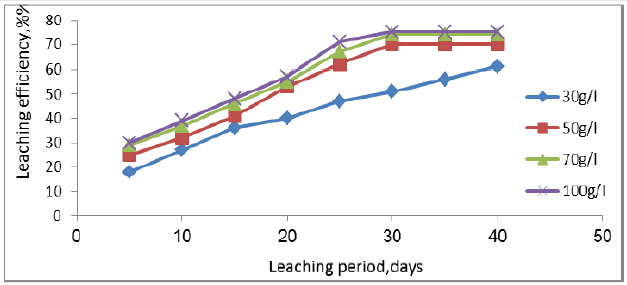
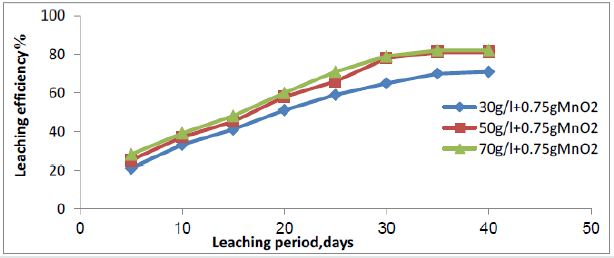
Acid concentration of lixiviant is an important variable of leaching process. To evaluate the effect of acid concentration over the recovery, time, and acid consumption, four leach solutions were prepared with different acid concentration, 30, 50, 70, and 100g/l acid strength. All lixiviant solutions were applied at the same flowrate (10 l/m2/h). The obtained results in Figure 8 indicate that the best acid concentration of sulphuric acid is 70g/l, which was found to give the highest leaching efficiency, 74.2% and acid consumed of 26.2g/kg ore. Leaching experiments were carried out on Qash Amir uranium mineralization to assess the potential of manganese dioxide as an oxidizing agent .Eh was adjusted to a desired potential value using. Figure 9 show that by using 0.75 g/l of manganese dioxide, the column leaching of uranium reaches 82% , and redox potential from 275 to 460mv after solution recycling and acid consumed was decreased to 22g/kg ore in 35days. In this regard Jing Huang et al shown that when add less MnO2, which play a major role in destructed gangue structure, it was enhanced the decomposition of uranium, if add more MnO2, the crystallization and the new manganese silicate crystals were promoted, the phase of uranium be repacked, therefore, the leaching rate of uranium was reduced [39].
Kinetic Models of Uranium Dissolution by Column Leaching
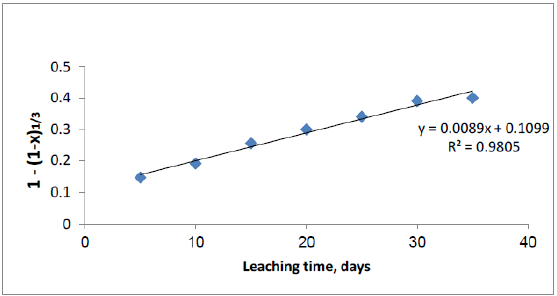
The percolation leaching of uranium from Qash Amir uranium mineralization is a solid liquid process, which can be described by the shrinking-core model, when the uranium mineralization particle is regarded as spherical. The shrinking-core model is the most popular kinetic model in hydrometallurgy [40,41]. Othusitse and Muzenda, stated that for the chemically controlled reaction at the interface (phase boundary-controlled reaction) the following equation can be used.
k·t = 1 – (1 – x)1/3
where k is the apparent rate constant (day-1), t is the leaching period (days), and x is the fractional conversion given by x = C/Co, C is the concentration of uranium in the post leaching solution (g/l), Co is the concentration of uranium in the raw material before experiments (g/ton).
By applying the equations to the previous experimental data and plotting the relationships between [1- (1- X)1/3] and the leaching time (days) of uranium as shown in Figure 10. The plot of 1-(1-x)1/3 vs. t is linear and the R squared values for particle diffusion control line are 0.98, from this result the shrinking-core model is verified.
Conclusion
The muscovite monzogranite (G. Qash Amir) is affected by deutric alteration and characterized by gradational contact with two-mica monzogranite, peraluminous in nature with visible primary and secondary uranium minerals, beryl and columbite. After 40 days columns leaching testes, uranium recovery of 74.2% was obtained at flow rate of 10 l/m2/h and acid consumption were achieved by 26.2 kg per ton of ore without oxidant. The studies indicate that the need for maintaining a redox potential of 460 - 475mVfor efficiency leaching of uranium from Qash Amir granite. Attaining these values of potential necessitates addition of 0.75kg of MnO2 to each ton of ore. Addition of MnO2 as oxidant lead to a significant increase in the column leaching efficiency to 87% and decreasing acid consumption to 22kg per ton of ore in 35 days. By applying the equations to the experimental data, the shrinking-core model is verified.
Acknowledgement
I would like to thank Prof. Kalied Fouad Mahmoud for his supervision and guidance throughout the production of this paper.
References
- Vail JR (1968) Tectonic control of dykes and related irruptive rocks in eastern Africa. In: Clifford, T.N., Gass, I.G. (Eds), African Magmatism and Tectonics. Hafner, Darien, Connecticut pp. 337-354.
- Bentor YK (1985) The crustal evolution of the Arabo-Nubian massif with special reference to the Sinai Peninsula. Precambrian Research 28(1): l-74.
- Hassan MA, Hashad AH (1990) Precambrian of Egypt. Said R (Eds.), The Geology of Egypt, Balkema Rotterdam pp. 201-245.
- El-Nisr SA, ElL-Sayed MM, Saleh GM ( 2002) Geochemistry and petrogenesis of Pan-African late- to post- orogenic younger granitoids at Shalatin-Halaib, south Eastern Desert, Egypt. Journal of African Earth Sciences 33(2): 261-282.
- Hussein AA ( 1990) Mineral deposits, R Said (Eds.), The Geology of Egypt, Rutterdamand Boston. Balkema pp. 734.
- Assaf HS, Ibrahim, ME, Amar SE, Saleh GM, Rashed MA ( 1998) Geological and mineralogical studies on the radioactive mineral’s occurrence at Qash Amir area , Southeastern Desert, Egypt.
- Assaf HS, Shalaby MH, Ibrahim ME, Amar SE, Rashed MA (1996) Ground radiometric reconnissances surveey, Shalatin-Halaib area, Southeastern Desert, Egypt.
- El Gammal E, Cherif O (1999) Granitoid series and tungsten deposits in the Eastern Desert of Egypt. 12 symposium on Precambrian and Development, Cairo, Egypt.
- Rashed MA (2001) Geology, petrology, and uranium potential of G Qash Amir-G. El Sela granitic mases, southern Eastern Desert, Egypt Msc. Thesis. Faculty of science, Mansoura Univ, Egypt.
- Masoud SM (2011) Geochemical characteristics of some per aluminous younger granites masses, Eastern Desert, Egypt. Journal of the faculty of Education. Ain shams university, Egypt.
- World Nuclear Association, 2011a. Sustainable Energy [Online].
- World Nuclear Association, 2013. Nuclear Basics [Online].
- International Energy Association (2011) Clean Energy Progress Report: Update June 2011, OECD/IEA, Paris.
- Chunlin Feng, Xiaowen Zhang, and Wei Huang (2012) “Leaching Characteristics of a Low-Grade Uranium Ore”, Uranium Ming and Metallurgy 31(1): 31-34.
- Xiaobo Wang, Guangyue Li, and Yongming Zhang (2009) Performance of Higher Column Leaching of Ore in Uranium Deposit”, China Mining Magazine 18(2): 72-75.
- Campbell MC (1985) In-place leaching of uranium at Denison Mines Limited. In: IAEA, Development of Projects for the production of uranium concentrates. Vienna, 25-28 November 1985. Vienna: IAEA pp. 151-164.
- Downes KW (1967) Recent developments in the treatment of uranium ores from the Elliot Lake district. In: IAEA, Processing of low-grade uranium ore. Vienna, 27 June -1 July 1966. Vienna: IAEA pp. 79-88.
- Sand W (1993) Controlled microbiological in-situ stope leaching of a sulphidic ore. Applied Microbiology and Biotechnology 40: 421-426.
- Dhawan N, Safarzadeh MS, Miller JD, Moats MS, Rajamani RK (2013) Crushed ore agglomeration and its control for heap leach operations. Miner Eng 41: 53-70.
- Padilla GA, Cisternas LA, Cueto JY (2008) On the optimization of heap leaching. Miner Eng 21(9): 673-678.
- Lunt D, Boshoff P, Boylett M, E1-Ansary Z (2007) Uranium extraction: the key process drivers. The Journal of Southern African Institute of Mining and Metallurgy 107: 419-426.
- IAEA OECD (2006) Nuclear Energy Agency, Uranium 2005, resources, production and demand, Joint Report.
- Júnior OG (1993) Bacterial leaching of uranium ore from Figueira-PR, Brazil, at laboratory and pilot scale. FEMS Microbiol Rev 11(1-3): 237-242.
- Seidel DC (1981) Extracting uranium from its ores. Cycle NMF (Edn.), International Atomic Energy Agency Vienna 23: 24-28.
- Ibrahim TMM (2007) Uranium Potentiality and its extraction from El-Sela shear zone, South Eastern Desert Egypt. J Sci Fac Sci Minufia Univ 21: 1-18.
- Ibrahim TMM (2011) Characterization of a new structural uranium deposit in El-Sela granite, South Eastern Desert, Egypt. J Geol Surv Egypt 31: 405-415.
- Yousef LA, Abdel Ghany MS Afifi SY (2013) Recovery of Uranium (VI) From Treated Technological Sample, El Sela Area, South Eastern Desert, Egypt. Arab Journal of Nuclear Science and Applications 46(3): 40-51.
- Khawassek YM (2012) Production of commercial uranium concentrate from El-Sela Shear Zone Mineralized Ore Material, South Eastern Desert Egypt, at Inshas Pilot Plant Unit. Nuclear Sci Scientific J 3(1): 169-179.
- Mohamed S Nagar, Shahin HA, Bahige M (2016) Column percolation leaching of uranium from El Sela, South Eastern Desert, Egypt. Research & Reviews: Journal of Chemistry 5(4): 32-41.
- Ibrahim ME, Zalata AA, Assaf HS, Ibrahim IH, Rashed MA (2005) El Sella Shear Zone, Southeastern Desert, Egypt; An Example of Vein-Type Uranium Deposit, the 9th international Mining, Petroleum, and Metallurgical Engineering Conference.
- Marczenko Z (1976) Spectrophotometric determination of elements. Ellis Horwood Ltd., Coll House, West ergate, Chichester, Sussex, England.
- Davies W, Gray W (1964) A rapid and specific titrimetric method for the precise determination of uranium using iron (II) sulphate as reductant. Talanta 11(8): 1203-1211.
- El-Agami NL, AbuBaker MA, Ibrahim ME, Rashed MA (1999) Mineralogical and geochemical studies for the mineralization of Halaib area, South Eastern Desert, Egypt. J Geol Sci 43(1): 27-38.
- Heikal MThS, Khedr MZ, Abd El Monsef M, Gomaa SR (2019) Petrogenesis and geodynamic evolution of Neoproterozoic Abu dabbab albite granite, central Eastern Desert of Egypt: petrological and geochemical constraints. J Afr Earth Sci 158: 103518.
- Cambon AR (1994) Uranium deposits in granitic rocks. Notes on the National Training Course on Uranium Geology and Exploration. Organized by International Atomic Energy Agency and Nuclear Material Authority pp. 8-20.
- Edwards CR, Oliver JA (2000) Uranium processing: A review of currents methods and technologies, Journal of Metals 52(9): 12-20.
- Ram R, Charalambous F, Tardio J, Bhargava SK (2011) An investigation on the effect of Fe (FeIII, FeII) and oxidation reduction potential on the dissolution of synthetic uraninite (UO2). Hydrometallurgy 109(1-2): 125-130.
- Ring RJ (1980) Ferric sulfate leaching of some Australian uranium ores. Hydrometallurgy 6(1-2): 89-101.
- Jing Huang, Mi Li, Xiaowen Zhang, Chunmei Huang, Xiaoyan Wu (2017) Extraction of uranium from tailings by sulfuric acid leaching with oxidants. Earth and Environmental Science 69: 012050.
- TIAN J, CHI R, YIN J (2010) Leaching process of rare earths from weathered crust elution-deposited rare earth ore, Transactions of Nonferrous Metals Society of China 20(5): 892-896.
- Levenspel O (2015) Predictive Models of Leaching Processes: A Critical Review, 7th International Conference on Latest Trends in Engineering & Technology (ICLTET'2015), Irene, Pretoria (South Africa).

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...