Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Review Article(ISSN: 2637-4609) 
Association Between Schiffs and Mannich Bases of 5-Substituted-1h Indole-2, 3 Diones and Evaluation of Antiepileptic Activity: Findings from PTZ Model Casual Pathway‐Based Multi‐Site Recruitment Volume 3 - Issue 3
Rahul Hajare*
- Indian Council of Medical Research, India
Received: July 23, 2018; Published: August 01, 2018
*Corresponding author: Rahul Hajare, Department of Quality Assurance, Pune University Pune, India
DOI: 10.32474/AOICS.2018.03.000165
Abstract
We report here novel Schiff bases of 5-substituted-1H Indole-2, 3 Dione comparing modified with aromatic primary amine and hydrazine. A recombinant with Mannich base has been synthesized. A structure of the synthesized compounds was analyzed for structure confirmed by mass spectral data. Compound 1a-p has screened for anti-convulsant activity, increased in latency time and reduction in duration of convulsion and standard drug when compared, sodium valporate 300mg/kg. PTZ dose of 90mg/kg produced severe clonic convulsions in 88% of the mice injected, with a mean onset time of 3.61 ± 0.5 min and 80% mortality. Clonic convulsive activity lasted 2.3±0.45 min and has postictal period of decreased motor activity and subsequently. Test result revealed that compounds at dose of 300 mg/kg, the severity and duration of the convulsive episode following PTZ has not observed.
Keywords: 1H Indole-2, 3 Diones; Schiffs base; Mannich base; Anti-convulsant
Introduction
Epilepsy has accepted group of disorders in which there are recurrent episodes of altered cerebral function associated with paroxysmal excessive & hypersynchronus discharge of cerebral neurons [1]. The clinical accompaniments of those episodes seizures vary in manifestation from brief lapses of awareness to prolonged bouts of unconsciousness incontinence. It has episode of neurological dysfunction a seizure. Isatin (1H-indole-2, 3-dione) and scaffold demonstrated a diverse array of biological activities. It has 5-halo derivatives have reacted to form the Schiff’s base, Mannich bases to form C-N, C=N bonds, from the spectral studies, Isatin undergo reaction at C-3 and N-1 position and showed the anti-convulsant activity.
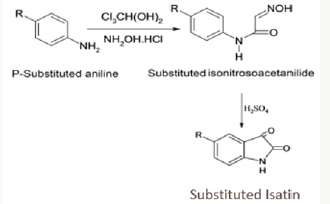
R & D of Iso nitroso acetanilide (Scheme I)
In round bottom flask took 9g (0.05 moles) of chloral hydrate in 86 mL of water added mixture 7g of sodium sulfate and solution of 3.1g (0.03moles) of aniline (4-halo substituted derivatives) in 30mL of water containing 4-5 mL of HCl added (dissolved amines). Finally, the solution of hydroxylamine in 50 mL of water added in the flask. Heat the mixture at 45 0C to dissolved precipitate and then boiled the solution vigorously for 1-2 min. Cooled the solution to room temperature. The precipitate obtained has filtered off. The yield has 80% (Scheme 1).
R & D of 5-Substituted-Indole-2, 3 Dione (Scheme I)
Heat the 3 mL of concentrated Sulphuric-acid at 70 0C. To this added 1 g of iso nitroso acetanilide with vigorous stirring. Heat the mixture to 80 0C for 1-2 min. Cool the mixture at room temperature. Added the 3-4 piece of ice or crushed ice having volume 3-4 times greater than the mixture with vigorous stirring, Yields was 72% melting point of different 5-substituted Isatin. 5-Cl- Isatin- 254-256Co, 5 NO2-Isatin- 180-182 Co, 5-Fl-Isatin-224-226 Co, 5-I-Isatin-280-282 Co, 5-CH3-Isatin- 264-266 Co.
R & D of Schiff’s bases of different 5-substituted 1-H-indole -2, 3-dione (Scheme II) (1a-p)
Equimolar quantities (0.004 mol) of isatin/5-substituted isatin and the aromatic primary amine/hydrazine has dissolved in 10 mL of warm ethanol and heated on a steam bath for 20-40 min. standing for approximately 24 h at room temperature, the crystalline product has separated by filtration, vacuum dried and recrystallized from ethanol (Scheme 2).
Scheme 2: Synthesis of Schiff’ base of different 5-substituted 1-H-Indole -2, 3-dione followed by synthesis of different Mannich base from Schiff’s base.
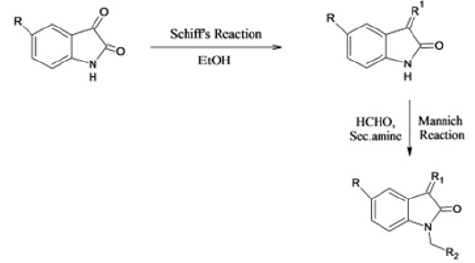
R & D of different Mannich base from Schiff’s base of different 1-H-Indol -2, 3-Dione (Scheme II) (1a-p)
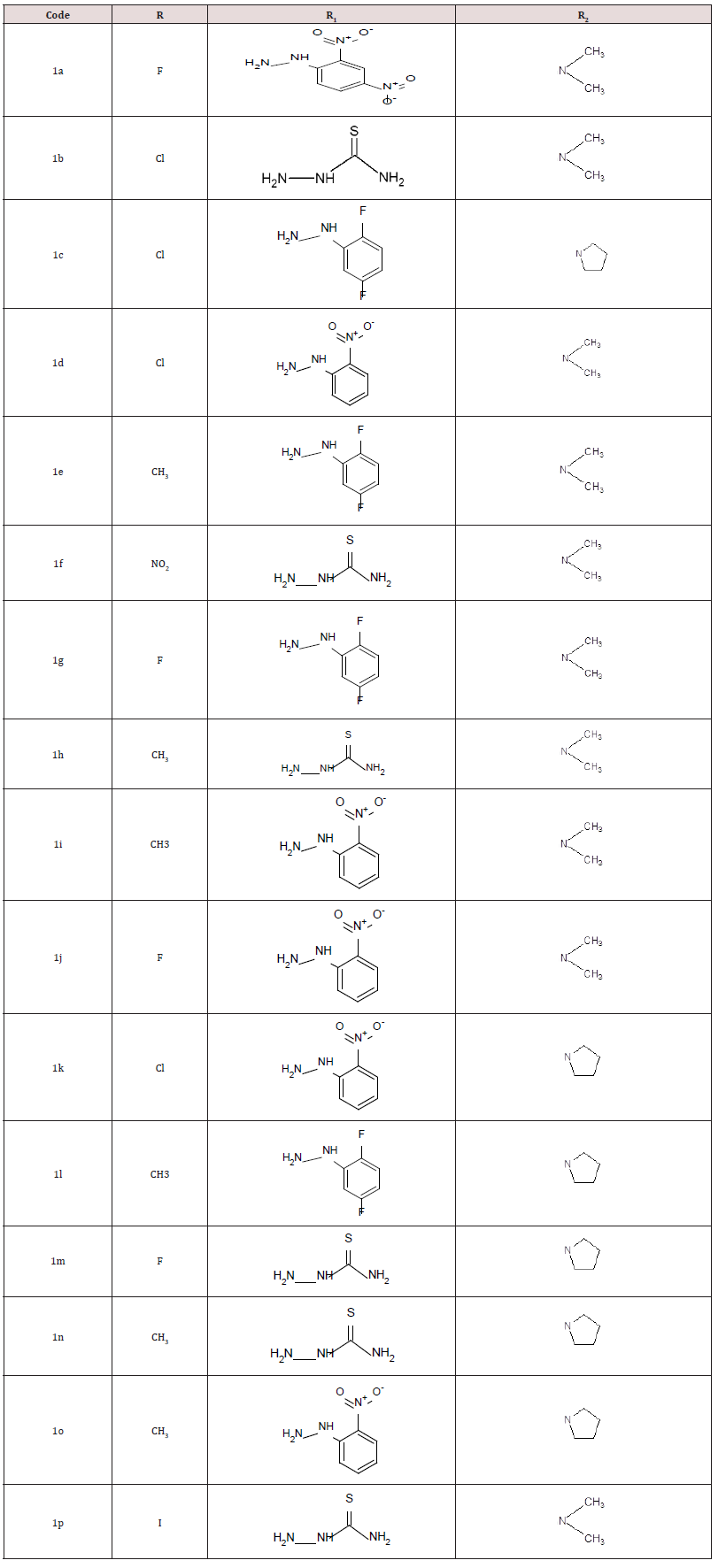
Molar absolute (0.004 mol) of diphenyl amine in 10mL of ethanol has added to slurry containing the appropriate isatin and aqueous formaldehyde solution dissolved in 10mL of ethanol. The reaction mixture has magnetically stirred for 1 h at room temperature and refrigerated for 48h. The product has separated by suction filtration, vacuum dried and recrystallized from ethanol. Final compound with their respective substitution of groups describe in Table 1 and physicochemical data of synthesized more novel 5-substituted 1-H-Indole-2,3-Dione described in Table 2.
a) Solvent for crystallization: ethanol.
b) Melting point of the compounds at decomposition.
c) Solvent system: benzene/methanol (9:1).
Preparation of Solutions
The PTZ solution (90 mg/kg) has prepared in saline solution. Sodium valporate (300mg/kg), has used as standard drug. Various test compounds have suspended in carboxy methyl cellulose (0.5%), suspensions have prepared freshly prior to the dosing.
Selection of Animals
Albino female mice of Wister strain weighing around 25- 35gm has collected and housed at the animal house in groups of six animals per cage at standard laboratory conditions at room temperature with 12:12 h light and dark cycle. All the animals have fed on standard laboratory diet and free access of water. The scientific animal ethical committee approved the present animal protocol [2]. The selected animals have kept in the cages for a week prior to dosing for all the limitations of the laboratory conditions.
Acute Toxicity Studies
LD50 of test compounds have performed as per the O.E.C.D guideline 423. Test compounds have suspended in 0.5% CMC solution. The compounds have administered orally at a dose level of 500,750 1000 and 1500 mg/kg body weight, to groups of 6 animals. After administration of test compounds, the mice have observed for gross behavioral neurological autonomic and toxic effects (3). The toxicological effects have observed in terms of mortality. No death occurred within 24h of dose of 500 and 1000mg/kg but at a dose of 1500mg/kg 50% mortality was observed. As dose was increased further up to 4000mg/kg, total mortality was found. Hence 1000 mg/kg dose was considered as LD50. 1/10th of the LD50 was considered as an effective dose i.e.150 mg/kg.tg
Evaluation of antiepileptic activity (PTZ model)
The compounds synthesized in the present investigation has subjected to pharmacological screening, to ascertain their anticonvulsant activity. The anti-convulsant activity was evaluated by using PTZ induced convulsion mice models [4,5]. In this method convulsion produce by intraperitoneal administration of pentylinetetrazole (PTZ) solution prepared in saline (90mg/ kg, 10mL/kg) was recorded over a period of 30 min, after pentylinetetrazole injection. The mice were treated orally with test (50,100,150,300mg/kg) suspended in 0.5% CMC or standard drug (sodium valporate 300 mg/kg, diazepam 4mg/kg, phenytoin 30mg/ kg), 30 min before i.p. injection of pentylinetetrazole. The time of convulsant recorded and permitted to express the protection of mice. The evaluations of anti-convulsant of the compounds has been listed in Table 3.
Table 3: All the results are expressed as Mean + S.D and were subjected to student t-test using Graph-pad

prism 4 software. Values are considered as significant when P< 0.05.
*P < 0.05 vs. pentylenetetrazole control (PTZ, 90 mg/kg i.p.).
**P < 0.01 vs. pentylenetetrazole control (PTZ, 90 mg/kg i.p.); students t-test
***P < 0.001 vs. pentylenetetrazole control (PTZ, 90 mg/kg i.p.
Statistical Analysis
All the results have expressed as Mean + S.D and has subjected to student t-test using Graph-pad prism 4 software. Values have considered as significant when P< 0.05.
Results and Discussion
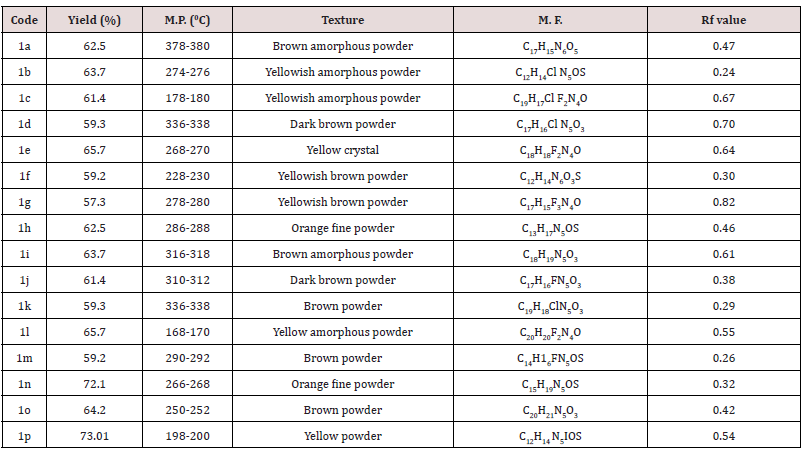
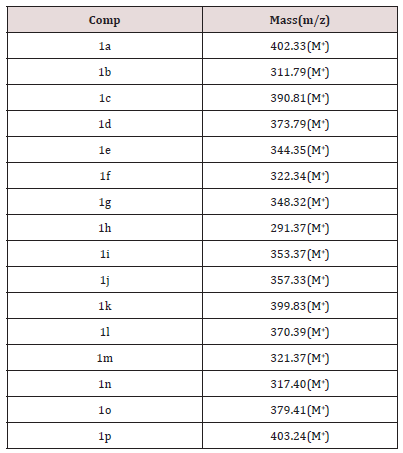
Synthesis of 5-substituted-1H Indole-2, 3 Diones have done by extended Sandmeyer method from Cl, NO2, I, CH3 and F aniline. The synthesized compounds have purified in ethanol. All these spectral analysis data showed the authenticity of the structure of the synthesized compounds. The mass spectra of compounds 1a-p showed molecular ion peaks M+ at m/z corresponding to their respective molecular masses, which agrees with their respective molecular formulas recorded in the Table 4. Anticonvulsant activity of the compounds 1b, 1c, 1f, 1h, 1j, 1l, 1m, 1n, 1o, 1p has examined using PTZ induced convulsion mice models. In this method convulsion produce by intraperitoneal administration of pentylinetetrazole (PTZ) solution prepared in saline (90mg/ kg,10 mL/kg) has recorded over a period of 30 min, after pentylinetetrazole injection. The mice have treated orally with test (50,100,150,300mg/kg) suspended in 0.5% CMC or standard drug (sod. valporate 300 mg/kg), 30 min before i.p. injection of pentylinetetrazole. The time of convulsant recorded and permitted to express the protection of mice. The evaluations of anti-convulsant of the compounds have listed in Table 5.
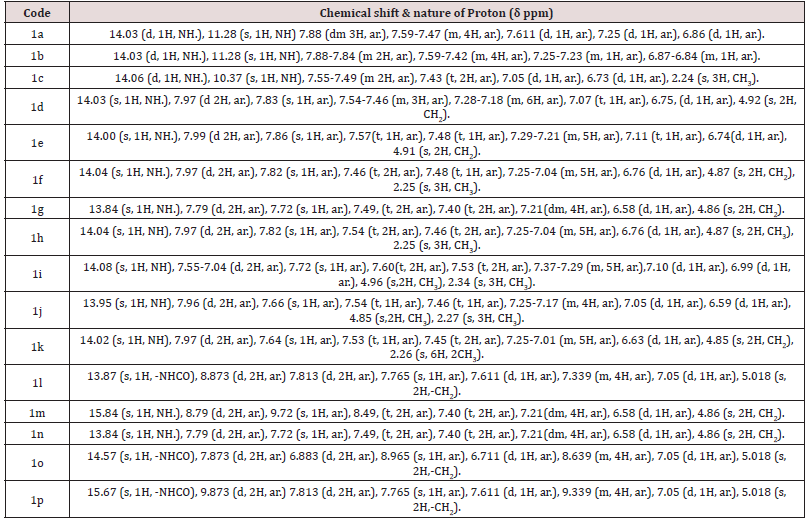
Table 4: Spectral characterization of synthesized different 5-substituted 1-H-indole-2,3- dione. 1HNMR Spectral characterization of synthesized different 5-substituted 1-H-indole-2,3- dione.
Conclusion
It can be concluded that the Schiff and N-Mannich base of 5-substituted-1H Indole-2, 3 Diones has synthesized easily. The pharmacological screening has done by pentylenetetrazole (PTZ) induced convulsion method, for this Sodium. Valporate (300 mg/ kg) has used as reference standard. Test compound has given to mice in different doses (50,100,150,300 mg/kg). The result suggested that all the compounds possess good anti-convulsant activity with increased in latency time and reduction in duration of convulsion as like the standard drug when compared Table 3. All tested compound shows moderate effect at 150 mg/kg dose and significantly effective at 300mg/kg dose. It concluded that the synthesized Schiff and N-Mannich isatin derivatives have shown excellent anti-convulsant activity.
References
- Verma M, Pandeya S, Singh k, StableS J (2004) Anticonvulsant activity of Schiff bases of isatin derivatives, Acta Pharm 54(1): 49-56.
- Lowenstein H (2001) Seizures and Epilepsy in Harrison’s principles of Internal medicine 15th Edn 2: 2354-2368.
- Losher W (1993) Sdimdt D: Central & Peripheral nervous system: New drugs for treatment of epilepsy curr Opin Invest Drugs 2: 1067-1095.
- Pajouhesh H, Parson R, POPP FD 72 (1983) Potential anticonvulsants VI: Condensation of isatin with cyclohexanone and other cyclic ketones, J Pharm Sci 72(3): 318-321.
- N Karali, Singh K (1994) Synthesis and anticonvulsant activity of some new thio semi carbazone and 4-thiazolidine derivatives bearing an isatin moiety, Farmaco 49(12): 819-822.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...