Lupine Publishers Group
Lupine Publishers
Research ArticleOpen Access
Advanced Oxidation Processes (AOPs) by Bismuth Oxyhalides for Environmental Remediation Using Solar Energy: Enhancing Material Property through Synthesis Volume 2 - Issue 2
ID Sharma1,2*, Chander Kant1, AK Sharma1 and KK Saini1*
- 1CSIR- National Physical Laboratory, India
- 2VSSI College, India
Received: March 01, 2018; Published: March 15, 2018
*Corresponding author: KK Saini, I D Sharma; CSIR- National Physical Laboratory, New Delhi-110012, India
DOI: 10.32474/AOICS.2018.02.000131
Abstract
To combat air and water pollution with green chemistry is one of the major issues. Several research groups, worldwide, are today engaged in the design and development of new photocatalytic materials which can act to make air and water free from harmful species with the use of freely available solar energy. These strategies have been briefly addressed here. We have also reported the synthesis of Bismuth oxyhalides BiOX(X=Cl, Br, I) by wt chemical method using Bismuth nitrate (Bi (NO3)3.5H2O) and respective potassium halides (KCl, KBr, KI) as precursor materials and mixture of deionized water and ethanol as solvent. Solvent composition is found to affect the photo activity of the three materials viz; BiOCl, BiOI and BiOBr differently.
Synthesized samples were characterized by X-Ray diffraction (XRD), UV Visible absorption and HRSEM techniques. Photo degradation ability of the synthesized samples was ascertained by studying the degradation of methyl Orange (MO) in aqueous solution under UV light and solar light exposure environments. It is observed that the material crystallizes in the form of thin plates. BiOCl and BiOBrare found to exhibit excellent photo- degradation ability in the normal sun light exposure. Role of solvent composition on different oxyhalides have been attributed to the synthesis environment provided by solvent, which affect the crystal facet exposure.
Introduction
Use of chemicals for various purposes and rapid industrialization for advancement of society release a variety of chemicals into the atmosphere due to which the vital resources of life on our planet earth get polluted. Persistent environmental pollutants and water pollution have attracted world-wide attention. Solar light can greatly contribute to the remediation of these environmental pollutants - from the partial decomposition to the complete destruction. This solar remediation action works either through direct photolysis where photo degradation is achieved through chemical bond cleavage by light, leading to the formation of smaller compounds or by the use of photo catalyst where photon triggers the processes of photocatalytic degradation through advanced oxidation processes. Advanced Oxidation Processes (AOP) use the highly oxidant and non selective hydroxyl radicals, which are able to react with almost all classes of organic compounds leading to their complete mineralization or to the formation of more biodegradable intermediates. Hydroxyl radicals are able to attack and destroy even the most persistent organic molecules that are not oxidized by the oxidants as oxygen, ozone or chlorine [1]. This method can be applied to a large set of different matrices, decontamination occurs through pollutants degradation instead of their simple phase transfer. AOP become even more attractive when they use the sunlight as source of energy. Such photocatalytic processes can proceed either through heterogeneous photo catalysis or homogeneous photo catalysis.
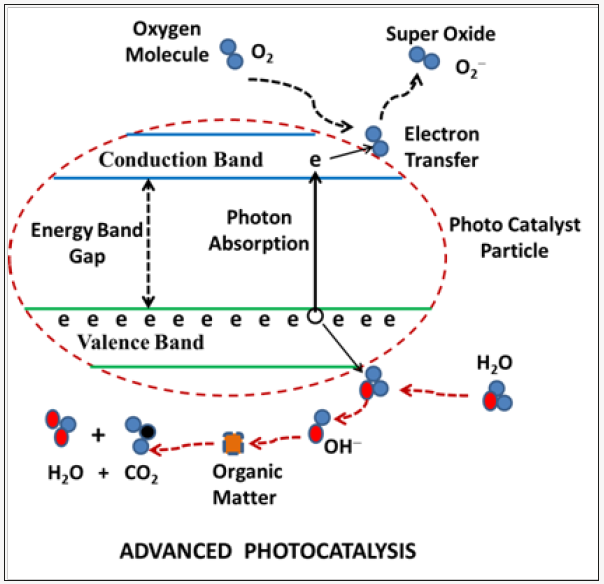
Heterogeneous photo catalysis produces both reducing and oxidizing species to promote mineralization of organic pollutants, using a semiconductor (TiO2, ZnO, etc) as photo catalyst. The photon absorbed by the catalyst produces an electron/hole pair in conduction/valence band as shown schematically in (figure 1). Excited electrons are transferred to chemicals (to be degraded) into the semiconductor particle environment (leading to its reduction) and at the same time the catalyst accepts electrons of oxidized specie by the valance band hole. The net flux of electrons in both directions is null and the catalyst stays unaltered. Detailed mechanism and the electron/hole generation processes of heterogeneous photo catalysis is addressed in several reviews [1-5].
Figure 1: Schematic depiction of advanced photocatalytic process on the surface of semiconductor particle.
Homogeneous photo catalysis - photo-Fenton process is extremely useful source of hydroxyl radicals and a potent oxidant of organic compounds. An aqueous hydrogen peroxide solution and Fe2+ (ferrous) ions, in acidic conditions (pH = 2-4), generate hydroxyl radicals in absence of light (Fe2+ + H2O2→Fe3+ + OH- +OH). When Fenton process occurs under light irradiation (λ≤580nm), degradation rate increases significantly by production of additional hydroxyl radicals and leads to the photo catalyst reduction by light [6,7]. This process allows more efficient use of sunlight as compared to TiO2 heterogeneous photo catalysis. The use of sunlight instead of artificial light for photo-Fenton activation significantly decreases the cost of treatment. Requirement of low pH, high consumption of hydrogen peroxide and the need of removing iron at the end of the treatment are disadvantages of this process. Yet this process is useful for industrial implementation.
Photo catalysis is an emerging technology that enables a wide variety of applications, including degradation of organics and dyes, antibacterial action, and fuel generation through water splitting and carbon dioxide reduction. Number of inorganic semiconducting materials have been explored as photo catalysts, in recent years [8,9]. Understanding the relationship between the physicochemical properties; the fundamentals in catalytic processes is important to design and synthesize useful photocatalytic materials [10,11]. Environmental problems and water pollution currently attract world-wide attention [12-14] and stress to develop highly active photo catalysts for degradation of pollutants, antibacterial action and fuel generation. However, most of the research has focused on the photocatalytic degradation of organic pollutants, with limited studies investigating the photocatalytic inactivation of microorganisms [15-17]. Photo catalysts are believed to affect cells also by generating reactive oxygen species (ROS), especially hydroxyl radicals (HO•) [18]. These ROS can result in lipid peroxidation, and damage to membrane integrity, DNA and proteins, subsequently leading to cell death [19]. However direct excitation of DNA by short UV radiation (UVC, UVB) can also result in cell death [20,21] but indirect mechanisms can do this at longer wavelengths (UVA, visible light).
Titanium dioxide (TiO2) has been extensively studied and utilized to degrade organic pollutants, H2 evolution and CO2 reduction since 1972, due to its chemical stability, non toxicity and low cost [22-24]. However, TiO2 has wide band gap energy (3.2 and 3.0 eV for anatase and rutile phases, respectively) [25] due to which TiO2 is only responsive to the UV region of the solar spectrum (~ 5-7%). Strategies have been developed to improve the photocatalytic performance of TiO2 for various applications [26].
In order to make full use of the richness of solar light, development of efficient optical photo catalysts is imperative. To date, many efficient photo catalysts have been explored, that may overcome limitations of TiO2. Layered materials viz; hexagonal boron nitride, layered double hydroxides perovskites, atomically thick graphene, transition metal dichalcogenides are at the center stage of these investigations due to their novel structure which provides enough room to tailor the desired property. Bismuth oxyhalides (BiOX) has received persistent attention because of their ability to remove contaminants from aquatic environments and other favorable properties from application point of view; e.g. high chemical and optical stability, non toxicity, low cost, and corrosion resistance. The BiOX (X = Cl, Br, I) are important V–VI–VII ternary compounds in a layered structure comprising of [Bi2O2]2+ slabs interleaved with double slabs of halogen atoms [27].
Internal static electric field, perpendicular to the layers, is generated inside the material, which ensures effective separation of photo-generated electron-hole pairs to reduce recombination losses. Valence band upper states, in these materials, are constituted by the p-orbitals of oxygen and respective halogen atoms, whereas 6p orbitals of bismuth constitute the conduction band lower states. Contribution of halogen atom p-states increases with increase in the element atomic number and so the dispersive characteristics of band energy level, thereby narrowing the energy band gap between top of the valence band and bottom of the conduction band. Therefore the band gap values and redox potentials of BiOX are highly associated with the atomic numbers of halogen, i.e. composition of the layered structure. Experimental studies and theoretical calculations have shown energy band gaps close to 3.4, 2.8 and 1.8 eV for BiOCl, BiOBr and BiOI respectively. Degradation of air or water pollutants by BiOCl with high throughputs have been reported, but under UV exposure. We have synthesized BiOX (X=Cl,Br,I) using mixture of deionized water and ethanol as solvent to achieve degradation performance like BiOCl under visible light exposure. We report our results in this communication. It is evident that the tunable layered structures endowed bismuth oxyhalides materials with excellent optical absorption ranging from UV to visible light, hence suggest that they have great potential for photocatalytic degradation under natural sunlight.
Experimental Details
Bismuth oxyhalides BiOX(X=Cl,Br,I) samples have been synthesized by wet chemical route using bismuth nitrate Bi(NO3)3•5H2O and potassium halides(KCl,KBr&KI) as precursors. Analytical grade procured from Merck India Ltd have been used in all synthesis and these were used as procured i.e. without further purification. To synthesize BiOCl; Bismuth nitrate and potassium chloride were taken in equimolar ratio. Both the salts were dissolved separately in mixture of deionized water (s≤10- 8 Ω-cm-1) and ethanol at room temperature. Different solvent mixtures containing water and ethanol in the ratio 20:80, 50:50 and 80:20 were prepared and used as solvent for bismuth nitrate and potassium halides. Complete dissolution of the material is achieved at 10% w/v concentration of solute in the solution. Mixing was done by slowly adding KCl solution to bismuth nitrate solution taken in conical flask and with constant stirring using magnetic stirrer. Potassium chloride solution was taken in a burette and fixed such as to drop the solution slowly into the conical flask. Flow rate of KCl solution was maintained at ~2.5ml/min-1 while stirring was going on using Teflon coated bit. We have maintained pH of the mixture at 2.0by adding ammonia solution (NH3.H2O). After adding whole KCl solution, stirring was further continued for 6, to ensure completion of the precipitation reaction for bismuth oxychloride. Three samples of BiOCl were synthesized with three different compositions of water & ethanol mixture (water: ethanol as 20:80, 50:50 and 80:20 v/v) as solvent. Resulting solid materials was collected by filtration on What man No. 1 filter paper and washed several times with distilled water for complete removal of undesired water soluble products. Solid compound so obtained is subsequently dried at 80 °C for 6 hours in an electric oven under normal atmospheric conditions. Three samples each of BiOBr and BiOI were also synthesized in the same manner where halogen salts were KBr and KI,
Structural and Basic Characterization
Structural analysis of the synthesized material was done on Bruker D8 Advance X-ray diffractometer which uses Cu Kα radiations (λ = 1.5418Å) to probe the sample. X-ray diffraction (XRD) spectra were recorded for 2θ values between 10-80° at a scan rate of 2 degrees per minutes. Average value of the crystallite size, in different samples, was estimated from strongest XRD peak using Scherrer relation (D = Kλ/d.sinθ; where k is constant, λ is wavelength of x-rays, d is width of the selected x-ray peak at half maxima (radians) and θ represents position of the selected peak).Photo activity of different samples was estimated from the degradation kinetics of methylene blue in aqueous solution by recording visible light absorption spectra, of the sample, with UVvisible spectrometer (SHIMADZU UV-1601) at room temperature after different durations of light exposure on the sample. Selected area scanning electron microscopy (SEM) studies are carried out on EVO-50 (ZEISS) scanning electron microscope, equipped with an energy-dispersive X-ray spectroscopy (EDX).
Photocatalytic Activity Measurements
Photocatalytic activity of the synthesized samples was evaluated by studying degradation kinetics of Methyl Orange (MO) in aqueous solution (0.02mM) under natural sunlight and UV light from mercury vapor lamp. 50mg of the synthesized samples (BiOCl, BiOBr or BiOI) was suspended in 200ml of aqueous MO solution taken in quartz container and exposed to light source from bottom. Solution was stirred constantly by bubbling dry nitrogen through the solution. About 2ml of MO solution was taken out from the quartz reactor after 30minutes, one hour, two hour, three hours and four hours of exposure durations. Amount of Methyl Orange (MO) present in the aqueous solution after the above exposure durations, was estimated by recording the absorption spectra of the extracted solution in visible range (400-800nm). Area under the absorption peak at 464nm wavelength was related to the amount of MO present in the solution. Kinetic constant for the dye degradation was calculated by assuming pseudo first order mechanism for the degradation reaction as per relation C = C0exp (- kt). Where C0 and C are concentrations of MO in the solution before exposure & after exposure for time duration‘t’ respectively and ‘k’ is rate constant for the degradation reaction.
Results and Discussion
Figure 2: X-ray diffraction Pattern of BiOCl, BiOBr & BiOI Samples synthesized with equivolume solvent ratio. (Water: ethanol: 50:50).
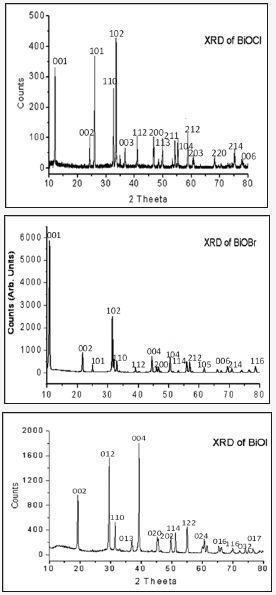
Figure 3: High resolution scanning electron micrographs (HRSEM) micrographs of the three samples BiOCl, BiOI & BiOBr synthesized using solvent with water to ethanol ratio in equal volumes.
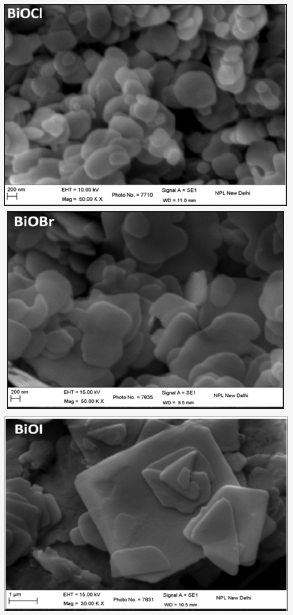
High resolution scanning electron microscopy micrographs (HRSEM) shown in (Figure 3), confirms the formation of welldefined platelet structure with varying plate sizes in all the three synthesized material samples. In BiOCl and BiOBr the plate size are very small - lateral dimensions in the range 200-400nm and thickness around 10nm or even less. BiOI sample crystallizes in thick and large size plates in comparison to the other two materials. This sample has plates with lateral dimension more than a micrometer with thickness more than 100nm.
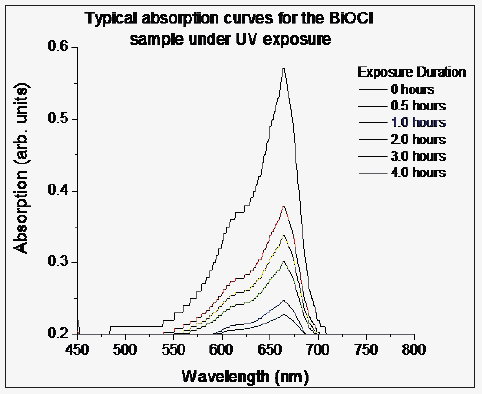
We assessed the photo degradation ability of the three materials synthesized using three different solvents (9 samples) by degradation of methyl orange in aqueous solution under UV exposure. UV light from mercury vapor lamp was allowed to excite the sample suspended in methyl orange solution taken in quartz container, from the bottom of the container. Solution was stirred by bubbling dry argon gas through the solution. 2ml sample of the solution was extracted after 0.5, 1.0, 2.0, 3.0 and 4.0 hours of UV exposure. Absorption spectra of these solutions were recorded in visible range (400-800nm). Representative absorption curves for BiOCl sample are shown in (Figure 4). The absorption peak intensity and therefore area under the curve decreases with increase in exposure duration and complete degradation of methyl orange in the solution has been observed after four hours of exposure.
Figure 4: Typical absorption curves of aqueous methyl orange (MO) solution exposed to UV radiations from mercury vapor lamp for different durations, in presence of BiOCl sample.
Plot for the degradation reaction kinetics, as shown in (Figure 5), was obtained from the absorption curves for all the samples synthesized using different solvent compositions. We have observed that BiOCl and BiOI exhibits maximum degradation kinetics if synthesized from water and ethanol in equal volumes as the solvent. Decreased photo degradation ability, of these samples was observed when synthesized with other two compositions of the solvent (i.e. water: ethanol: 80:20 & 20:80). In the case of BiOCl and BiOI samples the following sequence of photo degradation ability of the sample with solvent, is observed.
Figure 5: Kinetic degradation plots for BiOCl, BiOBr and BiOI samples synthesized using the three different ratios of water and ethanol in the solvent as water: ethanol: 50:50, 20:80 and 80:20.
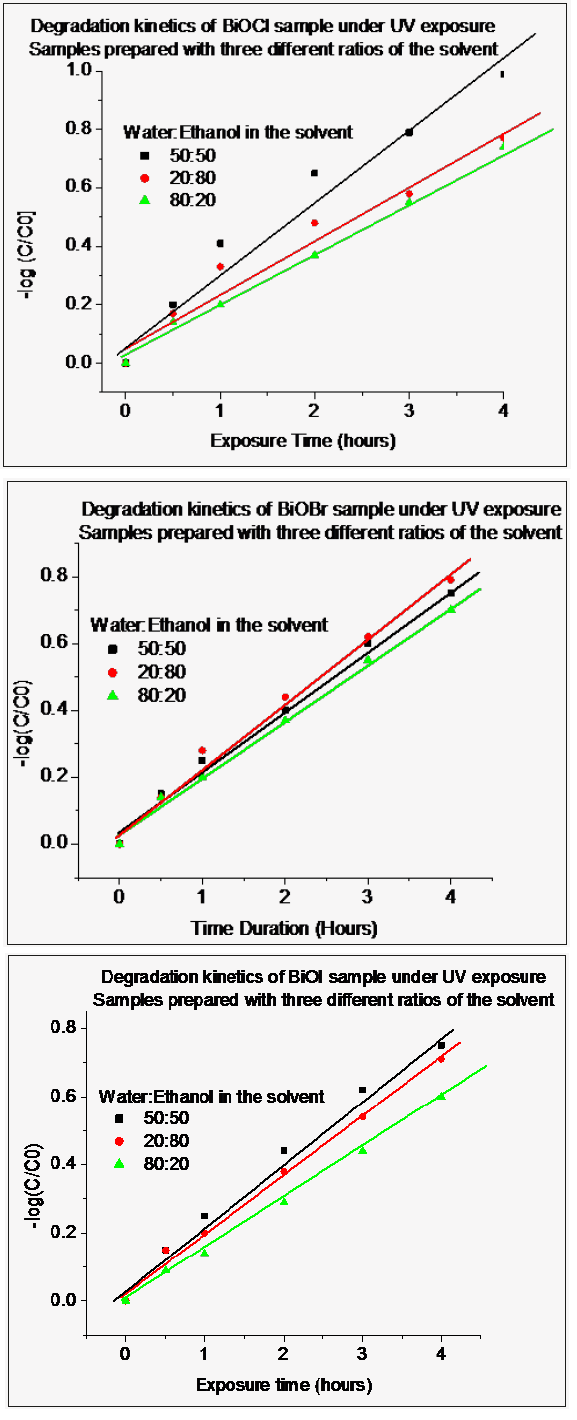
Water: ethanol: 50:50 (highest) >Water: ethanol: 20:80>Water: ethanol: 80:20 (lowest).
BiOBr sample exhibited different photo degradation trends; highest photo degradation ability is exhibited by the samples in which water and ethanol was taken in 20: 80 volume ratio, followed by the sample synthesized by taking water and ethanol in equivolume ratio as solvent. Least photo activity was observed in the BiOBr sample synthesized by taking water and ethanol volumes ratio as 80:20.BiOCl material was found to exhibit more photo degradation ability for methyl orange in aqueous solution under UV light exposure as compared to BiOI or BiOBr.
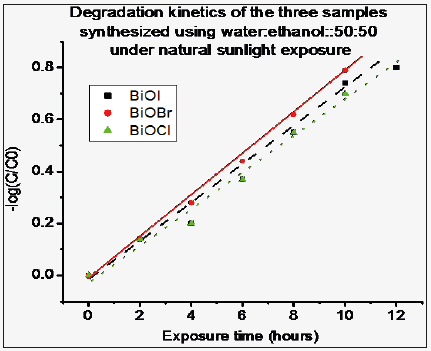
Kinetic studies for the degradation of methyl orange under natural solar exposure, by the three samples (BiOCl, BiOBr & BiOI) synthesized with solvent composition 50:50 are presented in Figure 6. However the value of kinetic constant is much less than that under UV exposure, but it is worth noting that BiOCl which have reported value of band gap as 3.52eV exhibits good degradation ability, comparable to the other two samples, under solar exposure (visible light) also.
Figure 6: Kinetic degradation plots for typical BiOCl, BiOBr and BiOI samples under natural sunlight exposure. These samples are with solvent as Water: Ethanol: 50:50.
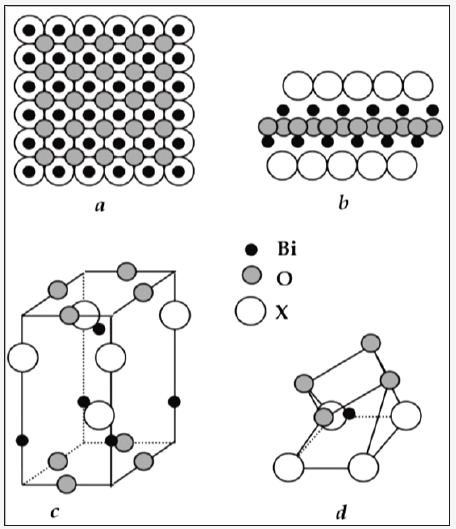
Single crystalline BiOX material has a layered 2D structure derived from the calcium fluorite (CaF2) structure as shown in Figure 7. The Bi atom is coordinated to a square anti prism with four O atoms in one base and four X (halogen) atoms in another. The O atom is tetrahedrally coordinated to four Bi atoms. The X atom is bonded with four Bi atoms in a planar square to form a pyramid and with its nonbonding (lone pair) electrons pointing to the other side of the square. These nonbonding electrons convert the three dimensional fluorite like structure into a two dimensional layered structure. In the three dimensional Bi2O3, the Bi atoms are coordinated to six O atoms. This structural difference between them is the major reason for BiOCl to have a wider optical band gap. The BiOX layers are stacked together by the Van der Waals interaction through the X atoms along the c-axis. Therefore, the structure is not closely packed in this direction. When one photon excites one electron from Cl 3p states to Bi6p states in BiOCl, One electron-hole pair (e-—h+ pair) is generated. The layered structure of BiOX can provide the space large enough to polarize the related atoms and orbitals. The induced dipole can separate the hole-electron pair efficiently, diminishing the probability of recombination, thereby enhancing photocatalytic activities. Also BiOX has an indirect transition band gap so that excited electron has to travel certain k space distance to be emitted to the valence band (VB). This k-space varies with X (X=Cl, Br, I), which further reduces the recombination probability of the excited electron and the hole especially in BiOCl. The energy band gap and variation in k-space explains variation in photo-activities of the three BiOX materials under identical irradiation conditions. The BiOX single crystals can be described as 2D structured materials. The open structure i.e. loose packed and indirect transition may benefit the hole-electron separation and the charge transport. Both these features are favorable to the photocatalytic reactions [28-34].
Figure 7: Different crystal structures of bismuth oxyhalides: (a) projection to (001) plane; (b) projection to (100) or (101) plane; (c) clinographic projection of the unit cell; (d) coordination polyhedron of the bismuth.
At first hand one may assume variation in crystallite size may result by using different solvent compositions in the synthesis of different samples of same oxyhalide which may be the reason for observed difference in photo activities by change in solvent compositions, but present HRSEM investigations do not support this. Recently morphology and photo-activity of BiOBr with a predominance of either {001} or {010} exposed crystal facets have been investigated [35,36]. Both these point out the importance exposed facet in enhancing the photo activity of the material but they don’t have consensus on facet. First group [35] have concluded that BiOBr with <010> exposed facet displayed a better photooxidative capability than BOBr with <001> exposed facet for both water oxidation and formic acid degradation (aqueous phase). The higher photo-oxidative capability of BiOBr<010> was attributed to the suppression of photo-induced electron/hole recombination. Additionally, the improved charge transfer efficiency and reduced charge transfer resistance in BiOBr<010> was revealed to be beneficial for enhancing photo electrochemical (PEC) performance. Second group [36] has concluded that it is the <110> facet of BiOBr which exhibit highest photo activity. These findings illustrate the use of crystal facet engineering to promote photocatalytic performance. Different solvent compositions used in the present synthesis provide different crystal growth environments to the BiOX samples. It is quite likely that this may result in different facets are favored to be exposed by particular solvent composition, which may explain the difference in photo-activity of a particular BiOX synthesized using different solvent compositions.
Conclusion
Present studies were aimed at to understand the role of water in ethanol used as solvent in the synthesis of three bismuth oxyhalides viz; BiOCl, BiOBr, and BiOI. It has been observed that different bismuth oxyhalides are affected differently by the water contents in ethanol. BiOCl compound exhibits best performance if it is synthesized with equi-volume ratio of water and ethanol as solvent for precursor materials. The other two compounds viz; BiOI & BiOBr, exhibit a trend that lesser the water, better is the compound with respect to its photo-activity. This trend is very clear in BiOBr but in case of BiOI there is little variation in the photo-activities of 50:50 & 20:80 (water: ethanol) composition. It will be too early to give much weight age to this small variation. The role of solvent in affecting the photo activity of sample is assigned to the exposure facet of the crystal favored by the solvent composition during synthesis. Another important conclusion of these studies is that we can get a BiOCl sample which exhibits good photo degradation ability for organic matter under natural sunlight exposure despite being a wide band gap semiconductor (~3.5eV). Looking at this observation, the bismuth oxyhalides can play an important role to combat environmental pollution using freely available energy from the Sun.
References
- Eckenfelder WW (2000) Industrial Water Pollution Control (3rd edn. McGraw Hill), ISBN 0-07-116275-5 Boston, EUA.
- Davis ML, Cornwell DA (1998) Introduction to Environmental Engineering (3rd ed. McGraw Hill), ISBN 0-07-115234-2, Nova York, USA.
- Kiely G (1998) Environmental Engineering (international edition, McGraw Hill), ISBN 0-07-116424-3, Boston, EUA.
- Metcalf, Eddy (2003) Wastewater Engineering Treatment and Reuse (4th ed. McGraw Hill), ISBN 0-07-124140X, Boston, EUA.
- Nevers N (2000) Air Pollution Control Engineering (2nd edn. McGraw Hill), New York, USA.
- Nogueira RFP, Trovo AG, da Silva MRA, Química Nova (2007) 30(2): 400- 408.
- Pignatello JJ, Oliveros E, MacKay (2006) A Critical Reviews in Environmental Science and Technology 36(1): 1-84.
- Chen X, Shen S, Guo L, Mao SS (2010) Semiconductor-based Photocatalytic Hydrogen Generation. Chem Rev 110(11): 6503-70.
- Mao SS, Shen S (2013) Nat Photonics 7: 944-946.
- Shen S, Mao SS (2012) Nanophotonics 1: 31-50.
- Shen S, Chen J, Cai L, Ren F, Guo L (2015) J Materiomics 1: 134-145.
- M Kapilashrami, Y Zhang, YS Liu, A Hagfeldt, J Guo (2014) Chem. Rev., 114, 9662-9707.
- L Kong, C Wang, H Zheng, X Zhang ,Y Liu (2015) J Phys Chem C 119: 16623-16632.
- N Becknell, Y Kang, C Chen, J Resasco, N Kornienko, et al. (2015) J Am Chem Soc 137: 15817-15824.
- Ren J, W Wang, L Zhang, J Chang, S Hu C et al. (2009) Commun10: 1940- 1943.
- Zhang LS, KH Wong, HY Yip, C Hu, JC Yu, et al. (2010) Environ Sci Technol. 44, 1392-1398.
- Wang WJ, Y Yu, TC An, GY Li, HY Yip, et al. (2012) Environ Sci Technol 46: 4599-4606.
- Malato S, P Fernandez-Ibanez, MI Maldonado, J Blanco, W Gernjak (2009) Catal Today 147: 1-59.
- Avery, SV Biochem J (2011) 434: 201-210.
- Cockell CS (1998) J Theor Biol 193: 717-729.
- He YY, DP Hader Photochem (2002) Photobiol Sci 1: 729-736.
- KS Yang, Y Dai, BB Huang (2007) J Phys Chem C 111: 18985-18994.
- C Kang, L Jing, T Guo, H Cui, J Zhou, et al. (2009) J Phys Chem C 113: 1006-1013.
- WQ Fan, XQ Yu, SY Song, HY Bai, C Zhang, et al. (2014) Cryst Eng Comm 16: 820-825.
- H Cheng, B Huang, Y Dai (2014) Nanoscale 6: 2009-2026.
- Shaohua Shen, Coleman Kronawitter, George Kiriakidis (2017) J Materiomics 3: 1-2.
- Fen Qiang Ma, Jing Wen Yao, Yan Feng Zhang, Yu Wei (2017) RSC Adv 7: 36288.
- SJ Peng, LL Li, PN Zhu, YZ Wu, M Srinivasan, et al. (2013) Chem Asian J 8: 258.
- Hong Deng, Junwei Wang, Qing Peng, Xun Wang, Yadong Li (2005) Chem Eur J 11: 6519.
- FA Bannister (1935) The Mineralogical Magazine and Journal of the Mineralogical Society. XXIV (149): 49.
- J Wang, Y Li (2003) Chem Commun 18: 2320.
- Keramidas KG, Voantsas GP, Rentzeperis PI, Kristalogr Z (1993) 205: 35.
- KL Zhang, CM Liu, FQ Huang, C Zheng, WD Wang (2013) Applied Catalysis B: Environmental 68: 125.
- K Zhao, L Zhang, J Wang, Q Li, W He, et al. (2013) Surface Structure- Dependent Molecular Oxygen Activation of BiOCl Single-Crystalline Nanosheets. American Chemical Society 135(42): 15750-15753.
- Xuelian Wu, Yun Hau Ng, Liang Wang, Yi Du, Shi Xue Dou, et al. (2017) J Mater Chem A 5: 8117-8124.
- Han AJ, Zhang HW, Chuah GK, Jaenicke S (2017) Applied Catalysis B: Environmental 219: 269-275.












