Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
A Novel 5-Bromoindolehydrazone Anchored Diiodo Salicylaldehyde Derivative as A Multifunctional Probe for Selective and Sensitive Detection of Tryptamine and F‒ Ions Volume 5 - Issue 3
Krishnaveni Karuppiah, Murugesan Sepperumal and Siva Ayyanar*
- Department of Inorganic Chemistry, School of Chemistry, Madurai Kamaraj University, India
Received:June 5, 2021 Published:July 07, 2021
*Corresponding author:Siva Ayyanar, Supramolecular and Organometallic Chemistry Laboratory, Department of Inorganic Chemistry, School of Chemistry, Madurai Kamaraj University, India
DOI: 10.32474/AOICS.2021.05.000213
Abstract
A new indole based chemosensor, 2-((5-bromo-1H-indol-2-yl) methylene) hydrazono) methyl)-4, 6-diiodophenol (BHDL) has been developed for the selective and sensitive detection of biogenic tryptamine and F‒ ions. The binding affinity of probe BHDL towards F‒/tryptamine (TryptA) has been investigated by UV-visible/fluorescence spectroscopy. In the presence of TryptA, probe exhibits strong enhancement in an emission band at 433 nm and the band at 555 nm underwent a blue shift accompanied by a decrease in intensity by the inhibition of Excited State Intramolecular Proton Transfer (ESIPT) functioned on BHDL. Interestingly, complexation with F‒ ions as well triggers an enhancement in a fluorescence band at 430 nm with the concomitant disappearance of the emission band at 555 nm due to the inhibition of ESIPT and deprotonation process initiated by the hydrogen bonding complex formation. Further, Density Functional Theoretical (DFT) calculations have been performed to support the mechanism functioned on the probe BHDL in the presence of TryptA/F‒.
Keywords:Chemosensor; BHDL; ESIPT; Tryptamine; Fluoride ion
Introduction
Fluorescent detection of biological and environmentally important species has been a recent area of focus due to its potential application in pharmacology, physiology, and environmental production [1,2]. Initially, several fluorescent sensors have been developed [3,4] merely for the detection of metal ions because of the selective binding ability of metal ions in water. Over the past decades, detection of anions and neutral analytes using fluorescent molecular system have witnessed an explosive growth in par with cation sensing. In particular, the design of multifunctional chemosensors has gradually become a new research focus in which a single receptor can independently recognize two or more guest species with diverse spectral responses via same or different channels. Among various ions, fluoride, being a strong electronegative element can interfere with the number of enzyme systems and thus acts as an enzyme inhibitor. It is present in biological fluids tissues, especially in bone and tooth [5]. The effect of association between the elevated levels of fluoride in drinking water and skeletal fluorosis has been well documented [6-8]. On the other hand, fluoride can have a beneficial effect on human health by treating osteoporosis and protecting dental health [9-13]. Thus, the detection of F- is essential to minimize its direct effects on human health. Biogenic amines are nitrogenous organic bases or deaminated derivative of amino acids. The natural resource of biogenic amines are grapes, food, and beverages, they can be synthesized by fermentation or microbial decarboxylation of amino acids [14] and a major important component in the production of hormones, alkaloids, nucleic acids and proteins. Among various biogenic amines, [15-22] tryptamine (TryptA) is a natural derivative can influence multiple processes within the central nervous system such as sleep, cognition, memory, temperature regulation and behavior [23,24]. Hence the detection and quantification of TryptA is of particular importance as it possess a strong pharmacological effect. Among various traditional analytical methods, detection based on fluorescent sensors stands as an effective tool to explore ions and neutral species owing to their simplicity, high selectivity, sensitivity, and versatility [25-33]. Moreover, recognition of miscellaneous targets with a single receptor would lead to a faster analytical processing and potential cost reductions. It is therefore highly desirable to develop a sensor that selectively recognize both TryptA and F‒ over other competing anions/biogenic amines through different optical modes. Being the core structure of the great number of inhibitor [34,35] and drug molecules [36,37] indoles have attracted a great deal of attention amid the scientific community due to their significant role in biological activity. Most of the indole derivatives are fluorescent and this property has been successively utilized for the design of indole-based receptors for selective chemosensing of metal ions and anions [38-44]. Herein, we have synthesized a new indole based dual mode chemosensor BHDL by simple condensation method and it has been utilized for the selective and sensitive detection of TryptA and F‒ in DMSO/H2O (9:1, v/v) medium. The probe BHDL displays excellent ratiometric responses in absorbance and strong “turn-on” fluorescence responses upon addition of both TryptA/ F‒ ions, respectively.
Experimental Section
Materials and Methods
All starting materials and reagents were purchased from Alfa Aesar, Sigma Aldrich, and used as received without further purification. The anions of Cl‒, Br‒, I‒, CN‒, H2PO4‒, HSO4‒, NO3‒, AcO‒ and F‒ were purchased as their tetrabutylammonium salts from Sigma-Aldrich Pvt. Ltd. Different category of amines like azane, methylamine, dimethylamine, trimethylamine, phenylethylamine, spermidine, spermine, tyramine, cadaverine, serotonin, histamine, tryptamine, L-tryptophan, dopamine, putrescine, D-glucosamine, glycine were purchased from Sigma-Aldrich Pvt. Ltd. Silica gel-G plates (Merck) were used for Thin-layer Chromatography (TLC) analysis with a mixture of petroleum ether and ethyl acetate as an eluent. 1H and 13C NMR spectra were obtained on a Bruker 300 MHz NMR instrument with TMS as internal reference using DMSO-d6 as solvents. Standard Bruker software was used throughout. 19F-NMR spectra were recorded at 293 K on BRUKER 400 MHz FT-NMR spectrometers using DMSO-d6 as solvents. Electro Spray Ionisation Mass Spectrometry (ESI-MS) analysis was performed in the positive/negative ion mode on a liquid chromatography-ion trap mass spectrometer (LCQ Fleet, Thermo Fisher Instruments Limited, US). Fluorescence microscopic imaging measurements were logged using Operetta High Content Imaging System (PerkinElmer, US).
Synthesis of (Z)-5-bromo-3-(hydrazonomethyl)-1Hindole (BMI)
The starting material BMI was prepared by the condensation between 5-bromoindole-3- carboxaldehyde (500 mg, 2.23 mmol) and excess of hydrazine hydrate in methanol (50 ml) for 5 h. The pale orange colored solid product was obtained by washed with ethyl acetate (yield 90 %).1H NMR (300 MHz, DMSO-d6) δ (ppm): 11.67 (s, 1H), 8.83 (s, 1H), 8.44 (s, 1H), 7.99 (s, 2H), 7.76 (s, 1H), 7.42 - 7.14 (m, 2H). 13C NMR (75 MHz, DMSO-d6) δ (ppm): 156.03, 136.63, 134.04, 127.10, 125.90, 124.83, 114.87, 113.98, 112.35.
Synthesis of 2-((Z)-(((Z)-(5-bromo-1H-indol-3-yl) methylene) hydrazono) methyl)-4, 6-diiodophenol (BHDL).
The probe BHDL was synthesized by condensation between precursor BMI (50 mg, 2.10 mmol) and 3, 5- diiodosalicylaldehyde (80 mg, 2.10 mmol) in methanol (50 ml) solution for 10 h. The orange-colored solid product was found and thoroughly rinsed with ethyl acetate. The pure solid product was obtained (yield 80 %). 1H NMR (300 MHz, DMSO-d6) δ (ppm):13.05 (s, 1H), 12.15 (s, 1H), 9.14 - 8.70 (m, 2H), 8.41 (s, 1H), 8.08 (t, 3H), 7.43 (d, 2H). 13C NMR (75 MHz, DMSO-d6) δ(ppm): 161.64, 160.07, 159.48, 158.24, 136.70, 135.90, 135.11, 133.41, 126.93, 126.26, 124.91, 121.60, 114.92, 114.48, 111.74, 111.16. MS (ESI): 591.60 (M-H).
UV-visible and fluorescence titrations
Sensing aptitude of BHDL towards different amines/anions in DMSO/H2O (9:1, v/v) system was investigated by absorbance and emission spectroscopy. Tetrabutylammonium salts of anions in dimethylsulfoxide were used throughout the studies. Optical mode analysis of BHDL was inspected with different amines like azane, methylamine, dimethylamine, trimethylamine, phenylethylamine, spermidine, spermine, tyramine, cadaverine, serotonin, histamine, tryptamine, L-tryptophan, dopamine, putrescine, D-glucosamine, and glycine. Absorption measurements were performed on JASCO V-630 spectrophotometer in 1 cm path length quartz cuvette with a volume of 2 mL at room temperature. Fluorescence measurements were made on a JASCO and F-4500 Hitachi Spectrofluorimeter with excitation slit set at 5.0 nm band pass and emission at 5.0 nm band pass in 1 cm × 1 cm quartz cell.
Computational details
Density functional theory (DFT) calculations were executed using Gaussian 09 program [45]. Further, to support the fluorescent enhancement of BHDL with tryptamine and fluoride ions, the optimized structures of BHDL, BHDL-TryptA and BHDL- F‒ were obtained by DFT-B3LYP method using 6-311G /LANL2DZ basis sets, respectively.
Results and Discussion
Synthesis of BHDL
In the synthetic route, simple condensation between 5-bomoindole-3-carboxaldehyde and hydrazine hydrate in excess yields the precursor BMI in the initial step. Successively, condensation process sustained between BMI and 3,5-diiodosalicylaldehyde in methanol. Finally, the chemoreceptor moiety BHDL was obtained with good yield as depicted in Scheme 1. Further to confirm the probe BHDL via diverse analytical methods such as 1H and 13C NMR and ESI-MS spectroscopy (Figures 1-4).
UV-vis and fluorescence studies of BHDL towards anions
To investigate the interactions of receptor BHDL with anions, absorbance and fluorescence intensity changes have been monitored in the presence of various anions such as I‒, Br‒, Cl‒, F‒, NO2‒, CN‒, AcO‒, HSO4‒ and H2PO4‒ in DMSO/H2O (9:1, v/v) system. Interestingly, in the absorption spectrum, addition of other anions was failed to alter the ground state behavior of the probe except F‒ ion which is shown in Figure 1. The free probe BHDL displays three absorption bands at 252, 372 and 492 nm (molar extinction coefficient: 9.9 × 104 M-1 cm-1, 5.6 × 104 M-1 cm-1 and 1.3 ×104 M-1 cm-1 respectively). The addition of the F‒ ion to the probe BHDL results in ratiometric changes in which the band centered at 372 nm gradually reached hypochromic shift and the absorbance at 492 nm attained hyperchromic shift along with a trivial increment in the absorbance of band obtained at 272 nm. The presence of well-defined isobestic points at 329 and 410 nm indicates that only two species coexisted at the equilibrium (Figure 2). The observed changes in absorbance in the presence of fluoride ion can be attributed to the formation of hydrogen bonding complexes with the -OH group of salicyl unit that eventually resulted in a deprotonation.
Figure 1: UV- visible absorption spectra of BHDL (1 × 10-5 M) with competing anions 5 equiv. in DMSO/H2O (9:1, v/v) system.
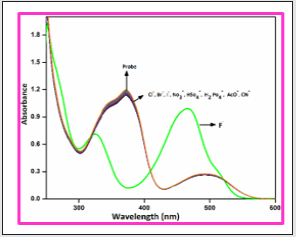
Figure 2: UV–visible absorption spectra of BHDL (1 × 10-5 M) with F- ions (0- 50 μM) in DMSO/H2O (9:1, v/v) system.
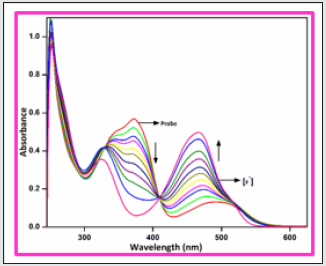
To gauge the sensitivity of the probe BHDL towards various anions, fluorescence titration experiments have been performed in DMSO/H2O (9:1, v/v) system. Interestingly, the fluorescence response of BHDL in the presence of other competing anions was inapt except F‒ ion in the same environment as shown in Figure 3. In probe BHDL, the phenolic -OH of diiodosalicyl moiety has an ability to excite the intramolecular hydrogen bonding towards imine nitrogen in ground state strongly initiate the ESIPT process as shown in Scheme 2. Under identical conditions, the fluorescence spectrum of the probe BHDL disclosed dual emissive bands centered at 430 and 555 nm when excited at 370 nm as shown in Figure 4 due to ESIPT operate on BHDL. The dual emissive band was perceived at 430 nm and 555 nm dispensed to the enol and the keto tautomer respectively owing to the ESIPT process. Upon titrated with F‒ ion towards probe displayed “turn -on” responses, initially the band obtained at 430 nm has attained slight enhancement in intensity and the emission band at 555 nm was blue shifted approximately 27 nm led to the appearance of the emissive band at 528 nm. Further incremental addition of F‒ ion gradually enhances the intensity of band at 430 nm with the expense of the band at 528 nm and finally disappeared completely. Moreover, enhancement of the fluorescence intensity induced by F‒ ions can be endorsed by the inhibition of ESIPT phenomena. The plausible ESIPT mechanistic pathway of BHDL with F‒ ion is described in Scheme 3. At the time of titration, the color of the complex turns into yellow color from orange, and it’s merely noticed by the naked eye (Figure 5).
Figure 3: Fluorescence spectra of BHDL (1×10-5M) with competing anions 5 equiv. in DMSO/H2O (9:1, v/v) system. Excitation at 370 nm. Slit width is 5 nm.
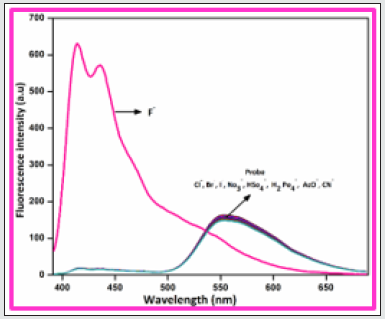
Figure 4: Fluorescence spectra of BHDL (1 × 10-5 M) with F- ions (0-50 μM) in DMSO/H2O (9:1, v/v) system. (Insert Fig. is emission spectrum of probe). Excitation at 370 nm. Slit width is 5 nm..
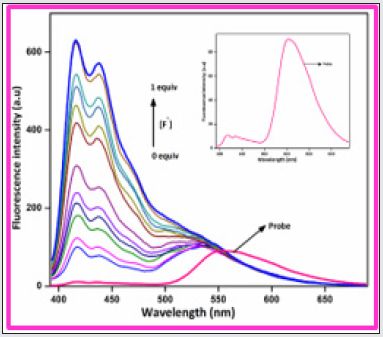
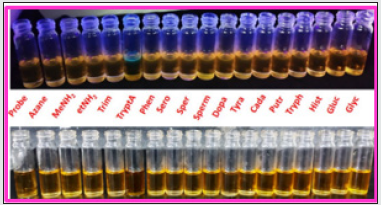
Figure 5: Naked eye detection of F- ions with BHDL under visible light (top) and fluorescence responses of F- ions with BHDL in UV-lamp (bottom).

UV-vis and fluorescence investigation of BHDL towards biogenic amines
In order to develop a bifunctional chemosensor, further we have tested the biogenic amines sensitivity of the probe BHDL in the presence of other important aliphatic, aromatic and biogenic amines such as azane, methylamine, dimethylamine, trimethylamine, phenylethylamine, spermidine, spermine, tyramine, cadaverine, serotonin, histamine, L-tryptophan, dopamine, putrescine, D-glucosamine, glycine and TryptA respectively in DMSO/H2O (9:1, v/v) system by absorption and fluorescence spectroscopy. The absorption spectral investigation of BHDL with competing biogenic amines reveals that nullified changes in the absorbance spectrum excluding TtyptA as shown in Figure 6. At first, the probe BHDL shows three absorption bands obtained at 251, 372 and 487 nm (molar extinction coefficient: 3.7×10-4 M-1 cm-1, 2.9 ×10-4 M-1 cm-1 and 0.75 × 10-4 M-1 cm-1 respectively). Upon the addition of TryptA, the probe BHDL exhibited a radiometric response in which the band at 372 nm was decreased with concomitant increase in absorption band at 487 nm along with new band at 284 nm as shown in Figure 7. Subsequent addition of TryptA to the probe BHDL, the absorption band at 487 nm underwent blue shift approximately 20 nm with two isobestic points at 331 nm and 408 nm and also with increment in absorbance of the band at 284 nm. From these observed results we conclude that both components are existing in equilibrium and also exhibit visible color changes in this state. Moreover, to analyze the fluorescence sensing potential of BHDL towards amines in DMSO/H2O (9:1, v/v) system. On the contrary, the addition of competing biogenic amines into the probe BHDL led to no discrepancy in the fluorescence intensity as shown in Figure 8 excluding TryptA. Under identical environments, initially the probe BHDL shows emissive bands at 433 and 555 nm when excited at 366 nm. Further adding increasing amounts of TryptA to BHDL led to stimulate specific fluorescence “turn-on” response with an increase in emission intensity at 433 nm and also the expense of intensity at 555 nm which is attributed to the nucleophilic substitution followed by the elimination of -OH group of salicyl moiety present in BHDL by TryptA. Upon continuous addition of TryptA led to increase in fluorescence intensity due to the inhibition of ESIPT resembled with the F‒ ion (Figure 9) and the plausible mechanistic pathway of BHDL with TryptA is presented in scheme 4. At the time of the occasion, the solution turns into a light brown color from the orange color as shown in Figure 10.
Figure 6: UV- visible absorption spectra of BHDL (2 × 10-5 M) with other amines 5 equiv. in DMSO/H2O (9:1, v/v) system.
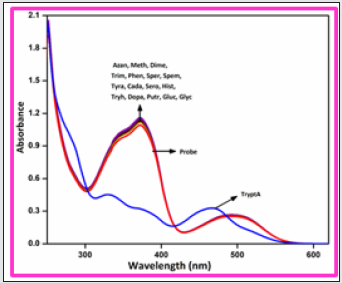
Figure 7: UV –visible absorption spectra of BHDL (2 × 10-5 M) with TryptA (0-100 μM) in DMSO/H2O (9:1, v/v) system.
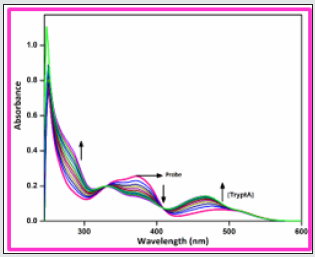
Figure 8: Fluorescence spectra of BHDL (2×10-5 M) with competing amines 5 equiv. in DMSO/H2O (9:1, v/v) system. Excitation at 366 nm. Slit width is 5 nm.
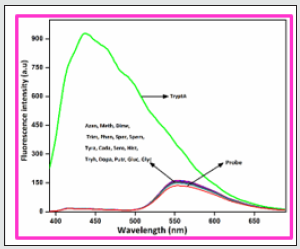
Figure 9: Fluorescence spectra of BHDL (2 × 10-5 M) with TryptA (0-100 μM) in DMSO/H2O (9:1, v/v) system. Excitation at 366 nm. Slit width is 5 nm.
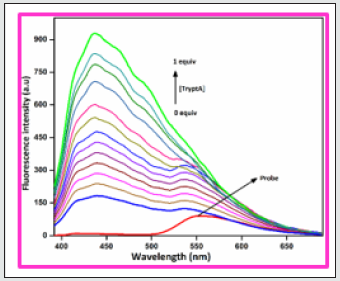
Figure 10: Naked eye detection of TryptA with BHDL (top) under UV-lamp and (bottom) fluorescence responses of F- ions with BHDL in visible light.
Competitive experiments
Figure 11: Selectivity investigation of F- ion with BHDL in the presence of other cations. Excitation at 370 nm, Slit width = 5 nm.
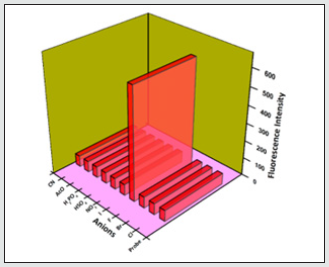
Figure 12: The red bars represent the change in fluorescence intensity of BHDL that occurs upon the consequent addition of competitive anions. The pink bars represent the addition of the competing amines to BHDL. Excitation at 370 nm, Slit width = 5 nm.
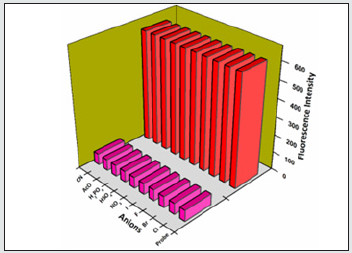
To analyze the selective sensing behavior of BHDL, competitive experiments have been performed in the presence of various concentrations of F‒/TryptA. The probe was treated with 5 equiv. of other competing anions/biogenic amines. In the presence of other competing anions/biogenic amines, there is a trivial intensity change in BHDL has been observed when compared with the solution containing only F‒/TryptA with the probe. This result suggests that BHDL allows selective F‒/TryptA detection in competing environment (Figures 11-14).
Figure 13: Selectivity investigation of TryptA with BHDL in the presence of other amines. Excitation at 366 nm, Slit width = 5 nm.
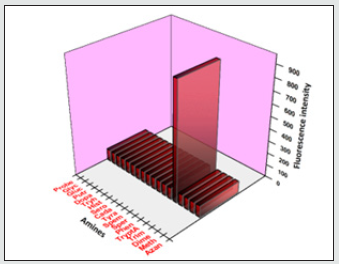
Figure 14: The maroon bars represent the change in fluorescence intensity of BHDL that occurs upon the consequent addition of competitive amines. The blue bars represent the addition of the competing amines to BHDL. Excitation at 366 nm, Slit width = 5 nm.
Determination of job`s plot, ESI-MS spectra, binding constant and detection limit
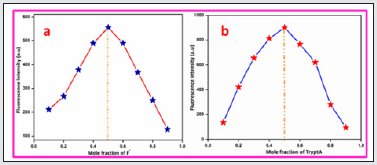
To further confirm the effective binding of BHDL with F‒/ TryptA, Job’s plot analysis has been performed using fluorescence titration experiment. The result shows that the probe BHDL binds with both F‒ and TryptA via 1:1 stoichiometric mode as shown in Figure 15(A & B). To find the linear fit analysis of BHDL against [TryptA] and [F‒] by absorption and fluorescence titration experiment and its intensity changes in a specific wavelength were plotted. When the changes in absorbance of the probe BHDL at 465 nm vs both [TryptA] and [F‒] ions yield good linearity. Similarly, the changes in fluorescence intensity displayed a good linear relationship between the fluorescence intensities noticed at 416 nm and 437 nm vs [TryptA] and [F‒] ions respectively (Figures 5-8). Furthermore, the binding constant values of BHDL with F‒/ TryptA has been calculated using modified Benesi-Hildebrand equation from UV-visible and fluorescence titration profile. The binding constant values of BHDL were calculated to be 9.26 ×10- 4 M/3.89 ×10-4 M from UV-visible titration analysis and 1.60×10-4 M/9.64×10-4 M from fluorescence titration analysis for F‒/TryptA respectively (Figures 9-12). From the binding constant values, the probe BHDL were efficiently bound with both F‒/TryptA. Furthermore, the probe BHDL might be sensing both F‒ and TryptA ions in low concentrations. The detection limits have been found to be 0.001×10-8 M and 0.002 ×10-8 M respectively.
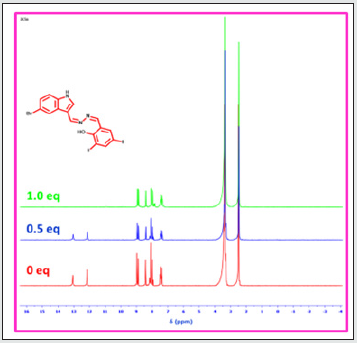
1H NMR titrations of BHDL with F‒ ions
Moreover, to find additional support of the binding propensity of probe BHDL complexed with F‒ ions, 1H NMR titrations were completed in DMSO-d6. The probe BHDL was titrated with F‒ ions (0-1 equiv.), the phenolic -OH and -NH protons signals appeared at 12.15/13.05 ppm was gradually reduced and disappeared with the addition of 0.5 equiv. to 1 equiv. of F‒ ion as presented in Fig. 16. The 1:1 binding of F‒ ion with BHDL was further confirmed by 19F-NMR analysis. The (HF2)‒ signal appeared at -143.11 ppm (Fig. S13, See ESI) which corresponds to deprotonation process was initiated by the hydrogen bonding formation of F- ion with -OH group presented in the diiodo moiety.
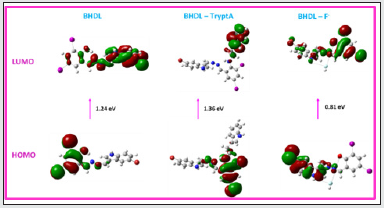
DFT studies of BHDL with TryptA/F‒ ion
Besides, to assist the fluorescence enhancement of probe BHDL binds with TryptA/F‒, DFT calculations were performed. The ground state optimized geometry of probe BHDL and BHDLTryptA and BHDL-F‒ complexes were found using the DFT/B3LYP- 6-311G/LANL2DZ basis set. From these result discloses that when BHDL complexed with TryptA/F‒ ion (Fig. 17) led to reduce the HOMO-LUMO energy gap value. In probe BHDL, the HOMO and are delocalized over the diiodo moiety whereas LUMO is spread over the indole unit. When complexed with TryptA, HOMO is dispersed over the diiodo moiety alone, although the LUMO is located in the TryptA moiety alone. It is clearly demonstrating that inhibition of ESIPT and deprotonation of -OH proton processes developed in BHDL when binds with F‒ yields the ratiometric responses in absorption band appeared at 372 nm and 492 nm and also responsible for the increment in fluorescence band obtained at 430 nm along with the reduction of emission band at 555 nm. Further the probe BHDL binds with F‒ ion, HOMO is centered at the indole unit alone while the LUMO placed at diiodo unit with lowering band gap. From these results F‒ ion has bound efficiently than TryptA.
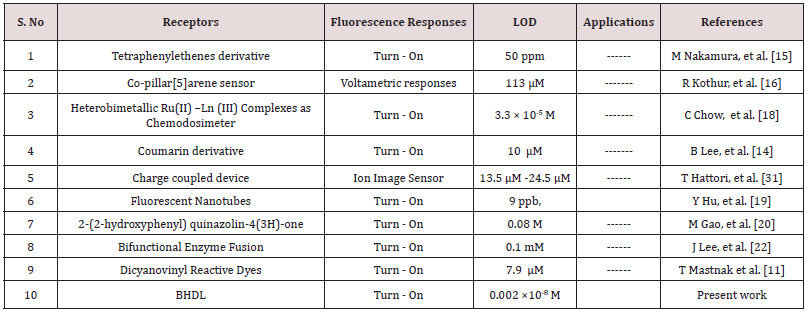
Comparison of BHDL with previously reported TryptA selective sensors
TryptA selective probes were compared with previously reported probes (Table 1). Based on these reports, our probe BHDL has the ability to detect TryptA in too low concentration range compare than permitted level recommended by the Environmental Protection Agency (EPA) and it was utilized to gauge TryptA ions in real system. Henceforth, our probe BHDL has possessed more admirable biological application and it can be used in environment sample analysis.
Conclusion
In summary, we have designed a 5-bromoindolehydrazone anchored diiodosalicylaldehyde derivative as a bifunctional probe BHDL that detects F‒ and TryptA in DMSO/H2O (9:1, v/v) medium. The probe BHDL has shown higher selectivity and sensitivity towards F‒/TryptA over competing anions/biogenic amines with an excellent and strong ratiometric changes in absorbance and “turnon” responses in fluorescence. These experimental results suggest that deprotonation process and inhibition of ESIPT mechanistic strategies were responsible for the optical responses of the probe in the presence of both F‒/TryptA and it can be efficiently supported by DFT calculations. The binding constant values of the complexes BHDL-F‒/BHDL-TrypA were found to be 9.26 ×10-4 M/3.89 ×10- 4 M from UV-visible titration profile and 1.60 ×10-4 M/9.64 ×10-4 M from fluorescence titration profile for F‒/TryptA respectively could demonstrated their stability and affinity of ghost/guest. The detection limits of probe BHDL with F‒/TryptA has been acquired to be 0.001×10-8 M and 0.002 ×10-8 M respectively might be used in real system.
Acknowledgments
We acknowledge the financial support from the Council of Scientific and Industrial Research (CSIR), HRDG, File No. 01(2901)/17/EMR-II, New Delhi and the Department of Science and Technology, SERB, Extramural Major Research Project (Grant No. EMR/2015/000969), Department of Science and Technology DST/TM/CERI/C130(G), New Delhi, India. Further, we also acknowledge the DST-FIST, DST-IRPHA, DST-PURSE, UGC-UPE and UGC-NRCBS, SBS, MKU for providing instrumental facilities.
References
- AW Czarnik (1994) Chemical Communication in Water Using Fluorescent Chemosensors. Acc Chem Res 27: 302-308.
- B Wang, E J Anslyn (2011) Chemosensors: Principles, Strategies, and Applications, John Wiley and Sons, New York, USA.
- R Martínez-Máñez, F Sancenón (2003) Fluorogenic and chromogenic chemosensors and reagents for anions. Chem Rev 103(11): 4419-4476.
- Y Zhou, JF Zhang, J Yoon (2014) Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem Rev 114: 5511-5571.
- MAR Buzalaf, GM Whitford (2011) Fluoride metabolism. Monogr Oral Sci 22: 20-36.
- S Ayoob, AK Gupta (2006) Fluoride in drinking water: A review on the status and stress effects. CREST 36(6): 433-487.
- DL Ozsvath(2009) Fluoride and environmental health : a review. Rev Env Sci Biotechnol 8: 59-79.
- RH Dreisbuch (1980) Handbook of Poisoning, Lange Medical Publishers. Los Altos, CA, USA.
- SK Kyung, YK Su, MY Hyoung, CN Kye (2007) Anion selective colorimetric chemosensor with nitronaphthalene urea derivative. Bull. Korean Chem Soc 28: 1815-1817.
- CA Baud, JM Very, B Courvoisier (1988) Biophysical study of bone mineral in biopsies of osteoporotic patients before and after long-term treatment with fluoride. Bone 9: 361-365.
- H Pollick (2018) The Role of Fluoride in the Prevention of Tooth Decay. Endocrinol Metab Clin NORTH Am 65: 923-940.
- KL Kirk (1991) Biochemistry of the Elemental Halogens and Inorganic Halides. Biochem Elem Halogens Inorg Halides 9: 6221.
- HS Horowitz (2003) The 2001 CDC Recommendations for Using Fluoride to Prevent and Control Dental Caries in the United States. J Public Health Dent 63: 3-8.
- F Sánchez-Jiménez, MV Ruiz-Pérez, JL Urdiales, MA Medina (2013) Pharmacological potential of biogenic amine-polyamine interactions beyond neurotransmission. Br J Pharmacol 170: 4-16.
- M Nakamura, T Sanji, M Tanaka (2011) Fluorometric sensing of biogenic amines with aggregation-induced emission-active tetraphenylethenes. Chem A Eur J 17: 5344-5349.
- R Reddy Kothur, B Anil Patel, PJ Cragg (2017) A co-pillar[5]arene sensor for linear biogenic amines. Chem Commun 53: 9078-9080.
- G Lu, JE Grossman, JB Lambert (2006) General but discriminating fluorescent chemosensor for aliphatic amines. J Org Chem 71: 1769-1776.
- CF Chow, MHW Lam, WY Wong (2013) Design and synthesis of heterobimetallic Ru(II)-Ln(III) complexes as chemodosimetric ensembles for the detection of biogenic amine odorants. Anal Chem 85: 8246-8253.
- Y Hu, X Ma, Y Zhang, Y Che, J Zhao (2016) Detection of Amines with Fluorescent Nanotubes: Applications in the Assessment of Meat Spoilage. ACS Sensors 1: 22-25.
- M Gao, S Li, Y Lin, Y Geng, X Ling, et al. (2016) Fluorescent Light-Up Detection of Amine Vapors Based on Aggregation-Induced Emission. ACS Sensors 1: 179-184.
- KS Hettie, X Liu, KD Gillis, TE Glass (2013) Selective catecholamine recognition with NeuroSensor 521: A fluorescent sensor for the visualization of norepinephrine in fixed and live cells, ACS Chem Neurosci 4: 918-923.
- JI Lee, JH Jang, MJ Yu, YW Kim (2013) Construction of a bifunctional enzyme fusion for the combined determination of biogenic amines in foods. J Agric Food Chem 61: 9118-9124.
- SD Brandt, S Freeman, P McGagh, N Abdul-Halim, JF Alder (2004) An analytical perspective on favoured synthetic routes to the psychoactive tryptamines. J Pharm Biomed Anal 36: 675-691.
- CPB Martins, S Freeman, JF Alder, T Passie, SD Brandt (2010) Profiling psychoactive tryptamine-drug synthesis by focusing on detection using mass spectrometry. TrAC - Trends Anal Chem 29: 285-296.
- M Gao, BZ Tang (2017) Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sensors 2: 1382-1399.
- AP De Silva, HQN Gunaratne, T Gunnlaugsson, AJM Huxley, CP McCoy, et al. (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97: 1515-1566.
- X Chen, X Tian, I Shin, J Yoon (2011) Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species, Chem Soc Rev 40: 4783-4804.
- D Wu, AC Sedgwick, T Gunnlaugsson, EU Akkaya, J Yoon, et al. (2017) Fluorescent chemosensors: The past, present and future. Chem Soc Rev 46: 7105-7123.
- AD Johnson, RM Curtis, KJ Wallace (2019) Low molecular weight fluorescent probes (LMFPs) to detect the group 12 metal triad. Chemosensors 7.
- MY Berezin, S Achilefu (2010) Fluorescence lifetime measurements and biological imaging. Chem Rev 110: 2641-2684.
- X Zhang, J Yin, J Yoon (2014) Recent advances in development of chiral fluorescent and colorimetric sensors. Chem Rev 114: 4918-4959.
- L He, B Dong, Y Liu, W Lin (2016) Fluorescent chemosensors manipulated by dual/triple interplaying sensing mechanisms, Chem Soc Rev 45: 6449-6461.
- X Li, X Gao, W Shi, H Ma (2014) Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem Rev 114: 590-659.
- SK Pal, Y Shukla (2003) Herbal medicine: Current status and the future. Asian Pacific J Cancer Prev 4: 281-288.
- G Bacher, T Beckers, P Emig, T Klenner, B Kutscher, et al. (2001) New small-molecule tubulin inhibitors. Pure App Chem 73: 1459-1464.
- AH Rosengren, R Jokubka, D Tojjar, C Granhall, O Hansson, et al. (2010) Over expression of Alpha 2A-Adrenergic receptors contributes to type 2 diabetes. Science 327: 217-220.
- LJ Scott, CM Perry (2000) Delavirdine: A review of its use in HIV infection Drugs 60: 1411-1444.
- Q Li, Y Guo, J Xu, S Shao (2011) Novel indole based colorimetric and “turn on” fluorescent sensors for biologically important fluoride anion sensing. J Photochem Photobiol B Biol 103: 140-144.
- PA Gale (2008) Synthetic indole, carbazole, biindole and indolocarbazole-based receptors: Applications in anion complexation and sensing, Chem. Commun pp. 4525-4540.
- L Wang, X He, Y Guo, J Xu, S Shao (2011) Tris(indolyl)methene molecule as an anion receptor and colorimetric chemosensor: Tunable selectivity and sensitivity for anions. Org Biomol Chem 9: 752-757.
- YL Sun, AT Wu (2013) Indole-based fluorescent sensors for selective detection of Hg2+. J Fluoresc 23: 629-634.
- HJ Huang, JL Chir, HJ Cheng, SJ Chen, CH Hu (2011) Synthesis of highly selective indole-based sensors for mercuric ion. J Fluoresc 21: 1021-1026.
- C Pan, K Wang, S Ji, H Wang, Z Li (2017) Schiff base derived Fe3+-selective fluorescence turn-off chemsensors based on triphenylamine and indole: Synthesis, properties and application in living cells. RSC Adv 7: 36007-36014.
- D Sain, C Kumari, A Kumar, S Dey (2016) Indole - based distinctive chemosensors for “naked-eye” detection of CN- and HSO4-, associated with hydrogen-bonded complex and their DFT study. Supramol Chem 28: 239-248.
- MJ Frisch, GW Trucks, HB Schlegel, GE Scuseria, MA Robb, et al. (2011) Gaussian 09 Citation.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...