Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Insight Into Equilibrium and Kinetics of Heavy Metal Remediation Potential Of 1, 3-Bis (Furan-2-Yl Methylene) Urea Volume 5 - Issue 4
Adeoye MD*, Alabi KA and Tewogbola KK
- Department of Chemical Sciences, Fountain University, Osogbo, Nigeria
Received:October 04, 2021; Published:November 09, 2021
*Corresponding author:Adeoye MD, Department of Chemical Sciences, Fountain University, Osogbo, Osun State, Nigeria
DOI: 10.32474/AOICS.2021.05.000220
Abstract
Heavy metals are common pollutants in water supplies due to effluents discharge from various industries; and their removal has received much attention in recent years. In this study, the application of synthesized 1,3-bis (furan-2-ylMethylene) Urea (BFMU)] for the remediation of heavy metal ions contaminated Osun River in Osogbo community, its uptake performance and binding efficiency were evaluated by varying the contact time and adsorbent doses. The kinetic experimental data were analyzed using first order and zero-order equation models. The level of heavy metal contamination in the water prior to treatment with BFMU followed the order Pb2+> Cd2+> Zn2+> Cu2+. The observed bands for the FTIR and UV spectra analysis of the (BFMU) sorbent before and after water treatment indicate high metal ions-BFMU interactions, hence, its affinity for these metals is in the order: Cu2+ > Zn2+ > Cd2+ > Pb2+. The sorption data correlated well with first order kinetic model with an instantaneous sorption approach.
Keywords: Metal Ion; 1, 3-bis (furan-2-ylMethylene) Urea; binding efficiency; sorption isother
Introduction
The contamination of soil and water resources with environmentally harmful chemicals through industrial wastes is one of the main health problems in industrial countries. Amongst the contaminants, heavy metals are of greater concern because of their high toxicity, bioaccumulation, and retention in the human body [1.2]. The bioaccumulation of heavy metals in the body is roughly a function of the deposited number of pollutants, the exposure time, and the effects of climatic factors. It had earlier been established that the presence of heavy metal (mercury, lead, arsenic, cadmium, copper, nickel, chromium e. t. c.), in the environment, even in moderate concentrations, is responsible for producing a variety of illnesses of the central nervous system, the kidneys, liver, bones or teeth [2,3]. Many researchers, in a bid to reduce water pollution and alleviate the problems associated with the presence of heavy metals are exploiting the use of nonconventional alternatives; including different plants, biomaterials and development of environmental friendly synthetic organic compounds, as alternative to the conventional methods, many of which has been found to be ineffective or require high operational costs, and may also result in large volumes of sludge causing disposal problems and further pollution [4]. Bis-imines are important due to the presence of azomethine (>C=N) moiety which has versatile ability to form coordinate bonds with many metal ions through its azomethine and phenolic groups [5-7]. Report on the evaluation of heavy metal adsorption on Azomethines derivatives is scarce; more details are therefore required on the kinetics and thermodynamics of their adsorption process. This study evaluates the present level of heavy metals in the contaminated Osun River water sample, and the viability of the synthesized bis-imines derivative (BFMU) as adsorbent for the heavy metals in water sample. The results obtained are herein reported.
Materials And Methods
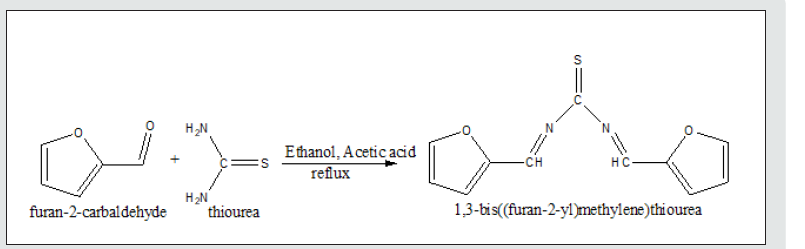
Synthesis and characterization of BFMU
BFMU was synthesized following the literature methods of Sonnekar et al. and Alabi et al, [7,8], as shown in (Scheme 1). The product was filtered, washed with distilled water and recrystallized in alcohol. The residue was collected and air dried for 3 - 4 days. The compound was characterized by 6405 UV/Visible Spectroscopy in acetic acid at room temperature in the region 200- 800 nm. Its Infrared spectroscopic analysis was also carried out with Schimadzu Fourier Transform Infrared (FT-IR) spectrophotometer in the range 500 - 4000 cm-1, using KBr disc. It 13C-NMR spectra analysis had earlier been reported by our group [8].
Water sampling and Treatment
Water samples from Osun River water located at Isale Osun axis of Asubiaro area (Lat 07, 44° N Long 04.74°E) in Osogbo, Osun state were collected. The physico-chemical, pH and temperature of the water sample were determined using Jenway 3505 pH- portable meter. The water sample was digested using the literature method of Adeoye et al. [9]. Metal analyses of the digested samples were determined with Solaar series 711047v1.22 Atomic Absorption Spectrophotometer (AAS). Detection limits were estimated from digested blank (deionized water) which was run during the analysis. The same procedure was repeated after the samples had been treated with blended BFMU.
Equilibrium and Kinetics Studies of the Heavy Metals Uptake Performance of BFMU
The equilibrium and kinetics experiments of the uptake performance of the synthesized BFMU for the detected heavy metal ions in the water samples were carried out using the literature method of Adeogun et al. [1]. Typically, by agitating the varying masses (0.05 -0.4 g) of the blended BFMU in 100 ml each of the water sample in glass̄-stoppered flasks kept on an isothermal shaker (orbital shaker) at 25±1 oC at varying time (0–3 hrs) at first, and then, for 48hrs equilibration time, at pH of 5.8. The mixtures were filtered at desired times, and at equilibrium time. The concentrations of the heavy metals in the filtrates were determined for the metal’s uptake capacities of the sorbents in each solution as a function of time. The amounts of metal ions adsorbed (in mg/g) by the adsorbent at equilibrium qe (mg/g) and at any time t, qt (mg/g) were calculated thus:

The linear forms zero order, pseudo-first and second order kinetics models, expressed in equations 3, 4 and 5 respectively were employed to determine the rate constant, k and the controlling mechanism of sorption process. These were done to further confirm the best fit kinetic model for the sorption process of BFMU (Table 1).
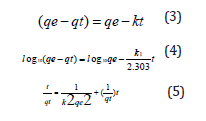
Where: ci and ct (in mg/ml) are the concentration of metal ions at initial and any time t respectively.
V = the volume of the solution (ml), w = the mass of adsorbent used (g); k0 (mol dm-3 min-1), k1(min-1) and k2(g/mg/min) are the rate constant of zero-order, pseudo first-order and pseudo second-order equations [10].
Table 1: Concentration of heavy metals before and after treatment of Osun River water sample with BFMU, ND Not detected.

Results and Discussion
The functionality presents in BFMU before and after metal ion adsorption due to the rotational and vibrational movement of the molecular groups and chemical bond of this molecule are as presented in the Table 2 and Appendix 1. The characteristics bands observed at 3155cm-1 is associated to C-Hstr. The peak observed at 1641cm-1 can be attributed to azomethine C=N stretching frequency while the observed bands at 1595 cm-1, 1228 cm-1, 1008 cm-1and 1539 cm-1 are assignable to C=O, C-O, C-N and C=C stretching frequencies respectively [11]. After remediation, there are changes in the positions of the observed band thus: the C-Hstr frequency was observed at 3128.64 - 3481 cm-1. The characteristics band for azomethine C=N peak previously observed at 1641cm-1 shifted to 1664 cm-1, with the appearance of additional peaks at 596cm- 1 and 468cm-1, indicated the coordination of metals with the adsorbent. The C=O stretching frequency now appear at 1591.33 and 1541.18 cm-1. The characteristic bands for C-O, C-N and C=C now appear at 1228cm-1, 1078 cm-1 and 1541cm-1 respectively. This observation attests to the coordination of metals to the adsorbent. Also, as indicated in (Figure 2.1), two bands occurring at 385 nm and 480 nm were observed in the UV spectra analysis of BFMU before treatment with the heavy metals contaminated water sample, while a band occurring at 370 nm was observed after the water sample had been remediated with BFMU. The observed blue shift signifies that there was probable metal coordination with the adsorbent at any of its different coordinating sites i.e., on the hetero atoms. The UV-Vis spectrum after the treatment clearly indicates the presence of metal ion with a band in the visible region around 650 nm
Figures 1: Ultraviolet visible (UV-VIS) absorption spectra of BFMU samples before (A) and after (B) treatment with Osun River water sample.

Evaluation of heavy metals in the Osun River water sample
The level of concentration of four heavy metal ions (Cu2+, Pb2+, Zn2+, and Cd2+) determined in the osun river water sample before and after treatment with the synthesized BFMU are as presented in (Table 1-5). The heavy metal contents in the studied water sample prior to treatment followed the order Pb2+> Cd2+> Zn2+> Cu2+, with the concentrations of Pb2+ and Cd2+ above the WHO and SON allowable standard of heavy metal in drinking water [12]. Similar results were reported by Adeoye et al. [9] for the Osun River water sample, although the concentrations of the heavy metals ions analyzed were different. The Cu2+concentration was greatly reduced and not within the detectable limits after remediation, indicating the higher affinity of BFMU for Cu ions.
Table 4: Comparison of the Zero and pseudo -first order adsorption rate constants and calculated and experimental qe values for different initial concentrations of the studied heavy metal ions.

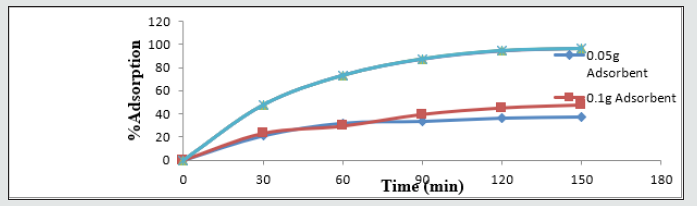
Effect of adsorbent dose and agitation time on the sorption of heavy metal ions
The time profile for the adsorption of metal ions at different starting doses of the adsorbent from the samples at 25 ᵒC is as presented in (Figures 1-4). The percentage of metal ions sorbed increased rapidly from 0% to about 85% over the first ninety (90) minutes of the contact time with the adsorbent. A further 10% (approximately) increase in the percentage of metal ion adsorbed was observed over the next 60 minutes of contact time for Cd2+, Pb2+, and Zn2+. However, all the Cu2+were adsorbed during the first 30 minutes, indicating the high affinity of BFMU for Cu ions. Also, the percentage of metal ions adsorbed increase significantly with increasing mass of adsorbent used. Although, there was no significant difference in the sorption capacities of BFMU at 0.05g and 0.1g doses but increased greatly at 0.2 g of adsorbent dose. Further increase in adsorbent doses to 0.3 g and 0.4 g has no significant increase in percentage adsorption for Cd and Zn metal ions over the entire contact time. However, there was no significant difference in the sorption capacities of BFMU for Pb2+ ion at 0.2 - 0.4 g adsorbent doses in the first contact time of 30 minutes. But beyond these, the adsorption rate of BFMU for this ion increased. The initial high percentage of metal ions adsorbed indicates an instantaneous sorption, which can be attributed to availability of more binding sites on the adsorbent, however, as these sites are progressively occupied, the sorption of metal ions was slowed down. Similar observations were made in the remediation studies of Adeoye [9] and Samra et al. [13] using chitosan and date pits respectively. The kinetic parameters (Table 4), (Figure 5) for the sorption process of heavy metal ions in the studied water sample by the synthesized BFMU shows that the experimental equilibrium sorption capacities, qe(exp), are in good agreement with the theoretical equilibrium sorption capacities (qecal) for the first order kinetic model than for zero order model. Also, the experimental sorption data correlated well to first kinetic model with regression values, R2 ranging from 0.94 to 0.97 (Table 3) than the zero-order kinetic model.
Figures 6: a) Zero order (b) Pseudo-first order kinetic model plots for sorption capacities of BFMU for heavy metals ions at 25 °C.

Conclusion
This study showed that the Osun River water is highly polluted with heavy metals, with the concentration of these metals above the maximum limits for drinking water quality as provided by the Nigerian Institute Standard (NIS), making it unsuitable for human consumption. The heavy metals concentration in the water sample is the order: Pb2+> Cd2+> Zn2+> Cu2+.1, 3-bis (furan-2yl methylene) urea has complexing potential for these heavy metal ions. However, it exhibits a selectivity character in adsorbing capacities. Maximum amount of heavy metal ions was absorbed by 1, 3-bis (furan-2-yl methylene) urea at about 90 mins (equilibrium time) for all the metal ions in the water sample. The affinity of the 1,3 bis (furan-2yl methylene) urea for these metal ions, as observed in the study follows the order: Cu2+> Zn2+> Cd2+>Pb2+. The adsorption kinetics follow first order and the experimental equilibrium sorption capacities determined from the contact time study were in good agreement with the theoretical equilibrium sorption capacities calculated from the kinetics model.
References
- Adeogun AI, Bello OS, Adeoye MD (2010) Biosorption of lead ions on biosorbent prepared from plumb shells (Spondiasmombin): Kinetics and equilibrium studies. Pakistan Journal of Science and Industrial Research 53(5): 246-251.
- Cleide STA, Dayene CC, Helen CR, Ione LSA, Luciana MC, et al. (2013) Bioremediation of Waters Contaminated with Heavy Metals Using Moringa oleifera Seeds as Biosorbent. Applied Bioremediation-Active and Passive Approaches Edited by Yogesh B. Patil and Prakash Rao 10: 226-252.
- Sharma P, Kumari P, Srivastava MM, Srivastava ST (2007) Ternary Biosorption studies of Cd(II), Cr(III) and Ni(II) on shelled Moringa oleifera seeds. Biores. Technol 98(2): 474-477.
- Paul OA (2013) Modelling of the Adsorption of Cu (II) and Cd (II) from Aqueous Solution by Iraqi Palm-Date Activated Carbon (IPDAC) Intern. J of Modern Chem 5(3): 136-144.
- Jarrahpour AA, Motamedifar M, Pakshir K, Hadi N, Zarei M, et al. (2004) Pure Chemical Compounds. Khimia: Moscow 9: 815.
- Adnan D (2013) Synthesis of Imine Compounds Derived from Acetylace-tone and Structure Study. International Journal of Chem Tech Research 5(1): 197-203.
- Sonnekar V, Jadhav W, Dake S, Pawar R (2013) Synthesis, Antimicrobial and Antifungal activities of novel Bis-imine derivatives. Journal of Pharmaceutical, Biological and Chemical Sciences 4(2): 1411-1418.
- Alabi KA, Abdulsalami IO, Adeoye MD, Aderinto SM, Adigun RA, et al. (2020) Synthesis, Characterization and Computational studies of 1, 3-bis [(E)-furan-2-yl) Methylene] urea and 1, 3-bis [(E)-furan-2-yl) methylene] thiourea. Physical Science Review 2(11): 1850.
- Adeoye MD, Azeez LA, Lawal AT, Olayiwola OA, Shitta OR, et al. (2014) Kinetic and equilibrium studies of the heavy metal remediation potential of Helix pomentia. African Journal of pure and Applied Chemistry 8(6): 123-133.
- Rifaqat AKR, Moonis AK, Fouzia R (2011) Batch and Column Studies for the Removal of Lead (II) Ions from Aqueous Solution onto Lignite. Adsorption Science & Technology 29(1): 83-98.
- Rajavel R, Maheswaran P, Akila E, Usharani M (2013) Synthesis, Spectroscopic Characterization, Biological Screening, and DNA Nuclease Activity of Schiff base Metal Complexes Derived from o-phenylenediamine. Intern J Chem & Phys Sci 1(6): 412-417.
- Majolagbe TA, Azeez L, Salawu HO, Adeoye MD, Lawal AT, et al. (2013) Assessment of heavy metal pollution in soils and wells around municipal dumpsites in Osogbo metropolis. Environmental Science: An Indian Journal 8(2): 56-61.
- Samra SE, Jeragh B, El-Nokrashy AM, El-Asmy AA (2014) Bio sorption of Pb2+ from Natural Water using Date Pits: A Green Chemistry Approach. Modern Chem Appl 2: 2.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...