
Lupine Publishers Group
Lupine Publishers
Menu
Opinion(ISSN: 2770-5447) 
Non-Compacted Cardiomyopathy: Is there a Need of a New Cardiomyopathy? Volume 2 - Issue 1
Francesco Bianco1,2*, Piergiusto Vitulli1, Valentina Bucciarelli1, Alvin Chandra1,2, Masatoshi Minamisawa2,3 and Sabina Gallina1
- 1Institute of Cardiology, Chieti, Italy
- 2Brigham and Women’s Hospital, Boston, USA
- 3Department of Cardiovascular Medicine, Matsumoto, Japan
Received: April 04, 2019; Published: April 09, 2019
Corresponding author: Francesco Bianco, Institute of Cardiology, Chieti, Italy
DOI: 10.32474/ACR.2019.02.000129
Abstract
Left ventricular non-compaction (LVNC) is a myocardial disorder, classically defined as a double-layered myocardium, consisting of a thick, spongy/hypertrabeculated, non-compacted endocardial segment and a thin, compacted, epicardial portion. The American Heart Association (AHA) classifies LVNC as a distinct primary genetic cardiomyopathy, while the European Association of Cardiology (ESC) as an unclassified cardiomyopathy. Despite the magnitude of the entire literature yield on this topic, to date the pathogenesis, prognosis, and treatment are still unclear. Prevalence and mortality can range respectively from 0.05% to 0.26% and 5% to 47%, but they are affected by the imaging criteria adopted for the diagnosis. In fact, LVNC has been for years incidentally discovered during autopsy of unexplained sudden cardiac deaths. Conversely, with the advent of increasingly sophisticated cardiac imaging techniques, the presence of hypertrabeculated myocardium has become very common. Both echocardiographic and magnetic resonance criteria have been proven to overestimate the diagnosis, which shares a peculiar phenotype with other pathologies. It is known that a hypertrabeculated left ventricle leads to a symptomatic triad consisting of heart failure, arrhythmias and thromboembolisms. Therefore, it is current opinion of the authors that a “non-compaction cardiomyopathy” (NC-CMP) seems to be the most comprehensive definition of a disease that, similarly to the other cardiomyopathies, and regardless of its etiology, beyond a peculiar phenotype shares a distinct symptomatology and deserves to be listed as an entity between cardiomyopathies.
Keywords: Left Ventricle Non-Compaction; Non-Compacted Cardiomyopathy; Cardiomyopathies; Heart Failure; Sudden Cardiac Death
Introduction
Left ventricular non-compaction (LVNC) is a myocardial disorder, classically defined as a double-layered myocardium, consisting of a thick and spongy or hypertrabeculated endocardial segment, defined as non-compacted, and a thin and compacted portion, laying epicardially [1]. The above-mentioned hyper trabeculations (HXTs) characteristically involve the left ventricle (LV), especially the apex, the lateral, infero-lateral and inferior wall [1,2], and less frequently the right ventricle [3]. Since its first reports, LVNC has always been considered a controversial pathology (Figures 1 & 2). In facts Grant and colleagues, for long accredited as discoverers in 1926, presented a case of persistent sinusoids instead of LVNC [1]. In addition, a lack of uniqueness among the World Health Organization [4], the European Society of Cardiology [5]. and the American Heart Association [6] for its classification, and the presence of trabeculae as a terminal phenotype of LV hemodynamic overload or other myocardial affections, has led several authors to debate on the real existence of a true form of uncomplicated primitive non-compacted cardiomyopathy [7,8].
Figure 1: Two different anatomo-pathological macroscopic sections of the left ventricle, presenting a non-compacted myocardium. Modified from Lorca et al. Int J Cardiol 2016.
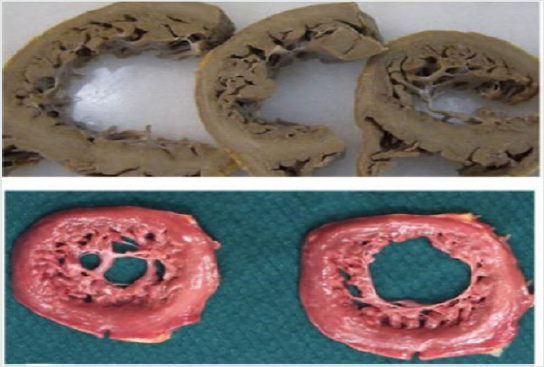
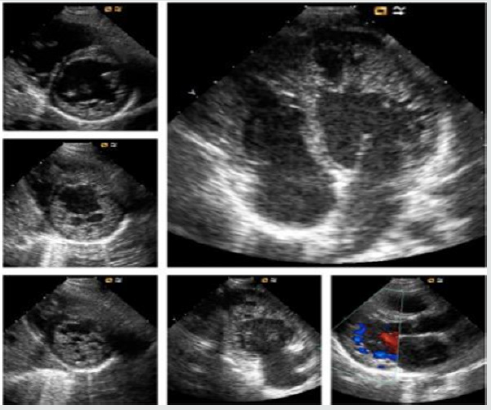
Figure 2: Echocardiographic features of a young adult of 16-years old, admitted in emergency room with acute signs and symptoms of acute heart failure.
Morpho Pathogenesis
The primitive hypothesis concerning LVNC is that the presence of HTXs is due to a failure in myocardial morphogenesis, in which the immature, non-compacted fetal myocardium normally undergoes a physiological compaction process during ontogenesis [1]. LVNC may occur isolated, in familial forms or associated with several congenital, genetic, neuromuscular and chromosomal conditions [9]. Mutations in the sarcomere gene, particular in MYH7, are the most common and non- sarcomere gene mutations (such as TAZ and NOTCH1) have also reported [10-12]. Some LVNC individuals have been detected by tracking asymptomatic relatives of affected patients [9], and therefore, a close correlation between genotype and phenotype has been recently underlined by 2 recent studies using NEXT-generation sequencing [13,14]. All these pathogenic variants were independent risk factors for cardiovascular events [13]. In addition, mutations in hyperpolarization-activated cyclic nucleotide channel 4 (HCN4) have also been reported in families with sinus node dysfunction and LVNC [15,16].
Epidemiology
Since only few studies focused on LVNC incidence, both prevalence and mortality are challenging to assess [1]. Among adults, it can be diagnosed in 0.05% - 0.26% of the cases, and approximately 0.14% of pediatric patient, with an overall mortality ranging from 5% to 47% in both populations [1]. Unfortunately, all the reports are affected by the diagnostic criteria adopted, imaging or autopsies, overestimating or underestimating the real prevalence [1,17] .In addition, and similarly with others cardiomyopathies, LVNC can be a subtle disease [17]; if not promptly diagnosed, patients may be asymptomatic for a long time and the onset may range from the early life to the adulthood [8].
Diagnosis
The advent of increasingly sophisticated cardiac imaging techniques set the spotlight on HXTs as a very common finding, instead of the rare disease that was previously considered [2,18,19]. Accordingly, Jenni, Chin and Stollberg defined different echocardiographic criteria, while Petersen, Jacquier, Captur and Stacey defined some cardiovascular magnetic resonance (CMR) criteria. 1 Unfortunately, they both showed very poor specificity [7,8], even if CMR overcame the ultrasound-related limits in morphologic assessment, and demonstrated a thigh correlation between late gadolinium enhancement myocardial fibrosis and clinical severity of the disease [20]. Nevertheless, there still a lack of an imaging-driven diagnostic gold standard [1,9].
Clinical Aspects and Treatment
Clinical findings are variable, including several grades of diastolic and systolic dysfunction, heart failure (HF), thromboembolic events, and malignant arrhythmias. 1 However, the most severe outcome is the sudden cardiac death. Atrial fibrillation, right/left bundle branch block, and repolarization abnormalities may be the only electrocardiographic features present at the moment of the diagnosis [2,8]. There is no specific therapy and LVNC management depends on the clinical manifestations; anticoagulation is indicated only if atrial fibrillation, heart failure, previous embolism, or intracardiac thrombus formation are present [1,21].
Discussion
LVNC has always been a controversial disease, with some unresolved issues. First of all, there is a heterogeneous genetic background and a wide spectrum of associated conditions that may occur contextually with HTXs. Secondly, LVNC real prevalence is still unknown. Certainly, the lack of any echocardiographic or CMR diagnostic gold standard, with frequent overestimation/ underestimation, does not help to clarify all the uncertainties. In addition, there are a wide variety of overlapping conditions that may occur with secondary myocardial HXTs, for example, a dilated cardiomyopathy or the end-stage hypertrophic cardiomyopathy (Figure 3). On the other hand, it is common experience that not all the above-mentioned cardiomyopathies and conditions can present an end-stage non-compacted phenotype and generalizing this aspect would be very simplistic. 8 In addition, Lorca and colleagues recently reiterated that non-compacted forms of cardiomyopathy do exist, especially during early stages of life, and they can be demonstrated in some forms of unexplained sudden deaths. 17 Furthermore, a multicenter longitudinal prospective study [7], despite its conclusions, and if carefully read between the lines, suggests that a significant proportion of asymptomatic patients meets all currently used imaging diagnostic criteria for LVNC.
Figure 3: Cardiac magnetic resonance imaging (CMR) exams of patients matching the currently imaging criteria for CMR (A, B, C, 2-chambers view; a, b, c, 4chambers view). (A, a) A 23-years-old male patients with a dilated cardiomyopathy. (B, b) a 38-years old woman with an end-stage hypertrophic cardiomyopathy. (C, c) a 45-years old woman admitted in emergency room for acute heart failure, and history of silent cerebral infarcts.
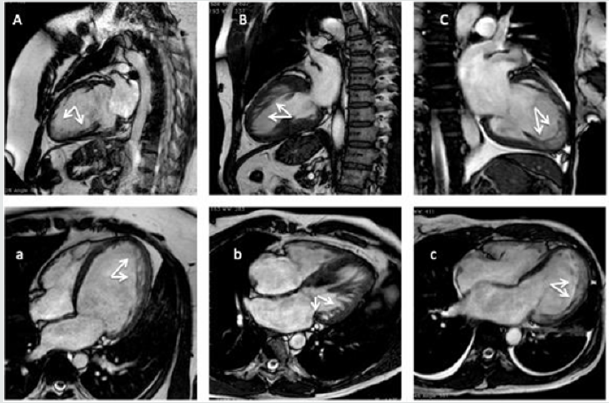
However, they demonstrated that outcomes are increased by symptoms and clinical conditions when associated with a noncompacted phenotype. Indeed, a recently published multicenter register provided an accurate estimation of genetic-phenotype association, and clinical and events of LVNC patients, concluding that the clinical course of symptomatic LVNC patient with a genotype-phenotype matching can be severe [13,14]. Given all these premises, it is reasonable considering the existence of a primitive non-compacted disease, congenital, and a mild form, with a late-onset, and/or acquired conditions. HF, thromboembolic events, and malignant arrhythmias seem to constitute the clinical triad for LVNC patients, and sudden cardiac death the most severe outcome. There is a tight genotype-phenotype correlation, with a wide spectrum of genes and mutation involved, as well as hypertrophic cardiomyopathy, for example, and the current imaging-derived diagnostic criteria probably need to be revised; perhaps it should be worth to combine some imaging and clinical criteria. At last, multicentric registries should be considered to help the real prevalence assessment of non-compacted forms of cardiomyopathies.
Conclusion
It is current opinion of the authors that a “non-compacted cardiomyopathy” (NC-CMP) seems to be the most comprehensive definition of a disease that, similarly to the other cardiomyopathies, and regardless of its etiology, beyond a peculiar phenotype shares a distinct symptomatology and deserves to be listed as an entity between cardiomyopathies.
References
- Finsterer J, Stollberger C, Towbin JA (2017) Left ventricular noncompaction cardiomyopathy: cardiac, neuromuscular, and genetic factors. Nat Rev Cardiol 14(4): 224-237.
- Stöllberger C, Finsterer J (2004) Left ventricular hypertrabeculation/ noncompaction. Journal of the American Society of Echocardiography 17(1): 91-100.
- Stacey RB, Andersen M, Haag J, Hall ME, McLeod G, et al. (2014) Right ventricular morphology and systolic function in left ventricular noncompaction cardiomyopathy. Am J Cardiol 113(6): 1018-1023.
- (1996) Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation 93(5): 841-842.
- Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F (2008) Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 29(2): 270-276.
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, et al. (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113(14): 1807-1816.
- Andreini D, Pontone G, Bogaert J, Roghi A, Barison A, et al. (2016) Long- Term Prognostic Value of Cardiac Magnetic Resonance in Left Ventricle Noncompaction: A Prospective Multicenter Study. J Am Coll Cardiol 68(20): 2166-2181.
- Weir-McCall JR, Yeap PM, Papagiorcopulo C, Fitzgerald K, Gandy SJ, et al. (2016) Left Ventricular Noncompaction: Anatomical Phenotype or Distinct Cardiomyopathy? J Am Coll Cardiol 68(20): 2157-2165.
- Ikeda U, Minamisawa M, Koyama J (2015) Isolated left ventricular noncompaction cardiomyopathy in adults. J Cardiol 65(2): 91-97.
- Ichida F, Tsubata S, Bowles KR, Haneda N, Uese K, (2001) Novel Gene Mutations in Patients With Left Ventricular Noncompaction or Barth Syndrome. Circulation 103(9): 1256-1263.
- Luxan G, Casanova JC, Martinez-Poveda B, Prados B, D’Amato G, et al. (2013) Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat Med 19(2): 193-201.
- Van Waning JI, Caliskan K, Hoedemaekers YM, van Spaendonck-Zwarts KY, Baas AF, et al. (2018) Clinical Features, and Long-Term Outcome of Noncompaction Cardiomyopathy. J Am Coll Cardiol 71(7): 711-722.
- Sedaghat Hamedani F, Haas J, Zhu F, Geier C, Kayvanpour E, et al. (2017) Clinical genetics and outcome of left ventricular non-compaction cardiomyopathy. Eur Heart J 38(46): 3449-3460.
- Wang C, Hata Y, Hirono K, Takasaki A, Ozawa SW, et al. (2017) A Wide and Specific Spectrum of Genetic Variants and Genotype-Phenotype Correlations Revealed by Next-Generation Sequencing in Patients with Left Ventricular Noncompaction. J Am Heart Assoc 6(9).
- Milano A, Vermeer AM, Lodder EM, Barc J, Verkerk AO (2014) HCN4 mutations in multiple families with bradycardia and left ventricular noncompaction cardiomyopathy. J Am Coll Cardiol 64(8): 745-756.
- Schweizer PA, Schroter J, Greiner S, Haas J, Yampolsky P, et al. (2014) The symptom complex of familial sinus node dysfunction and myocardial noncompaction is associated with mutations in the HCN4 channel. J Am Coll Cardiol 64(8): 757-767.
- Lorca R, Martin M, Reguero JJ, Diaz-Molina B, Moris C, Lambert JL, Astudillo A (2016) Left ventricle non-compaction: The still misdiagnosed cardiomyopathy. Int J Cardiol 223: 420-421.
- Duncan RF, Brown MA, Worthley SG (2008) Increasing identification of isolated left ventricular non-compaction with cardiovascular magnetic resonance: a mini case series highlighting variable clinical presentation. Heart Lung Circ 17(1): 9-13.
- Sandhu R, Finkelhor RS, Gunawardena DR, Bahler RC (2008) Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction. Echocardiography 25(1): 8-12.
- Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M (2011) Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail 13(2): 170-176.
- Arbustini E, Favalli V, Narula N, Serio A, Grasso M (2016) Left Ventricular Noncompaction: A Distinct Genetic Cardiomyopathy? J Am Coll Cardiol 68(9): 949-966.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...


