Lupine Publishers Group
Lupine Publishers
Menu
Research Article(ISSN: 2770-5447)
Iron-Based Nanoparticles, an Accurate and Powerful Sniper Targeting SARS- Cov-2 Volume 3 - Issue 3
Nura A Mohamed1,2*, Isra Marei2,3, Sergio Crovella1, and Haissam Abou-Saleh1,4
- 1Department of Biological and Environmental Sciences, College of Arts and Sciences, Qatar University, Qatar
- 2Department of Cardiothoracic Pharmacology, National Heart and Lung Institute, Imperial College, SW7 2BU, London, UK
- 3Department of Pharmacology, Weill Cornell Medicine-Qatar, Doha-Qatar
- 4Biomedical Research Center, Qatar University, Doha, Qatar
Received:November 20, 2020; Published: December 02, 2020
Corresponding author: Nura A Mohamed, Department of Biological and Environmental Sciences, College of Arts and Sciences, Qatar University, Doha-Qatar
DOI: 10.32474/ACR.2020.03.000162
Abstract
For the past few months, the world has been facing another coronavirus disease, COVID-19, caused by the SARS- CoV-2, giving the rise of a pandemic. In the vast majority of infected individuals, SARS-CoV-2 causes a mild ailment, but in some subjects, it progresses to severe disease or even death, with some groups being at high risk. However, SARS-CoV-2 is not the first coronavirus that caused serious, sometimes fatal, disease. Almost 20 years ago, SARS-CoV and later MERS were coronaviruses that led to severe diseases but did not result in pandemics. Some of the therapeutic lessons learned during the SARS-COV and MERS epidemics are being used now to treat COVID-19 patients, such as the still debated use of Chloroquine. However, there were other important preclinical studies performed around the time of SARS-COV and MERS epidemics that may also be useful applications in the COVID-19 context. This review highlights the benefits that could be gained by revising the non-conventional therapeutic approaches used in the previous coronavirus epidemics in improving the detection, treatment, and prevention tools and developing patient treatment follow-up strategies. Specifically, this review discusses the utilization of iron oxide nanoparticles due to their attractive properties. It also highlights the therapeutic opportunities and future directions of the iron oxide nanoparticles to be eventually employed in the current coronavirus pandemic.
Keywords: SARS-Co V-2; COVID-19; Nanomedicine; Iron Oxide Nanoparticles
Abbreviations:ACE Inhibitors= ACEIs; Acute Lung Injury= ALI; Acute Respiratory Distress Syndrome= ARDS; Angiotensin Converting Enzyme-2= ACE2; Angiogenesis II= AngII; Angiotensin Receptor Blockers= ARB; Antisense Oligonucleotide= ASO; Black, Asian and Minority Ethnic= BAME; Cardiovascular Diseases= CVDs; Computed Tomography= CT; Diabetic Melitus= DM Intensive Care Unit= ICU; Interferon-α= IFN-α; Interferon-β= IFN-β; Iron Oxide NPs= IONPs; Mas Receptors= MasR Middle East Respiratory Syndrome Coronavirus= MERS-CoV; Nanoconjugates= NCs; Nanoparticles= NPs; Nanozymes= IONzymes; Nitric Oxide= NO; Norwegian University of Science and Technology= NTNU; Phosphodiesterases-5= PDE5; Poly (amino ester) with carboxyl groups (PC)-coated magnetic NPs= pcMNPs; Prorenin receptor- Ang II type 1 receptor= PRR-ACE-Ang II-AT1R; Pulmonary Arterial Hypertension= PAH; Reactive Oxygen Species= ROS; Receptor Binding Domain= RBD; Renin Angiotensin System= RAS; Reverse Transcription Polymerase Chain Reaction= RT-PCR; Royal Gwent Hospital= RGH; Severe Acute Respiratory Syndrome Coronavirus= SARS-CoV; Spike protein= S; Transmembrane Protease Serine 2= TMPRSS2; Tumour Necrosis Factor= TNF; World Health Organization= WHO
Introduction
Outbreaks caused by infectious diseases represent a tremendous challenge to humanity, with coronaviruses accounting for a relevant part of them[1]. Coronaviruses are a single-stranded positive-sense RNA family that can affect several vertebrate hosts. In the past, these viruses were known to cause minor to mild upper respiratory tract diseases with symptoms similar to the common cold in many cases. However, in the last 17 years, three aggressive human coronavirus pathogens appeared as we witnessed the emergence/re-emergence of zoonotic diseases causing epidemics and pandemics. These include the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV)1 with 8098 infected cases and 774 [2] deaths, the Middle East respiratory syndrome coronavirus (MERS-CoV) with 2494 infected cases and 858 [3] deaths, and the most recent outbreak of the novel coronavirus (SARS-CoV-2 (COVID-19) which was first reported in Wuhan-China in December 2019 and was considered a pandemic on March 11th 2020 [4]. As of November 20th, 2020, there are 57,236,335 reported cases, of which 39,722,802 recovered and 1,365,634 died[5]; the current pandemic originated enormous global health, social and economic crises1. SARS-CoV-2 succeeded in crossing species barriers to infect humans and can now be effectively transmitted from one person to another1. So far, 7 coronaviruses affecting humans are known: SARS-CoV, MERS-CoV, and SARS-CoV-2 causing symptoms that start with influenza-like signs that either stays mild and disappears or escalates to severe lung injuries with multiorgan failure, acute respiratory distress syndrome (ARDS), and acute lung injury (ALI); the remaining 4, namely HKU1, NL63, OC43, and 229E, are associated with mild respiratory symptoms [4, 6]
The death rate of SARS-CoV-2 increases by age and the
presence of other comorbidities1. SARS-CoV-2 mechanism of action
affects three host components: blood, inflammation, and cellular
components. In the blood component, the viral proteins “ORF10
and ORF3a” coordinate to attack the heme part of the hemoglobin
(1-beta chain), which results in the dissociating of the iron to form
porphyrin, decreasing the amount of the functional hemoglobin,
which leads to the development of anemia and respiratory distress
symptoms [7]. In the inflammation component, it was noticed that
the overproduction of proinflammatory cytokines -such as IL-6,
IL-1β, and tumor necrosis factor (TNF)- could cause a SARS-CoV-2-
induced cytokine storm in addition to the accumulation of the fibrin
and thrombin [8]. The sudden cytokine increase elevates the risk
of developing vascular hyperpermeability and multiorgan failure,
eventually leading to death. The inflammatory component of the
SARS-CoV-2 includes a significant upsurge in the reactive oxygen
species (ROS), triggering redox imbalance, mitochondrial and
lysosomal dysfunction, and rendering the cells even less resistant
to infection. Such a sequence of events can result in serious and
permanent long-term cellular damage, which accelerates the
immune system’s aging process and affects tissues/organs (i.e. lung)
[8]. In addition, the inflammatory component is responsible for the
formation of the ground-glass-like figure of the COVID-19 lung.
This indicates that developing immunomodulators or therapeutic
strategies to target the inflammatory component, ROS, and the
overactive cytokines could be one of the SARS-CoV-2 therapeutic
options [7, 9]. The cellular component is the most crucial one in the
pathogenesis of SARS-CoV-2, which is why we will be discussing it
in depth. So far, angiotensin-converting enzyme-2 (ACE2) receptors
have been considered as the primary cellular entry point for three
strains of coronavirus: NL63, SARS-CoV, and the novel SARS-CoV-2
[10]. ACE2 receptors are expressed ubiquitously in the blood
vessels, heart, lung, kidneys, gut, brain, testis [11], and salivary
glands [12]. It is expressed in rodents and humans, and present
bound to the cell membrane with low levels in the plasma [11].
The nasal epithelium is now considered the portal entry for
the SARS-CoV-2 infection and transmission, where higher viral
loads were found in symptomatic and asymptomatic patients. The
virus’s entry depends on the binding of the spike (S) protein to a
specific cellular receptor, which then initiates a series of cellular
proteases. A crucial and limiting factor for the viral entry and the
initial infection is the ACE2 receptor facilitated by host cell-derived
serine protease, the transmembrane protease serine 2 (TMPRSS2).
It is worth noting that TMPRSS2 could be used as an alternative
entry route for the SARS-CoV-2 using cathepsin B/L [13, 14]. The
S protein’s high affinity towards the ACE2 is now considered an
important determinant factor for the viral replication rate which
in turn determines the severity of the disease [15]. Datasets for
multiple human tissues, including airways, were retrieved from
published/unpublished databases that could be found on European
Genome–phenome Archive (https://www.ebi.ac.uk/ega/home),
GEO [16], and MedGen. These data were analyzed by both Vieira et
al. [17] and Deprez et al. [18]. Results showed a high level of ACE2
expression in nasal secretory cells, multiple types of epithelial cells
across the airway, and the alveolar epithelial type II cells13. Looking
back at the history and importance of the ACE2 receptors, we will
find that the renin-angiotensin system’s (RAS) classical view has
dramatically changed since the discovery of the ACE2 receptors.
Since then, ACE2 was classified as a membrane-bound receptor; it
cleaves the angiogenesis II (AngII) to generate the active peptide
Ang (1–7). The cellular actions of the Ang (1–7) are then mediated
by Mas receptors (MasR) [19] to perform cardio- protective effects.
ACE2 Expression Modulators
ACE2 expression is modulated by the following factors: age, ethnicity, sex, and the existence of other health comorbidities: in SARS-CoV2, these factors have an impact on the susceptibility and severity of the disease11. An in vivo study evaluating the age effect on the expression of several cardiac markers between two groups of 24 month-old and 12 month- old mice showed that 24 month-old ones expressed significant enhancement of the prorenin receptor - Ang II type 1 receptor (PRR-ACE-Ang II-AT1R) axis with a reduction in the ACE2/ Ang (1–7)/MasR axis [20]. Global studies from China, Italy, Spain, UK, and the USA showed that elder people have low expression of ACE2 in the lung11 and therefore are more susceptible and fragile to the disease, which could be attributed to the virus ability to further lower the ACE2 baseline expression in the ACE2 low producing cells [11]. This might be attributed to the fact that aging is a major factor in the development of cardiovascular diseases (CVDs) due to endothelial cell dysfunction and inflammation [21]. Also, the fetal dataset retrieved and analyzed from GEO16 revealed the low to no expression of the ACE2 with no co-expression of the TMPRSS2, which might partially explain why SARS-CoV-2 represents a low risk to young generations [13, 14, 22]. In terms of ethnicity and sex, it was shown that the diverse genetic basis in the Black, Asian, and Minority Ethnic (BAME) groups could indeed affect ACE2 functions. Also, it has been shown that other ethnic groups have some structural variations of the ACE2, that confirmed its protective effects due to its low binding affinity to the viral S protein [11]. This could be the reason why people from the BAME origins have high SARS- CoV-2 susceptibility due to the increased affinity of their ACE2 variation to the viral S protein showing more disease severity, which might contribute to the death rate [23-25]. Additionally, Baumer et al. [26] showed that 35% of the SARS-CoV-2 cases that were admitted to the intensive care unit (ICU) and 35% of the deaths in the Royal Gwent Hospital (RGH), Newport, Wales, were of BAME ethnic descent. Another study showed that men had a higher chance of getting SARS-CoV-2 as their cells express a higher percentage of ACE2 compared to women [23, 24].
Risk factors such as elder age and comorbidities (namely, CVDs, Diabetic Mellitus (DM), obesity) are known to worsen SARS-CoV-2 severity, eventually leading to death. Patients with the above-mentioned risk factors share one feature that worsens their SARS-CoV-2 condition, which is the involvement of the endothelial cells in the progression of the SARS- CoV-2 with endothelial cells having different expression profiles of the ACE2 in patients with different comorbidities. The up or downregulation of the expression of this receptor can either benefit or harm the SARSCoV- 2 patients depending on which group they belong to; As it determines the patient’s response to the viral invasion. ACE2 is generally expressed by endothelial cells and plays an important role in vasodilation, anti-hypertrophy, and anti-fibrosis [27]. Under normal circumstances, the downregulation of ACE2 leads to an increased function with the ACE/AngII axis inducing antiapoptotic, thrombotic, proinflammatory, and vasoconstriction effects; while the upregulation of the ACE2/ Ang (1–7)/MasR axis exhibits various cardiovascular protective effects including the vasodilatory, anti-proliferative, anti-atherogenesis, antithrombosis, and antifibrotic effects. Dysregulation between these two axis was reported in SARS-CoV-2 infected patients from high-risk groups. An example of the dysregulation between the two axis was reported in pulmonary arterial hypertension (PAH); with idiopathic or heritable PAH patients expressing an increased level of AngII levels with markedly decreased levels of phosphorylated status (Ser-680) of ACE2 whereas, PAH due to congenital heart disease showed decreased levels of serum ACE2 and Ang (1–7). In contrast, DM and other CVDs such as hypertension are considered high-risk factors for the severity of SARS-CoV-2 due to the significant increase in the expression of the ACE2 receptors increasing the viral entry points [28]. Lately, evidence has emerged, linking obesity to the poor prognosis of patients with SARS-CoV-2. This is attributed to comorbidities associated with obesity; these comorbidities are caused by the endothelial dysfunction leading to hypertension and inflammation [29, 30]. The downregulation of the ACE2 in PAH and old age groups might seem beneficial at first glance, but it also means that once infected, the virus will cause a further deficiency in the low ACE2 bassline, which will worsen the condition. In this category, using the ACE inhibitors (ACEIs) and Angiotensin Receptor Blockers (ARB) could be harmful [24], unlike in DM and other CVDs where they might help as ACE2 is highly expressed in these conditions [31,32]. Surprisingly, respiratory allergies, asthma, and controlled exposure to allergen were not considered high risks for developing severe SARS- CoV-2 despite having a low expression of ACE2 [33]. The fact that PAH, despite having a decreased level of ACE2 expression, is still considered a high SARS-CoV-2 risk group indicates that there might be other factors beyond ACE2 expression, modulating the response of the respiratory allergies, asthma and controlled exposure to allergen groups to SARS-CoV-2 that we are not aware of.
Therapeutic Lessons from Past Coronaviruses
The fact that SARS-CoV-2 shares several similarities with SARSCoV as it belongs to the β-coronavirus genus, and its receptorbinding domain (RBD) resembles the SARS-CoV one indicates that they may share the same ost-cell receptor6. As such, some medications used to treat SARS-CoV were considered for SARSCoV- 2, like Chloroquine. Chloroquine is an immune modulator used in treating parasitic malaria infections; it inhibits the binding of the parasite to the ACE2 receptors, with some studies showing the antiviral activity of the drug against SARS-CoV-2 [34]. The fact that this medication can act on the ACE2 receptors promoted its use in SARS-CoV and SARS-CoV-2 [35] as it works either by inhibiting the viral fusion through the cell membrane, preventing the glycosylation of the host cell’s receptors, or preventing the assembly of the virus in the endoplasmic reticulum [34]. It also inhibits the autophagy process [36, 37]. Ribavirin is another SARSCoV antiviral drug that was employed for SARS-CoV-2 treatment [38]. The Ritonavir combination with lopinavir, other SARS-CoV antiviral drugs were also evaluated as a therapeutic option. Due to the involvement of inflammation in COVID-19, there have been signs of the benefit of using interferon-α (IFN-α) and interferon-β (IFN-β) treatments. Furthermore, some MERS-CoV in vitro and animal in vivo experiments demonstrated that combining the ribavirin and INFs treatments improved the clinical outcomes [39]. However, translating these findings to be used in the routine clinical treatment of SARS-CoV-2 infected patients needs further investigations [39]. The fact that ACE2 is expressed by various cardiovascular cells also indicates that any imbalance between the ACE axis can harm the heart, with the increase in the ACE/AngII being associated with heart failure while ACE2/ Ang (1–7)/MasR can prevent such event. Formulating ACEIs and ARB can protect from cardiovascular complications. However, their role in the lung is still unknown and requires further investigation to understand better if they can be used to ameliorate the ALL induced by SARS-CoV-211. SARS-CoV-2-related endothelial cell dysfunction was shown to be accompanied by a reduction in nitric oxide (NO) production. NO was shown to significantly inhibit the viral replication cycle by inhibiting the synthesis of the viral proteins and RNA [40], which gave the scientists another possible therapeutic angle by improving the bioavailability of NO. This is done by either supplementing patients with NO or improving the intrinsic NO bioavailability by inhibiting its degradation through the use of phosphodiesterases-5 inhibitors (PDE5) such as Sildenafil [41]. The NO bioavailability can be improved by packaging it in nanocarriers, which increased the interest in combating the virus by implementing nanomedicine tools. Nanomedicine offers many attractive properties that can overcome the current limitations and surpass the present conventional therapeutic and detection tools.
Current SARS-CoV-2 Nanomedicine Applications
Viruses are often considered nature’s devil nanocarriers. They
package their genetic materials, protect it from the immune system,
prolong its presence in the biological system, deliver it to the host
cell, and then transfer it from infected cells to other destinations.
They are nature’s evolutionary work of art with many unique
features, making them smart, attractive, capable, yet dangerous
nanoparticles (NPs). Many researchers in the nanomedicine field
proposed the use of virus-based NPs as gene therapy tools [42].
Viral nanocarriers are double-edged swords that should be studied
thoroughly for their benefits/damages. Indeed, to win the battle of
COVID-19, we need to think and implements tools that reassemble
the virus’s nature. Therefore, the use of nanomedicine to face
SARS-CoV-2 is now being evaluated. The nanomedicine community
today, more than ever, can significantly contribute to this battle
with nanomaterials being developed and thoroughly investigated.
Governments/countries, research/academic institutes, health
organizations, and charity foundations are providing substantial
funds to aid in developing nano-formulations that could be used as
diagnostics tools, nanocarriers for therapeutics (drugs and vaccine)
as well as developing non-conventional nano-therapies. Ongoing
nanomedicine research includes the development of a rapid pointof-
care diagnostic tool that will enable us to screen a higher number
of persons to capture the silent careers and improve the detection
threshold to detect the virus at the early infection stages.
Having such a detection tool will aid in the viral surveillance
monitoring to identify regions with increased infection rate using a
time effective procedure. In addition, creating a low-cost detection
nanocarrier means that low-income communities will have access
to such detection tools. Another angle by which we can fight SARSCoV-
2 is reusing existing therapeutic agents while innovating new
ones. By understanding the basic interaction between the SARSCoV-
2 and the host cell, we can develop a nanocarrier that can either
block the interaction or act as a new host for the virus. We can also
use that interaction to our benefit and design a nano-vaccine. Despite
being at the preliminary stages, antiviral nanomedicine therapies
are promising, cost-effective, and have high-quality properties that
could open new avenues in the prevention, diagnosis, and treatment
of COVID-19. The physical size of SARS-CoV-2 makes the relevance
of nanotechnology clearer, which is also supported by the antiviral
research using nanomaterials [43]. Due to the global pandemic and
emerging need for developing prevention and treatment strategies
for COVID-19, the World Health Organization (WHO) adopted the
strategy of repurposing existing nanomaterials to develop drug/
vaccine nano-therapies, detection tools, and antiviral coatings.
Nano-Detection Tools
Current Nano-based diagnostic tools suggested for SARS-CoV-2 include a nano-based-colorimetric bioassay that was developed using gold-NPs; these NPs were capped with a thiol-modified antisense oligonucleotide (ASO) that is specific for N-gene (nucleocapsid phosphoprotein) of the SARS-CoV-2. Capping the gold-NPs is important to ensure the efficient detection of SARSCoV- 2 in COVID-19 patients within 10 minutes [44]. Another study reported the use of polymer-stabilized multivalent gold-NPs functionalized with sialic acid derivative to make it interact with the viral spike glycoprotein [45]. Reverse Transcription Polymerase Chain Reaction (RT-PCR)-based methods are considered SARS-CoV2 gold standard detection tool. However, in their systematic review, Rodriguez et al. [46] showed that this detection tool has falsenegative rates of 2-33%46 in repeat sample testing. Also, Cohen and Kessel [47] showed that the SARS-CoV-2 RT-PCR detection tool has false-positive rates 0-16% [47] in repeat sample testing. To overcome the false positive/negative results, a study showed the possibility of increasing the RT-PCR precision using fluorescentlabeled- NPs that are conjugated to viral RNA specific probes [48]. Additionally, another group developed a Nano plasmonic sensor chip that has an extraordinary time efficiency (<15 min) and sensitivity (LOD = 370 vp/mL) as it detects the entire SARSCoV- 2 virus [49]. Finally, the Norwegian University of Science and Technology (NTNU) formed a collaboration with St. Olavs Hospital to develop an iron oxide nanoparticle-based detection Tools [50].
Nano-Based Vaccines
Nano-based vaccines are also drawing attention in facing the
current pandemic. The use of nanotechnology in the development
of vaccines is called ‘Nanovaccinology’; Nanovaccinology is
considered an alternative and effective tool that can substitute the
conventional vaccines. This is attributed to:
i) their tailorable surface properties and improved stability,
ii) immuno-stimulatory properties,
iii) high payloads,
iv) tunable sizes, which determines the cellular uptake rate,
and
v) controllable drug release kinetics [51, 52]. Materials used
in the synthesis of the NPs, their surface chemistry, and size are
important factors in determining which cells will be activated and
the potential immune response that will be triggered [53, 54].
They will also determine the vaccine release rate, pharmacokinetic
properties, biodistribution, and the bioavailability of the
immunogenic agents. Vaccines are developed from:
i) inactivated/killed pathogens (first-generation vaccines),
ii) synthetic
peptides (second-generation vaccines),
iii) DNA vaccines (third-generation vaccines), and/or iv) liveattenuated microorganisms [53]. The efficacy of these conventional vaccines depends upon using appropriate delivery systems, therefore conjugating them with NPs can improve their efficacy. Nano-based vaccines can benefit from the added NP’s properties in guiding the vaccine to the immune cells enhancing the antigen uptake and therefore boosting the host’s immunity, and due to the high cellular uptake of some NPs, this can lead to the induction of humoral and cellular responses50. A study showed the possibility of using iron oxide NPs (IONPs), which are currently being employed to treat anemia as nanovaccine due to their in vitro antiviral activity [55]. Additionally, other promising nanomaterials are being studied with 4 COVID-19 nano-based formulations currently in trials, ClinicalTrials.Gov [56]. One nano-vaccine that is currently being investigated employs lipid nanoparticles to carry viral mRNA [57]. Additionally, other studies showed the effectiveness of using Chloroquine NPs [58] as well as the currently ongoing inhaled NO NPs trial [59]. Furthermore, scientists in the nanomedicine field are currently trying to promote nanomaterials in treating and preventing pneumonia caused by SARS-CoV-243. Nanoconjugates (NCs) Based Stem Cell Therapy Patients suffering from SARS-CoV-2 can develop virus-induced lung injuries and, to some extent, present abnormalities in liver function. The fact that some patients suffered from life-time damages in these organs led to considering the use of the Nanoconjugates (NCs) Based Stem Cell Therapy [60]. The main two challenges facing stem cell therapy are i) the low cell retention and survival rate, which in turn affects their repair capacity, and ii) the difficulty monitoring cellular behavior and fate [61]. Due to the NPs superior physical and chemical properties, NCs Based Stem Cell Therapy can overcome these limitations. In this approach, NPs are loaded/conjugated with functional agents (e.g., dye, gene, targeting ligands….etc) that could be easily taken up by the desired or studied stem cell type to genetically engineer them61. Alternatively, NPs could be used to selectively label stem cells to monitor their behavior and determine their fate; additionally, cells can be labeled with NPs that are surface modified with materials (targeting ligands) that could enhance their retention rate in the desired tissues [61]. NCs were shown effective in autoimmune disorders, CVDs, cancer, …..etc. and are currently being investigated to overcome SARS-CoV-2 induced organ damages [60]. Nano-coating tool NPs from the metals and metal oxides such as iron oxide [62], zinc oxide[63], silica (SiONPs) [64], gold (AuNPs) [64], silver (AgNPs) [65], and cuprous oxide (CuONPs) [66], possesses antibacterial and antiviral properties on their own. For that purpose, surfaces and equipment are coated with them to prevent any bacterial or viral contaminations. Especially ventilators, which are important equipment that are used for treating patients with sever SARS-CoV-2; this will help in better managing ventilator-associated pneumonia [67, 68]. Nowadays, the SARS-CoV-2 nanomedicine research is interested in implementing inorganic NPs of specifically the iron oxide nanoparticle to be used as a detection tool, therapeutic and theragnostic agents in this pandemic for the many attractive properties they have39.
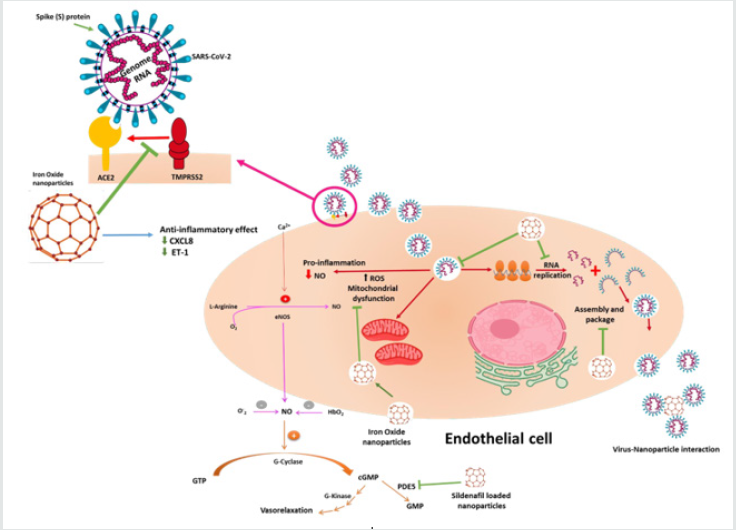
Could iron oxide NPs be the magic bullet for SARSCoV- 2?
From the above ongoing SARS-CoV-2 nanomedicine research,
we chose to discuss the super magnetic and iron oxide NPs as they
have great potential in helping us fight SARS-CoV2. Previous results
evaluating iron oxide NPs showed that they have:
i) anti-inflammatory effects on human endothelial cells and
human smooth muscle cells [69],
ii) anti-inflammatory effect on mouse macrophages, and
iii) showed no toxicity to endothelial cells from different
origins [70]. With one prototype being loaded with the PDE5
inhibitor, Sildenafil69which can be used as a SARS-CoV-2
therapeutic option. We understand the importance of the
vasculature component to the recovery from SARS-CoV-2,
particularly the endothelial and inflammatory cells [70].
Therefore, we and others emphasized on the use of this ironbased
nano-formulation for the detection and future treatment
of COVID-19 disease by benefiting from the current knowledge
of SARS-CoV-2 viral invasion, replication and survival cycles as
demonstrated in (Figure 1).
In terms of SARS-CoV-2 detection and COVID-19 therapeutic
option, there is an interest in iron oxide NPs to be further
investigated. SARS-CoV-2 current detection method relies on
using RT-PCR; despite being accurate, this method is hampered
by the sample processing steps. Zhao et al. developed a rapid,
inexpensive, and safe detection procedure by using the poly
(amino ester) with carboxyl groups (PC)-coated magnetic NPs
(pcMNPs). The pcMNPs, combine the lysis and binding steps into
one step, enabling purifying the viral RNA from several samples
using manual or automated methods within ≥ 20 min [71]. In
support of these findings, another study by Chen et al. showed that
iron oxide NPs offer a better viral detection tool [72]. In terms of
anti-inflammation/antioxidant effects, iron oxide NPs were shown
to have enzyme-like activity, making them classified as nanozymes
(IONzymes). Nano-enzymes are favored over natural enzymes as
their activity can be modified by tailoring the nanoparticle’s size,
shape, and surface properties. The IONzymes, being the most
typical nano- enzyme, can perform two enzymes like function, the
peroxidase, and catalase activities. The nanoparticle’s composition,
surface modification, and the environment pH determines which
enzymatic activity IONzymes will perform73. For example, at acidic
pH conditions, they exhibit peroxidase-like activities. Because of
this activity IONzymes are used as biomarker detection tools in
several diagnostic immunoassays that are capable of detecting the
presence of Ebola, diabetes and certain tumours [74]; in addition,
IONzymes have antibacterial effects and improve wound healing
process74. Under neutral conditions,
IONzymes exhibit catalase-like activities, reducing the ROS,
therefore improving the anti-inflammatory processes [74].
Recent studies showed that SARS-CoV-2 infection is accompanied
by a significant increase in the ROS activity, which indicates the
therapeutic benefit of using IONzymes in the management of SARSCoV-
2 infection [8, 75]. This catalytic activity was previously shown
effective in the peroxidation of the viral lipid envelope, inactivating
the enveloped viruse [73]. Furthermore, the antioxidant effect of
the iron oxide NPs can be further enhanced through the surfacefunctionalization
of the NPs with naturally occurring antioxidant
such as gallic acid [76] and dextane conjugated trypsin [77].
In terms of their cardiovascular benefit, a study conducted by
Duan et.al showed that iron oxide NPs could be used in treating
CVDs associated with oxidative stress as they functioned as an autophagic-related antioxidant in HUVECs [78]. In addition, iron
oxide NPs were shown to have serine protease inhibition activity,
indicating that they might inhibit the TMPRSS2 that is required in
the binding of the SARS-CoV-2 to the ACE2 without disturbing the
ACE/AngII and ACE2/ Ang (1–7)/MasR axis balance. This inhibition
can provide an extremely valuable option in the treatment and
prevention of the SARS-CoV-277. The exact interaction between
the TMPRSS2 and the iron oxide NPs, however, still needs further
investigation. In addition, iron oxide NPs were shown to possess
antiviral effects against H1N1 influenza A virus, as they inhibit the
virus from binding to host cells (i.e. lung epithelial cells) [79]; also,
a recent study reported that iron oxide NPs can induce membrane
lipid peroxidation in synthesized liposomes, ending the viral
replication cycle, which makes it a universal antiviral strategy [73].
Furthermore, it was shown that iron on its own is considered an
attractive component to the virus as it is essential for viral survival,
cellular invasion, and replications [7]. This explains the fact that
anemia is associated with SARS- CoV-2 as the virus attacks the
hemoglobin and breaks its down [7].
Therefore, thinking like a virus and creating attractive NPs for the virus might be the way to go. Having an intact nano-formulation that is hard for the virus to breakdown yet can attract the virus and interact with it might help contain the virus and prevent its rapid spread. Iron oxide NPs can attract the virus due to the presence of the iron, they are available in many forms, their stability can be tailored to range from days to months, and they possess antiviral activity with the possibility of loading/tagging them with antiviral drugs. The viral attraction to the iron oxide nanoparticle is currently being used as an antiviral tool and for rapid viral detection [71, 72]. Iron oxide NPs were shown to affect the cellular component of the SARS-CoV-2. As we know, the involvement of the endothelial cell dysfunction was proven to worsen and complicate the SARSCoV- 2 infection. Therefore, using NPs that can be taken up by the endothelial cells, reducing its dysfunctionality would be highly beneficial. So far, iron oxide NPs have been studied to mark and track endothelial cells. Some of them were shown to have no toxicity and reduce the endothelial cell’s inflammatory markers (CXCL8 and ET-1) [70]. A particular prototype, nanoMIL-89, was loaded with the PDE5 inhibitor sildenafil (generating Sil@nanoMIL-89) and investigated as an alternative tool in PAH treatment. This prototype was shown to have the above-mentioned anti-inflammatory effects on the endothelial cells, it also reduced the vascular smooth muscle cell proliferation as in PAH these cells undergo an increased proliferation rate70 and finally prolonged the Sildenafil half- life [69]. As we know, PAH is considered one of the high-risk groups in SARS-CoV-2, and developing a tool with several therapeutic properties could reduce the risk of disease worsening. Furthermore, Xiong et al. have shown and discussed the cardioprotective property of the iron oxide NPs [80]. Finally, some iron oxide NPs were shown to accumulate in the lung [70]. The lung is the most vulnerable organ to the SARS-CoV-2 infection as it has a wide alveolar epithelial cell surface expressing ACE2 receptors that is prone to viral invasion. Once infected, the lung will lose its elasticity due to the reduction in the surfactant quantity; and the consequences of the imbalance in the ACE/AngII axis and ACE2/ Ang (1–7)/MasR axis. Leading to an impairment in the gas exchanges and fibrosis, eventually causing severe bilateral peripheral pneumonia giving the lung its COVID-19 ground glass figure detected by the Computed Tomography (CT) scan 11. The high affinity of the iron oxide NPs to the lung, without causing lung oedema or showing lung toxicity in vivo, makes it interesting and valuable tool to be investigated in this pandemic [81,82].Their accumulation in the lung can be used in treating pneumonia. Caaman o and Morales showed in their study that due to their antibacterial effect, iron oxide NPs improved the antibacterial activity of erythromycin67, which, if tailored, could be used in treating COVID-19 related pneumonia. In addition, it was shown previously that iron oxide NPs inhibited the influenza A virus entry to the epithelial cells, which are the main SARS-CoV-2 entry host [79]. The possibility of aerosolizing these NPs to be inhaled adds more weight to this application, especially for patients under ventilation. Because the inhalation route will provide direct access to the most affected organ (i.e. the lung), increasing the local concentration which will improve the drug’s efficacy and avoid the systemic side effects at the same time [39]. In our opinion, NPs are an exciting adjuvant strategy that should be considered particularly when developing vaccines for infectious diseases that so far do not have effective ones, such as SARS-CoV-2. With proper research, nanomedicine could provide enormous potential for SARS-CoV-2 prevention, diagnosis, and treatment. This requires the interdisciplinary collaborations between virologists, biologists, chemists, engineers as well as consulting clinicians to implement nanomedicine in: i) developing affordable and rapid SARS- CoV- 2 diagnostic tools to be globally available (e.g. nano-antiviral sensors), (ii) developing nano-formulations (e.g. iron oxide NPs) that can prevent the viral replication and interfere with the RNA synthesis, iii) using nanomaterials that can prevent the interaction between the virus and ACE-2, and finally (iv) to use that knowledge in developing new Nano-based vaccines. Several NPs are currently being studied to be used as a detection tool, with iron oxide NPs attracting the COVID-19 scientific research. The fact that they have antimicrobial effects makes them potential strong candidates in developing contamination-safe equipment and tools.
Conclusion
Life will never be as we knew it before. This pandemic has proven that to win this fight against COVID-19, we need to focus our efforts on improving our scientific community and research capabilities. It showed us that building human capacity in research is the only way to win this battle, and that enriching the knowledge of our communities is our best weapon. Our communities must come together in sticking to the guidelines until we learn to coexist again naturally. Furthermore, our scientists, including virologists, biologists, chemists, engineers, clinicians, and healthcare workers from all over the world, need to concentrate their efforts on translating and deploying advances in the diagnosis/ treatment/prevention strategies, including nanomedicine, to the frontline.
Acknowledgements
This publication was made possible by the post-doctoral research award [PDRA3-0324-17001 and PDRA4-0129-18003] awarded for NAM and IM, respectively from the Qatar National Research Fund (a member of The Qatar Foundation). The contents herein are solely the responsibility of the author.
Conflict of Interest
All authors declare no conflict of interest
References
- Vanessa Monteil , Hyesoo Kwon, Patricia Prado, Astrid Hagelkrüys, Reiner A Wimmer, et al. (2020) Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell.
- (2003) World Health Organization (WHO).
- World Health Organization (WHO)-MERS-CoV.
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF (2020) The proximal origin of SARS-CoV-2. Nat Med. United States World meter p. 450-452.
- World Meter
- Daniel Blanco-Melo BEN-P, Wen-Chun Liu RM, Maryline Panis, David Sachs, Randy A, et al. (2020) SARS-CoV-2 launches a unique transcriptional signature from in vitro, ex vivo, and in vivo systems. bioRxiv.
- Wenzhong Liu HL (2020) COVID-19:Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism. ChemRxiv.
- Lippi A, Domingues R, Setz C, Outeiro TF, Krisko A (2020) SARS-CoV-2: At the Crossroad Between Aging and Neurodegeneration. Mov Disord 716-720.
- Mendoza Ferradas FJ, Garcia Del Barrio L, Bastarrika G (2020) Extent and Quantification of Inflammation Burden in COVID-19 by Computed Tomography. Arch Bronconeumol.
- Khan IH, Zahra SA, Zaim S, Harky A (2020) At the heart of COVID-19. J Card Surg 1287-1294.
- Verdecchia P, Cavallini C, Spanevello A, Angeli F (2020) The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med 76: 14-20.
- Pascolo L ZL, Melato M, Tricarico PM, Crovella S (2020) TMPRSS2 and ACE2 Coexpression in SARS-CoV-2 Salivary Glands Infection. J Dent Res 10: 1120-1121.
- Sungnak W, Huang N, Becavin C, et al. (2020) SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26(5): 681-687.
- Dwight L McKee , Ariane Sternberg , Ulrike Stange , Stefan Laufer , Cord Naujokat (2020) Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res 157: 104859.
- Robson B. COVID-19 (2020) Coronavirus spike protein analysis for synthetic vaccines, a peptidomimetic antagonist, and therapeutic drugs, and analysis of a proposed achilles’ heel conserved region to minimize probability of escape mutations and drug resistance. Comput Biol Med.
- Simon LM SH (2019) Single cell RNA sequencing analysis of fresh resected human lung tissue. In: Munich HZ, (ed.)
- Jian Jiang , Eirini S Fasouli, Mirjana Efremova, Roser Vento-Torm, Carlos Talavera-López, et al. (2019) A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med 25(7): 1153-1163.
- Deprez Mea (2019) A single-cell atlas of the human healthy airways. Biorxiv.
- Zhao W, Zhao T, Chen Y, Sun Y (2015) Angiotensin 1-7 promotes cardiac angiogenesis following infarction. Curr Vasc Pharmacol 13(1): 37-42.
- Yoon HE, Kim EN, Kim MY, Cheol Whee Park , Yoon Sik Chang, et al. (2016) Age-Associated Changes in the Vascular Renin-Angiotensin System in Mice. Oxid Med Cell Longev 2016: 6731093.
- Burton-Freeman IEaBM (2014) Age associated endothelial dysfunction: Role of oxidative stress, inflammation and Western Diet. Nutrition and Aging 2(2014): 197–211.
- (2020) AACE2 angiotensin I converting enzyme 2 [Homo sapiens (human)] -. Gene – NCBI.
- Cao Y, Li L, Feng Z (2020) Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV 2) receptor ACE2 in different populations. Cell Discov 6: 11.
- Li G, Hu R, Zhang X (2020) Antihypertensive treatment with ACEI/ARB of patients with COVID-19 complicated by hypertension. Hypertens Res. England 43(6): 588-590.
- Patel A AA, Ariyanayagam D, Killington K, Denny SJ, Mughal N, et al. (2020) Investigating the association between ethnicity and health outcomes in SARS-CoV-2 in a London secondary care population. PLoS One 15(10): e0240960.
- Baumer T PE, Dhadda A, Szakmany T, Tamas Szakmany (2020) Epidemiology of the First Wave of COVID-19 ICU Admissions in South Wales-The Interplay Between Ethnicity and Deprivation. Front Med (Lausanne) 7: 569714.
- Xia H, Lazartigues E (2010) Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep 12(3): 170-175.
- Pal R, Bhansali A (2020) COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res Clin Pract 162: 108132.
- Watanabe M, Risi R, Tuccinardi D, Baquero CJ, Manfrini S, et al. (2020) Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev : e3325.
- Engin A (2017) Endothelial Dysfunction in Obesity. Adv Exp Med Biol 960: 345-379.
- Berglund LM, Lyssenko V, Ladenvall C, Per-Henrik Groop , Allan Vaag, et al. (2016) Glucose-Dependent Insulinotropic Polypeptide Stimulates Osteopontin Expression in the Vasculature via Endothelin-1 and CREB. Diabetes 65(1): 239-254.
- Hadi AR Hadi, Jassim Al Suwaidi (2007) Endothelial dysfunction in diabetes mellitus. Vascular Health and Risk Management 3(6): 853-876.
- Jackson DJ, Busse WW, Bacharier LB, Peter J Gergen , Patrice Becker, et al. (2020) Association of Respiratory Allergy, Asthma and Expression of the SARS-CoV-2 Receptor, ACE2. J Allergy Clin Immunol 146(1): 203-206.
- Here’s How Nanomedicine Provides Insights into Chloroquine’s Efficacy Against COVID-19.
Chloroquine%E2%80%99s-Efficacy-Against-COVID-19
- Silva LS, Silva-Filho JL, Caruso-Neves C, Pinheiro AA (2015) New Concepts in Malaria Pathogenesis: The Role of the Renin-Angiotensin System. Front Cell Infect Microbiol 5: 103.
- Edelstein CL VM, Dong Z, Zheng Dong (2020) Autophagy inhibition by chloroquine and hydroxychloroquine could adversely affect acute kidney injury and other organ injury in critically ill patients with COVID-19. Kidney Int. 98(1): 234-235.
- Mario Mauthe IO, Cecilia Rocchi, Xingdong Zhou, Morten Luhr, Kerst-Jan Hijlkema, et al. (2018) Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14:8: 1435-1455.
- Khalili JS, Zhu H, Mak NSA, Yan Y, Zhu Y (2020) Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J Med Virol 92(7): 740-746.
- Sportelli MC, Izzi M, Kukushkina EA, Rosaria Anna Picca, Nicoletta Ditaranto, et al. (2020) Can Nanotechnology and Materials Science Help the Fight against SARS-CoV-2? Nanomaterials (Basel) 10(4): 802.
- Akerstrom S, Mousavi-Jazi M, Klingstrom J, Leijon M, Lundkvist A, et al. (2005) Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol 79(3): 1966-1969.
- Dal Moro F, Livi U (2020) Any possible role of phosphodiesterase type 5 inhibitors in the treatment of severe COVID19 infections? A lesson from urology. Clin Immunol 214: 108414.
- Guenther CM KB, Lam MT, Robinson TM, Zhao J, Suh J, et al. (2014) Synthetic virology: engineering viruses for gene delivery. Wiley Interdiscip Rev Nanomed Nano bio technol 6(6): 548-558.
- Tang Z ZX, Shu Y, Guo M, Zhang H, Tao W, et al. (2021) Insights from nanotechnology in COVID-19 treatment. Nano Today 36.
- Moitra P AM, Dighe K, Ketan Dighe, Matthew B Frieman, Dipanjan Pan (2020) Selective naked eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 14(6): 7617-1727.
- Baker AN RS, Guy CS, et al. (2020) The SARS-CoV-2 spike protein binds sialic acids and enables rapid detection in a lateral flow point of care diagnostic device. Chem Rxiv.
- Ingrid Arevalo-Rodriguez DB-G, Daniel Simancas-Racines, Paula Zambrano-Achig, Rosa del Campo, Agustin Ciapponi, et al. (2020) False-negative results of initial RT-PCR assays for COVID-19: a systematic review. Med Rxiv (preprint).
- Cohen AN KB (2020) False positives in reverse transcription PCR testing for SARS-CoV-2. medrxiv (preprint).
- Edouard Alphandéry (2020) The potential of various nanotechnologies for coronavirus diagnosis/treatment highlighted through a literature analysis. Bioconjugate Chem 31(8): 1873-1882.
- Huang L DL, Zhou J, Chen S, Chen F, Zhao C, et al. (2021) One-step rapid quantification of SARS- CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosens Bioelectron. 1.
- Devi J (2020) Nanoparticles As a Powerful Tool to Fight with COVID-19. SSRN. 3641672.
- Zaman M GM, Toth I (2013) Nanovaccines and their mode of action. Methods 60(3): 226-231.
- Zhao L SA, Wibowo N, Chun-Xia Zhao , Neena Mitter, Chengzhong Yu, et al. (2014) Nanoparticle vaccines. Vaccine 32(3): 327-337.
- Lugade AA BD, Pradhan V, Vandana Pradhan, Galina Elkin, Shaker A Mousa, et al. (2013) Single low-dose un-adjuvanted HBsAg nanoparticle vaccine elicits robust, durable immunity. Nanomed Nanotechnol Biol Med 9 (7): 923-934.
- Tang Z KN, Zhang X, Liu Y, Hu P, Mou S, et al. ( 2020) A materials-science perspective on tackling COVID-19. Nat Rev Mater 14: 1-14.
- Abo-Zeid Y IN, McLean GR, Hamdy NM (2020) A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur J Pharm Sci 1 : 493.
- gov. U.S National Library of Medicine. Chan WCW (2020) Nano Research for COVID-19. ACS Nano 14: 3719-3720.
- Pelt J, Busatto S, Ferrari M, Thompson EA, Mody K, et al. (2018) Chloroquine and nanoparticle drug delivery: Apromising combination. Pharmacol Ther 191: 43-49.
- Parma Phorum. Mallinckrodt, Novoteris’ nitric oxide will start COVID-19trial.
- Desai D SP (2020) Nanoconjugates-Based Stem Cell Therapy for the Management of COVID-19. Stem Cell Rev Rep 7: 1-10.
- Zhu K LJ, Wang Y, Lai H, Wang C (2016) Nanoparticles-Assisted Stem Cell Therapy for Ischemic Heart Disease. Stem Cells Int 1384658.
- Roberto Vazquez-Munoz aJLL-R (2020)Nanotechnology as an Alternative to Reduce the Spread of COVID-19. Challenges 11: 15.
- Tavakoli A A-PA, Mm Sadeghi G, Mohammad Farahmand , Davod Javanmard, Seyed H Monavari, et al. (2018) Polyethylene glycol-coated zinc oxide nanoparticle: an efficient nano weapon to fight against herpes simplex virus type 1.Nanomedicine (Lond) 13(21): 2675-2690.
- Lysenko V LV, Lokshyn M, et al. (2018) Nanoparticles as antiviral agents against adenoviruses. Adv Nat Sci: Nanosci Nanotechnol 9: 025021.
- Fujimori Y ST, Hayata T, Tsuruo Nakayama, Ryuichi Sugamata, Kazuo Suzuki, et al. (2011) Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ Microbiol 78(4): 951-955.
- Hang X PH, Song H, Hongyuan Song, Zhongtian Qi, Xiaohui Miao, et al. (2015) Antiviral activity of cuprous oxide nanoparticles against hepatitis C virus in vitro. J Virol Methods 15: 222.
- Marlen Aparicio Caamano aMCM (2016) Iron Oxide Nanoparticle Improve the Antibacterial Activity of Erythromycin. Journal of Bacteriology & Parasitology 7: 2.
- Armijo LM WS, Kopciuch M, Brandt YI, Rivera AC, Withers NJ, et al. (2020) Antibacterial activity of iron oxide, iron nitride, and tobramycin conjugated nanoparticles against Pseudomonas aeruginosa biofilms. J Nanobiotechnology 18(1): 35.
- Nura A Mohamed HAS, Yu Kameno, Isra Marei, Gilberto de Nucci BA-S, Fisnik Shala, et al. (2019) Metal-organic framework (MOF) nanomedicine preparations of sildenafil designed for the future treatment of pulmonary arterial hypertension. bioRxiv 718478.
- Mohamed NA, Davies RP, Lickiss PD, Magdi H Yacoub 2, Nicholas S Kirkby, et al. (2017) Chemical and biological assessment of metal organic frameworks (MOFs) in pulmonary cells and in an acute in vivo model: relevance to pulmonary arterial hypertension therapy. Pulm Circ 7(3): 643-653.
- Zhen Zhao HC, Wenxing Song, Xiaoling Ru, Wenhua Zhou, Xuefeng Yu (2020) A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2 bioRxiv preprint.
- Chen HW, Fang ZS, Chen YT, Chen-Ying Chien, Yuan-Chih Chang, et al. (2017) Targeting and Enrichment of Viral Pathogen by Cell Membrane Cloaked Magnetic Nanoparticles for Enhanced Detection. ACS Appl Mater Interfaces 9(46): 39953-39961.
- Qin T, Ma R, Yin Y, Juqun Xi, Qi Liu, et al. (2019) Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics 9(23): 6920-6935.
- Chen Z, Yin JJ, Zhou YT, Lina Song, Mengjie Song, et al. (2012) Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 6(5): 4001-4012.
- Livan Delgado-Roche , Fernando Mesta (2020) Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Archives of Medical Research 51(5): 384-387.
- Shah ST, W AY, Saad O, Abeer A Alhadi , Lina A Al-Ani, et al. (2017)Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials (Basel) 7(10): 306.
- Mandal SM, Porto WF, De D, Sanat K Roy, Octavio L Franco, et al. (2014) Screening of serine protease inhibitors with antimicrobial activity using iron oxide nanoparticles functionalized with dextran conjugated trypsin and in silico analyses of bacterial serine protease inhibition. Analyst 139(2): 464-472.
- Duan J, Du J, Jin R, Qiyong Gong , Bin Song, et al. (2019) Iron oxide nanoparticles promote vascular endothelial cells survival from oxidative stress by enhancement of autophagy. Regen Biomater 6(4): 221-229.
- Kumar R, Nayak M, Sahoo GC, Mamta Chawla Sarkar, Yousuf Ansari, et al. (2019) Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J Infect Chemother 25(5): 325-329.
- Xiong F, Wang H, Feng Y, Yu Zhang 1, Ning Gu, et al. (2015) Cardioprotective activity of iron oxide nanoparticles. Sci Rep 5: 8579.
- Price DN, Stromberg LR, Kunda NK, Muttil P (2017) In Vivo Pulmonary Delivery and Magnetic-Targeting of Dry Powder Nano-in-Microparticles. Mol Pharm 14(12): 4741-4750.
- Hasenpusch G, Geiger J, Wagner K, Olga Mykhaylyk, Frank Wiekhorst, et al. (2012) Magnetized aerosols comprising superparamagnetic iron oxide nanoparticles improve targeted drug and gene delivery to the lung. Pharm Res 29(5): 1308-1318.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...