
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Case Report(ISSN: 2638-5945) 
Two Case of Breast Carcinoma Developing Reactive Axillary Lymphadenopathy After CoronaVac (Sinovac) Vaccine Volume 5 - Issue 3
Elif Şahin1*, Devrim Cabuk1, Busra Yaprak Bayrak2, Furkan Caliskan3, Arzu Serpil Arslan3, Zehra Onesu4, Beyza Capa4, Yasemin Bakkal Temi1, Umut Kefeli1 and Kazım Uygun1
- 1Department of Medical Oncology, Kocaeli University Faculty of Medicine, Kocaeli, Turkey
- 2Department of Pathology, Kocaeli University Faculty of Medicine, Kocaeli, Turkey
- 3Department of Radiology, Kocaeli University Faculty of Medicine, Kocaeli, Turkey
- 4Department of Internal Medicine, Kocaeli University Faculty of Medicine, Kocaeli, Turkey
Received: September 1, 2023 Published: September 15, 2023
Corresponding author: Elif Sahin MD, Department of Medical Oncology, Kocaeli University Faculty of Medicine, 41000, Kocaeli, Turkey
DOI: 10.32474/OAJOM.2023.05.000215
Introduction
Breast cancer is the most frequent malignancy in women and a leading cause of cancer-related death worldwide. In individuals with poor prognostic characteristics, recurrence rates in this disease might reach 30-70%. Relapses might manifest as a local tumor, unilateral axillary lymphadenopathy (LAP), or distant metastases [1]. Until April 2023, 13.3 billion doses of Coronavirus disease-2019 (COVID-19) vaccine have been administered worldwide, and 5.5 billion (68%) people have been vaccinated at least one dose [2]. In Turkey, a total of 152.7 million doses of COVID-19 vaccine have been administered, and approximately 58 million (68%) people have been vaccinated at least one dose [2,3]. Axillary LAP has been documented in the literature as a side effect of COVID-19 vaccinations. Younger people, women, and those who had taken the second dose were more likely to experience this side effect [3-5]. Both the patient and the doctor are concerned when an axillary LAP develops in a breast cancer patient due to the possibility of recurrence. The management of these individuals lacks conclusive data, and as experience grows, the strategy will become clearer. By presenting two Breast Cancer (ca) cases with axillary LAP discovered roughly 6-8 weeks after the second CoronaVac (Sinovac) injection, we intended to add to the body of information on this topic.
Case-1
A 43-year-old female patient applied to an external center with the complaint of a palpable mass in the left breast in January 2021. On mammography, 2 cm spiculated contoured mass was detected in the left breast. Tru-Cut Biopsy (bx) was performed on the mass of the patient who applied to us in February 2021. The pathology of mass was reported as invasive, Non-Specific Type (NST), grade-2, ki67: 20%, ER: 70%, PR: 5%, CerB-B2 score: 3/3. Left lumpectomy + Sentinel Lymph Node Biopsy (SLNB) was performed to the patient on 22.02.2021. Pathological examination revealed 1.9 cm invasive ca, NST, grade-2, ki67: 18%, ER: 80%, PR: 50%, CerB-B2 score: 3/3. The surgical margin was clear and the two sentinel lymph nodes were tumor free. The patient was given adjuvant paclitaxel + trastuzumab for 12 weeks. When she came for the last course of treatment, physical examination revealed palpable LAP in the left axilla and breast USG was performed. On USG (18.06.21), a 15x11 mm LAP with no visible hilum and suspected metastasis was seen in the left axilla. There were no signs or symptoms of infection. Hemogram and CRP were normal. CA 15-3 and viral markers were negative. When questioned, it was learned that the patient had COVID-19 vaccine in her history. She had 3μg Sinovac COVID-19 vaccine administered to the left deltoid muscle on 09.04.21 and 10.05.2021. On 21.06.2021, tru-cut bx was performed for suspected LAP and pathology was reported as ‘benign fibroadipose tissue, lymphocytic cell infiltration (no lymph node floor was found)’ (See Figure 1, A and B). With the joint decision of the Radiology and Oncology clinics, adjuvant traztuzumab treatment was continued and radiotherapy was applied to the left breast. On the control breast USG (27.08.2021); There were no signs of recurrence and no axillary LAP. She has completed adjuvant trastuzumab therapy and her follow-up continues.
Figure 1: Histopathology of the LAPs: Case 1.A. Fibroadipose tissue with non-neoplastic breast ducts (x100, H&E). Case 1.B. Fibrinoid material and lymphocytic cell infiltration (x200, H&E). Case 2.C. Fibroadipose tissue containing congested vascular structures (x100, H&E) Case 2D. Focal fat necrosis (x200, H&E).
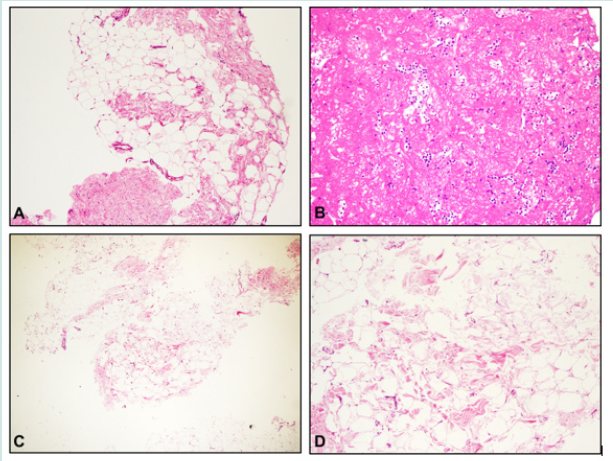
Case-2
In September 2018, on the screening mammography of a 44-year-old female patient with a history of breast ca in her aunt, grouped microcalcifications were observed in two foci in the upper outer quadrant of the right breast. On 30.01.2019, the patient applied to us and she underwent excisional bx after marking with USG. Lobular Carcinoma in Situ (LCIS) foci were detected in the pathology, and the surgical margin was 1 mm. ‘Intensely enhanced non-mass enhancement (lobular ca?) with the same signal with a 2x1.5 cm breast parenchyma at a distance of 6 cm from the areola at 11 o’clock on the right breast’ was observed on the control breast MRI dated 01.11.2019. All the options were explained to the patient, and bilateral subcutaneous mastectomy + bilateral SLNB was performed at 10.01.20 with the decision of the patient. Invasive lobular ca focus in the right breast with 1 mm, grade 2, ki 67: 4%, ER: 90% +, PR: 90% +, CerB-B2: - and LCIS and ductal carcinoma in situ (DCIS) in 1.5 cm area foci (ER+), sclerosing adenosis were observed in the left breast, no tumor was found in two sentinel lymph nodes removed from both axillae. Postoperative tamoxifen treatment was started and she was followed up. In the breast USG taken on 02.07.2021; a 4x1.5 cm LAP with hilus echogenicity and asymmetric cortical thickening in the left axilla was observed. There were no signs or symptoms of infection. Hemogram and CRP were normal. Ca 15-3 and viral markers were negative. When questioned, it was learned that the patient had 3μg Sinovac COVID-19 vaccine administered to the left deltoid muscle on 08.04.2021 and 08.05.2021. On July 2, 2021, a tru-cut bx was performed for left axillary LAP. It was reported as ‘congested fibroadipose tissue containing focal fat necrosis foci (no lymph node floor was found)’ (See Figure 1, C and D). It was decided to take on breast MRI (14.07.2021) and no mass or LAP was observed, postop changes were seen (BIRADS 2). The patient received the first dose of Pfizer-BioNTech COVID-19 vaccine on 09.08.2021, upon the recommendation of the Ministry of Health of the Republic of Turkey for additional doses of vaccine to people in the risk group. On the control breast USG performed on 27.08.2021; no recurrent mass and pathological LAP were observed. A second dose of Biontech was performed 3 months later and LAP did not develop. Routine follow-up continues.
Discussion
It is known that ipsilateral axillary LAP may develop as an adverse effect in individuals who have been vaccinated in the deltoid muscle. However, since axillary LAP in a patient with breast cancer can be an important manifestation of cancer recurrence, the management of breast cancer patients with a history of vaccination poses a diagnostic dilemma for clinicians [6]. The incidence of axillary tenderness and swelling was reported as 11-16% (4.3 -5% in the placebo arm) after Moderna mRNA-1273 vaccine, 2.43% after Pfizer BioNTech BNT162b2 vaccine, and 0.9% after inactivated CoronaVac (Sinovac) vaccine [4,5,7]. These studies included in the patients who complained of axillary tenderness or swelling and did not include those who had asymptomatic LAP. Therefore, the actual frequency of LAP may be higher than reported in studies. Two different studies with PET/CT in the literature confirmed this notion. In the first study, the frequency of reactive LAP detected after an average of 10 (3-20) days after the second dose of COVID-19 vaccine was found to be 26% [3]. In the other study, the frequency of reactive LAP was found to be 29% after 42- 71 days after the second dose of vaccine, and it has been reported that LAPs can persist until the end of the study (post-vaccine 10th month) [8]. In our cases, axillary LAP was detected 40 and 55 days after the second dose of vaccine, respectively, in line with this study. The first control imaging was performed after an average of 8 weeks (approximately 16 weeks after vaccination) in the first case, and 2 weeks (approximately 10 weeks after vaccination) in the second case, and the LAPs were observed to have disappeared. The frequency of LAP following MRNA vaccinations is much higher than that following inactive vaccinations, as previously mentioned. This might be a result of the immune system being more activated following MRNA vaccinations. In both cases, LAP developed after the inactive vaccine. Our second case had 2 doses of Sinovac vaccine and 3 months later had an additional BioNTech vaccine. In this case, after the 2nd dose Sinovac vaccine 4.5 cm LAP developed, but within two weeks, this LAP disappeared. Although the BioNTech vaccine was applied 18 days before the second USG control, there was no LAP on the imaging. Also, there was no LAP after the second dose of BioNTech vaccine. This was notable because it was anticipated that the immune response to the BioNTech vaccination would be more pronounced. However, the mechanisms of each vaccine differ, and unidentified factors can alter these processes.
If a recent immunization history exists, a personal plan must be made when the axillary LAP is identified in breast cancer patients. When selecting whether to perform a biopsy, a variety of considerations should be taken into account, including the patient’s age, condition, expectations/requests, additional clinical factors, imaging data supporting malignancy, and more. In order to help clinicians manage such patients, the Society of Breast Imaging (SBI) has made recommendations [9]. These recommendations state that the vaccination history should be investigated; the name, date, and vaccination arm should be noted, and they should be taken into account when making judgments. Imaging should be perform at least 4-6 weeks after immunization, and it is advised to reexamine the patient after 4–12 weeks. Furthermore, if the LAP does not shrink in the follow-up, it is advised to perform a biopsy even if there is little reason to suspect metastasis (1). NCCN (National Comprehensive Cancer Network) guideline recommendations are also consistent with these recommendations [10]. Immunization should not cause a delay in imaging if there is an urgent clinical indication [11]. According to Becker et al., the vaccine can be administered contralaterally to the site of primary cancer, thus making interpretation easier in some cases [12]. Reactive alterations are expected to be prominent in the histology of enlarged lymph nodes. Strangely, despite tru-cut biopsy being performed on the LAP under USG guidance, in both of our cases, only fibroadipose tissue and lymphocyte infiltration were observed in the biopsy samples. Since the suspicion of metastasis is weak and a second biopsy may cause morbidity, repeat biopsy was not considered by agreement with the patients. The pathology findings in our cases suggest that the Sinovac vaccination may have caused fibroadipose tissue proliferation and tissue lymphocyte expansion.
Conclusion
It should be noted that in breast cancer patients with new axillary LAP, the etiology of the LAP could be a vaccine. Patients should be questioned regarding their history of COVID-19 infection and vaccination. Select patients with a recent COVID-19 immunization history and a low suspicion of recurrence may be candidates for USG follow-up at intervals of 4-12 weeks. LAPs that do not shrink in size and show a persistent suspicious pathological appearance should be biopsied to rule out metastasis.
Compliance with Ethical Standards
a. Informed consent: Informed consent was obtained from the patients.
Funding and/or conflicts of interests/competing interests
There is no conflict of interest.
References
- Cardoso F, Senkus-Konefka E, Fallowfield L, Costa A, Castiglione M (2010) Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(5): v15-9.
- Coronavirus (COVID-19) Vaccinations - Our World in Data.
- Adin ME, Isufi E, Kulon M, Pucar D (2021) Association of COVID-19 mRNA Vaccine with Ipsilateral Axillary Lymph Node Reactivity on Imaging. JAMA Oncol 7(8): 1241-1242.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 384(5): 403-416.
- Riad A, Sağıroğlu D, Üstün B, Pokorná A, Klugarová J, Attia S, et al. (2021) Prevalence and Risk Factors of CoronaVac Side Effects: An Independent Cross-Sectional Study among Healthcare Workers in Turkey. J Clin Med 10(12): 2629.
- Özütemiz C, Krystosek LA, Church AL, Chauhan A, Ellermann JM, Domingo-Musibay E, et al. (2021) Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients. Radiology 300(1): E290-E294.
- Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, et al. (2021) Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med 385(12): 1078-1090.
- Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, et al. (2021) Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond6 Weeks after mRNA COVID-19 Vaccination. Radiology 300(3): E345-347.
- Grimm L, Srini A, Dontchos B, Daly C, Tuite C, Sonnenblick E, et al. (2021) Revised SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination Society of Breast Imaging Patient Care and Delivery Committee 2020-3.
- Malignancies H Malignancies ST. NCCN : Cancer and COVID-19 Vaccination Recommendations of the National Comprehensive Cancer Network ® (NCCN ®) COVID-19 Vaccination Advisory Committee* NCCN : Cancer and COVID-19 Vaccination pp. 1-15.
- Tu W, Gierada DS, Joe BN (2021) COVID-19 Vaccination-Related Lymphadenopathy: What To Be Aware Of. Radiol Imaging cancer 3(3): e210038.
- Becker AS, Perez-Johnston R, Chikarmane SA, Chen MM, El Homsi M, et al. (2021) Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radiology Scientific Expert Panel. 300(2): E323-E327.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




