
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Research Article(ISSN: 2638-5945) 
p21 WAF1/CIP1 is a Downstream target of ELK-1 Growth / Tumor Suppressor Pathway in Breast and Androgenindependent Prostate Cancers Volume 4 - Issue 5
Jingyao Xu1, Zerak Kabir1, Kartik Aysola1, Yuli Chai1, Nina Wyatt1, Shai Waldrip1, Alexis Clark1, Michelle Lee2, Vaishali Reddy2, Manan Shah2, Eric Chang2, Joel Okoli3, E Shyam P Reddy1 and Veena N Rao1*
- 1Cancer Biology Program, Department of OB/GYN, USA
- 2Internal Medicine, USA
- 3Surgery, Morehouse School of Medicine, Atlanta, USA
Received:July 1, 2021 Published: July 30, 2021
Corresponding author: Veena N Rao, Professor and Co-Director Cancer Biology Program, GCC Distinguished Cancer Scholar, Department of OB/GYN, Morehouse School of Medicine, RW D-335, 720 Westview Drive, Atlanta, Georgia 30303-3031. Phone 404- 756-5755, Fax 404-756-8828
DOI: 10.32474/OAJOM.2021.04.000197
Abstract
African American men have the highest risk of developing prostate cancer and have more than twice the mortality rate compared to Caucasian men in the US. ELK-1 belongs to the ETS family of ternary complex factors that plays a major role in cell proliferation, tumorigenesis, as well as differentiation. We have previously shown ELK-1 to not only suppress growth but also to augment the growth suppressive function of BRCA1 proteins in human breast cancer cells. In the current study we have demonstrated for the first time Elk-1 to inhibit the growth of androgen-independent prostate cancer cells. ELK-1 transfected prostate and breast cancer cells were slow growing and inhibited in their capacity to form colonies in soft agar. The p21WAF1/CIP1 protein was found to be expressed at elevated levels both in ELK-1 transfected breast and prostate cancer cells. The p21WAF1/CIP1 promoter activity was also enhanced in the presence of increasing concentrations of ELK-1 protein. Furthermore, ELK-1 protein by binding to the ETS binding motif in the p21WAF1/CIP1 promoter induced the expression of the protein in a p53-independent manner. This work suggests for the first time ELK-1 to function as a growth /tumor suppressor of androgen independent human prostate and breast cancer cells by activating p21WAF1/CIP1 independent of the presence of p53. These findings can provide a novel mechanism that can bypass the p53 defect in tumors with endogenous p53 mutations thus developing effective therapy for patients with advancedstage androgen independent prostate and breast cancers.
Keywords:Elk-1, Breast cancer, Androgen Receptor, Androgen-independent Prostate Cancer, Growth / Tumor Suppressor, p21 WAF1/CIP1
Abbreviations:ELK-1: ETS like gene; AIPC: Androgen-independent prostate cancer; AR: Androgen Receptor; AA: African American; TCF: ternary complex factors; CRPC: Castration resistant prostate cancers; SRE: serum response element; SRF: Serum response factor.
Introduction
According to the American Cancer Society (ACS), it has been estimated that 191,930 men will develop and 33,330 will die of prostate cancer in 2020 [1]. One of the most affected ethnicities are African Americans (AA), having the highest incidence and mortality rates of prostate cancer. Risk factors that have been seen are increasing age, African ancestry, a family history of the disease, and certain inherited genetic conditions [1]. In support of this data, other studies have also shown that African American men have among the highest incidence of prostate cancer (PCa) worldwide. African American men are more likely to develop PCa at any age, as well as develop the disease earlier in life than men from other racial and ethnic groups [2]. There are two forms of prostate cancer: androgen-dependent prostate cancer and androgen-independent prostate cancer. Although all prostate cancers begin in an androgendependent state, over time, after androgen deprivation therapy is administered, some cells can survive and become androgenindependent prostate cancer (AIPC) by Androgen Receptor (AR) gene amplifications, AR gene mutations, changes in expression of AR co-regulatory proteins, changes in expression of steroidgenerating enzymes, and ligand-independent activation of AR [3].
Due to the lack of effective targeted therapies for the androgenindependent form, it is considered the fatal point of the disease [3].
In contrast to prostate cancer, Non-Hispanic White women have a higher incidence of breast cancer; however, African American women have a higher mortality rate [4]. Recent data from the ACS states that the estimated number of invasive breast cancer in the United States for 2020 are 276,480 [5]. These findings prompted us to look for molecular pathways that could potentially be used to treat breast and androgen-independent prostate cancers. ELK-1 gene identified and cloned by Rao, et al. [6] belongs to the ETS family of ternary complex factors (TCFs), i.e., SAP1, NET/ERP/SAP2/ELK- 3 [7, 8, 9]. The ELK-1 gene codes for at least two alternately spliced products. ELK-1 and Δ ELK-1, which function as transcriptional activators, are substrates for MAP kinases and JNK protein kinases [10, 11, 12, 13]. The ELK-1 protein is a ternary complex factor (TCF) which, in association with serum response factor (SRF), forms a ternary complex on the serum response element (SRE) of the c-Fos promoter and regulates c-Fos transcription [7]. ELK- 1 and Δ ELK-1 proteins were shown to induce apoptosis in breast cancer cells when treated with calcium ionophore [14]. Δ ELK-1 is an alternatively spliced variant of ELK-1, which has lost the SRF interaction domain, negative regulatory DNA binding domain, and part of the ELK-1 DNA binding domain [15]. Δ ELK-1 protein has lost the capacity to form an SRF-dependent ternary complex with SRE and to activate Fos transcription [15]. Δ ELK-1 might function by competing for some of the ELK-1 target sequences, thereby blocking the transcriptional activation of Fos by ELK-1. ELK-1 appears to fall in the category of genes that encode activators and repressors through the mechanism of differential splicing [10]. We have previously also looked at the interaction of ELK-1 with the tumor suppressor BRCA1 and found both BRCA1a and BRCA1b isoforms to interact in vivo with the ETS domain of the ELK-1 protein (16). We have found ELK-1, but not Δ ELK-1 and SAP1a protein, to enhance BRCA1a-mediated growth suppression in MCF-7 breast cancer cells [16, 17]. ELK-1 has been shown to have not only an impact on breast cancer, but prostate cancer as well. ELK-1 was also shown to be required for androgen receptor-dependent growth in various hormone-dependent and castration-resistant prostate cancer models [18]. The amino-terminal domain of AR docks at two sites on ELK-1 to co-activate essential growth genes [18]. A unique feature of prostate cancers is its dependence on androgen, which acts by binding to and activating transcriptional signaling by the androgen receptor (AR) [18]. ELK-1 is an AR-tethering protein that is obligatory for androgen/AR-dependent malignant growth in a variety of well-established prostate cancer/castration-resistant prostate cancer (CRPC) models. With the knowledge that ELK-1 has an impact on androgen-dependent prostate cancer, we wanted to assess how ELK-1 would impact AIPC. Cyclin-dependent kinase inhibitors (CDKIs) are proteins that bind to and inhibit the activity of CDKs. Two major classes of CDK inhibitors are p16 family (p15, p16, p18 and p19) that binds to and inhibits the activities of CDK4 and CDK6, and the p21 family (p21, p27, p28 and p57) which can bind to a broad range of CDK-cyclin complexes and inhibit their activities [20]. CDKIs are capable of suppressing growth, and studies show that p21 can function as a tumor suppressor protein [19]. p21 is a mediator of p53 tumor suppressor activity and works as an inhibitor of cell cycle progression by inhibiting the activity of cyclin-dependent kinase (CDK)–cyclin complexes and proliferating cell nuclear antigen (PCNA). Recent data suggests a tumorigenic role for p21 in certain contexts that relies on its ability to suppress apoptosis and promote the assembly of type-D cyclins with CDK4 and CDK6 [20, 21] also noted that p21 is a tumor suppressor, but also can behave as an oncogene in certain cellular conditions; however, other studies indicated that knock down of p21 & p27 resulted in an increased rate of tumor growth and aggressive prostate carcinoma phenotype [19]. ELK-1 was also shown to transactivate the p21 gene independent of p53 and EGR-1 genes in HaCaT cells that were exposed to sodium arsenate [22]. The putative role of p21 in growth arrest mediated by p53 makes it an important gene with respect to the understanding of loss of growth control in transformation processes [23]. In another study CARM1, a coactivator-associated arginine methyltransferase 1, along with p300, another co-activator, was shown to cooperate with BRCA1 and p53 to induce expression of p21 WAF1/CIP1 in response to DNA damage [24]. Our previous work also demonstrated BRCA1 isoforms BRCA1a and BRCA1b to transactivate p21 expression in both a p53-dependent and independent manner (25, 26). We have found ELK-1 to be one of the downstream targets of BRCA1 growth suppression pathway in breast cancer cells [16]. Since ELK-1 functions as a growth suppressor, we can hypothesize that it can function as a growth / tumor suppressor by inducing the expression of p21WAF1/CIP1 in breast and prostate cancer cells. In this study, we have investigated whether ETS-like gene ELK-1 functions as a growth/tumor suppressor in MCF-7 breast cancer and PC-3 androgen-independent prostate cancer cells. Our results suggest ELK-1 to function as a potential growth/tumor suppressor of breast and androgen-independent prostate cancer cells by inducing the expression of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 independent of p53.
Materials and Methods
Cell Culture
MCF-7 pcDNA3, MCF7 ELK-1#13, MCF7 ELK-1#16, MCF7 ΔELK- 1, PC-3 pcDNA3, PC-3 ELK-1 #3, PC-3 ELK-1 #3, PC-3 ELK-1 #6 and PC-3 ΔELK-1 cells were maintained in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS). The stable cell lines were generated as described previously [16, 27].
Expression Constructs
The pcDNA3 ELK-1, pcDNA3 ΔELK-1, pSG5-ELK-1, and pSG5- ELK-1 DN (aa 1-82) expression plasmids were cloned as described previously [16].
Antibodies and Reagents
The antibodies used in this study were primary polyclonal ELK-1 antibody (NEB) and p21 polyclonal antibody (Santa Cruz Biotechnology).
Western Blot Analysis
ELK-1 transfected MCF-7 cells and PC-3 stable cells were maintained in Eagle’s Minimum Essential Medium (MEM) and ATCCformulated F-12K Medium (Catalog No. 30-2004) supplemented with 10% Fetal Bovine Serum (FBS). Cells were lysed in lysis buffer and the extracts were separated on 4-20% SDS-PAGE gradient gel and subjected to Western blotting using ELK-1 polyclonal antibody and p21 antibody as described previously (16,27).
Colony suppression assay
PC-3 cells were transfected with 20 μg of plasmid DNAs (pcDNA3, pcDNA-ELK-1 or pcDNA3 Δ ELK-1) using the calcium phosphate mammalian transfection system from Promega. The next day, cells were washed twice in PBS and re-fed with fresh medium (F-12K Medium supplemented with 10% FBS). Twentyfour hours later, the cells were trypsinized and plated into six 10 cm petri dishes containing complete medium and 600 μg/mL G418. Cells were fed with medium containing G418 every 3-4 days. Cells were fed approximately 18-21 days after transfection in methanol acetic acid (3: 1) for 1 hour at -20oC and stained with crystal violet as described previously [16].
Soft agar assay
Soft agar assay was carried out using 0.3% agar / DMEM or Ham’s F-12K /10% FBS and plated on a base of 0.5% agar/ DMEM or Ham’s F-12K as described previously (31). MCF-7 and PC-3 stable cells were plated at a concentration of 1X104 per 35 mm plate in soft agar containing complete media and kept at 37oC in a CO2 incubator, adding a few drops of media every week. Colonies greater than 80 μm in diameter were scored after 3 weeks. Each soft agar assay was performed in triplicate.
EMSA
EMSA was carried out as described previously [7,10] but using 32P- labelled ETS binding motifs in the p21 promoter and in vitro translated full-length ELK-1 protein. Rabbit polyclonal antisera against ELK-1 and the appropriate pre-immune serum was used [15]. Nuclear extract from human HeLa cells were obtained from Sigma-Aldrich.
Transcriptional Activation Assays
COS-7 or MCF-7 or MEF p53-/- cells were co-transfected with 6 μg of WWP-Luc and 200 ng, 400 ng, and 1 μg of ELK-1, ELK-1-DN (aa 1-89) expression plasmids using the Stratagene transfection kit. The cells were harvested after 26 hours of transfection and assayed for luciferase activity. Luciferase activity was measured using a scintillation counter with the Promega kit as described previously [16]. The luciferase activity shown represents relative activity compared to the vector alone. The experiments were repeated at least three times and the experiments represent the mean of three independent experiments.
Results and Discussion
p21 WAF1/CIP1 protein levels are upregulated in ELK-1 transfected MCF-7 and PC-3 cells.
Figure 1: Expression of ELK-1 and p21WAF1/CIP1 protein levels in ELK-1 transfected MCF-7 and PC-3 cells (A) Western blot-analysis of total cellular ELK-1 and p21WAF1/CIP1 protein levels in MCF-7 ELK-1 transfectants. Total cellular extracts from MCF-7 pcDNA3 (lane 1), MCF-7 ELK-1#16 (lane 2) and MCF-7 ELK-1#13 (lane 3) were electrophoretically separated, transferred to PVDF membrane and subjected to immunoblot analysis using an ELK-1 antibody (NEB) and p21 antibody (Santa Cruz Biotechnology, Inc.). The apparent molecular weight of the prestained protein standards is shown. The molecular weights of the 62 kD ELK-1 and 21 kD p21 proteins are shown. (B) Total cellular extract (~90μg) from PC-3 pcDNA3 (lane 1); PC-3 ELK-1#13 (lane 2) were subjected to western blot analysis as described in (A). Equal amount of protein was loaded on the gels using actin antibody as a control.
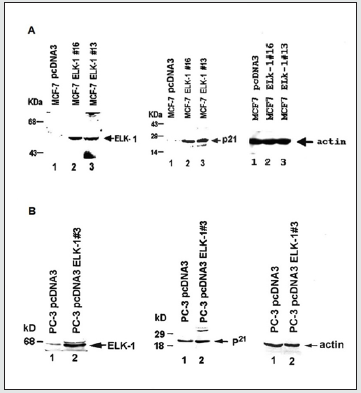
As mentioned earlier, we have previously shown ELK-1, unlike Δ ELK-1 and SAP1a, to function both as a growth suppressor and to enhance BRCA1a/1b-mediated growth suppression of MCF- 7 breast cancer cells. To investigate whether ELK-1 induces the expression of p21 WAF1/CIP1 in both MCF-7 and PC-3 cells, we developed stable MCF-7 and PC-3 cell lines expressing ELK-1 and subjected them to Western blot analysis using ELK-1 and p21 antibodies. Our results (Figure 1) showed increased expression of endogenous p21 WAF1/CIP1 protein in both the stable MCF-7 (Figure 1A) and PC-3 cell lines (Figure 1B) that were transfected with ELK-1 compared to cells transfected with pcDNA3 vector alone by western blot analysis. These results suggest that one of the mechanisms by which ELK-1 could function as a growth suppressor of breast and prostate cancer cells could be via induction of p21 WAF1/CIP1 protein expression.
ELK-1 inhibits the growth of PC-3 prostate cancer cells
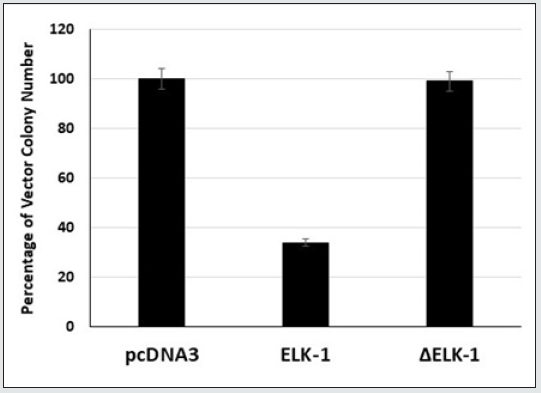
ELK-1, unlike Δ ELK-1 protein, was previously shown by us to function as a growth suppressor of human breast cancer cells [16]. To investigate whether ELK-1 suppresses the growth of androgenindependent PC-3 prostate cancer cells, we transfected pcDNA3, ELK-1 and Δ ELK-1 cDNAs into PC-3 prostate cancer cells and analyzed their effect on G418 colony formation. We have used PC-3 cell lines because they are extremely aggressive, are negative for androgen receptor (AR) and prostate-specific antigen (PSA) and do not respond to hormonal therapy [28]. The expression of pcDNA3 ELK-1 resulted in the reduction in the number of G418 colonies formed compared to pcDNA3 vector (Figure 2), unlike Δ ELK-1 (pcDNA3 Δ ELK-1). These results indicate ELK-1, unlike Δ ELK-1, can function as a growth suppressor of androgen-independent human prostate cancer cells.
Figure 2: Elk-1 inhibits the growth of PC-3 prostate cancer cells. Cells were transfected with pcDNA3 or pcDNA3 ELK-1 or pcDNA3ELK- selected with G418 and the resulting colonies were stained and counted. The number of colonies obtained by pcDNA3 clone was considered as 100%. Each experiment was repeated at least three times and done as described previously [16, 26].
Overexpression of ELK-1 inhibits in vitro growth in soft agar of MCF-7 and PC-3 cells
Since ELK-1 was found to suppress the growth of MCF-7 and PC-3 cells, we next tested if ELK-1 can inhibit the growth of MCF-7 and PC-3 cells in vitro in soft agar. All the ELK-1 stable MCF-7 and PC-3 transfectants were greatly inhibited in their capacity to grow and form colonies in soft agar, unlike the Δ ELK-1 transfectants (Figure 3A, 3B). These results show ELK-1 to function as a tumor suppressor in vitro in human breast and androgen-independent prostate cancer cells.
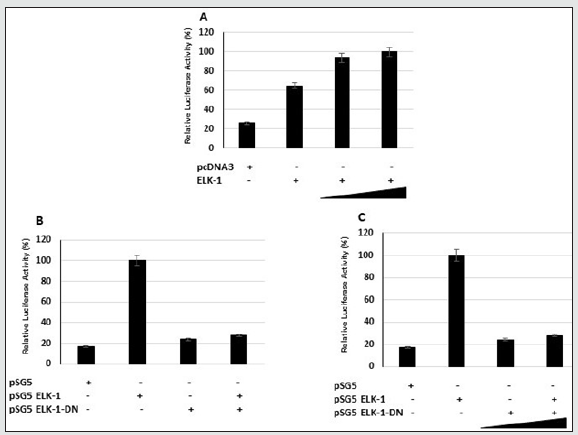
ELK-1 activates the p21 WAF1/CIP1 promoter activity independent of p53
Cell proliferation is closely linked to cell cycle progression, which is regulated by a complex network involving cyclins, cyclindependent kinases (CDKs), and cyclin-dependent kinase inhibitors (CKIs). The CKIs can be divided into two structurally related groups. The INK4 family, which includes p14, p15INK4b, p16INK4a, p18INKc, and P19INK4d that inhibit cyclin D-Cdk4/6 complexes and maintains Rb in its active anti-proliferative state. The other group of CKIs, the CIP1/KIP family, include p21 WAF1/CIP1, p27KIP1, and p57KIP2, which inhibit kinase activities of cyclin E-Cdk2, and cyclin D-Cdk4/6. Functional disruption of p16INK4a and p21 WAF1/CIP1 occurs in prostate tumors. p21 WAF1/CIP1 has been implicated in growth arrest, cellular senescence, and terminal differentiation. Induction of p21 WAF1/CIP1 expression has been linked to growth inhibition by p53 and p21 WAF1/CIP1. Expression of p21 WAF1/ CIP1 has also been found to signal growth arrest independent of p53 in cells undergoing differentiation. The altered growth and increased apoptosis observed in MCF-7-ELK-1 transfectants suggested that p21 WAF1/CIP1 and p16INK4A could be potential downstream targets. p21 WAF1/CIP1 has been reported to be a transcriptional target of overexpressed ETS factor EIAF [29]. In fact, alterations in the p16/Rb cell cycle checkpoint occur frequently in prostate cancers [30]. Since we observed increased levels of p21 WAF1/CIP1 in both stable MCF-7 ELK-1 and PC-3 ELK-1 cells (Figure 2), it suggests that ELK-1 could transcriptionally induce the expression of p21 WAF1/CIP1. We therefore tested the ability of ELK-1 to activate reporter genes containing the p21 WAF1/CIP1, p15, and p16INK4a promoter luciferase reporters in COS-7 and MCF-7 cells. We observed ELK-1 to activate the p21 WAF1/CIP1 promoter activity at a higher level compared to p15 and p16INK4a (Figure 4A, 4B). Interestingly, we found ELK-1 binding motifs (5’-AGGAAG/C-3’) in the mouse and rat p21 WAF1/CIP1 promoters [23]. We next transfected the p21 luciferase reporter with an increasing concentration of full-length ELK-1 into COS-7, MCF-7, and MEF p53-/- cells. There was a dose-dependent increase in the p21 luciferase reporter plasmid expression in COS-7 and MEF p53- /- cells (Figure 5). Using an ELK-1-DN (aa-1-89) expression plasmid in the presence of ELK-1 inhibited the p21 luciferase reporter expression in MCF-7 cells (Figure 5B). These results indicate that ELK-1 transactivates the p21 WAF/CIP promoter by binding to the p21 WAF1/CIP1 promoter and independent of the presence of p53.
Figure 3: Over expression of ELK-1 inhibits in vitro growth in soft agar of MCF-7 cells (A) and PC-3 cells (B). 1 x 104 cells per dish of MCF- 7 or PC-3 pcDNA3 vector transfectants or ELK-1 or Δ ELK-1 transfectants were analyzed for anchorage independent growth as described previously (31). The figure shows clonogenicity of both pcDNA3 vector, ELK-1 and ΔELK-1 transfectants. Each experiment was repeated at least three times and the bars represent standard deviation.
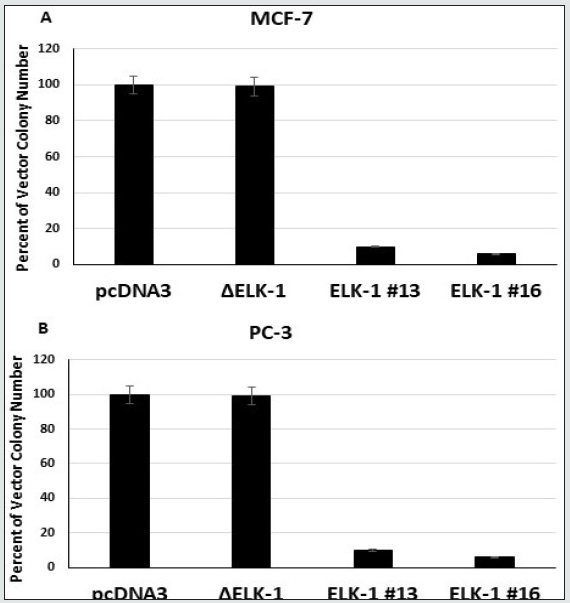
Figure 4: ELK-1 activates the p21WAF1/CIP1 promoter activity more than p15 and p16 in Cos-7 (A) and MCF-7 cells (B). Cells were cotransfected with expression vectors for pcDNA3 or pcDNA3-ELK-1 with luciferase reporter plasmid. The cells were harvested 36 hours after transfection and assayed for luciferase activity. The experiments represent mean of three independent experiments.
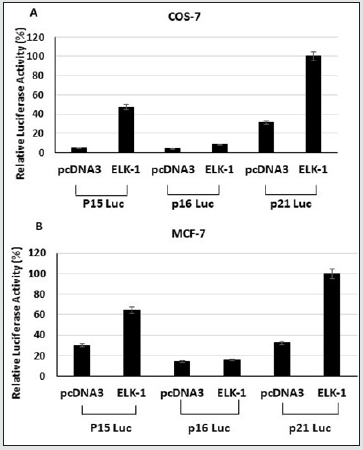
Figure 5: Elk-1 transactivates the p21 WAF1/CIP1 promoter in COS-7 (A), MCF-7 (B) and MEF p53-/- cells (C). COS-7 cells or MCF-7 cells or MEF p53-/- cells were co-transfected with 6ug of WWP-Luc and 200ng, 400ng, and 1ug of Elk-1, Elk-1-DN (aa 1-89) expression plasmids and luciferase activity was measured by scintillation counter using the Promega kit. The luciferase activity shown represents relative activity compared to vector alone. The experiments were repeated at least three times.
ELK-1 binds to the ETS binding motifs in the p21 WAF1/ CIP1 promoter
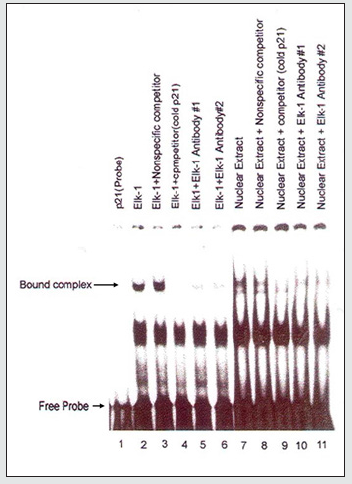
To investigate whether ELK-1 binds to the p21 WAF1/CIP1 promoter sequences, we analyzed the p21 WAF1/CIP1 promoter sequences for the presence of potential ELK-1 binding sites using the GGAA consensus binding sequence determined for the ETS proteins [29]. We next investigated whether ELK-1 binds to the ELK-1 binding motifs in the p21 WAF1/CIP promoter using EMSA. We obtained fragments corresponding to the promoter sequences of p21 WAF1/CIP by PCR. This fragment was 32p-labelled and used for EMSA with invitro translated full length ELK-1 protein as described previously [7,10]. We observed binding of full length in vitro translated ELK-1 to p21 WAF1/CIP1 promoter sequences, which was competed on using ELK-1 antibodies (Figure 6). The same scenario was seen using HeLa cell nuclear extract which was also competed by ELK-1 antibodies (Figure 6). These results further confirm our hypothesis that ELK-1 induces the expression of p21 WAF1/CIP1 by binding to the ETS binding site in the promoter.
Figure 6: ELK-1 binds to the ETS binding motifs in the p21WAF1/CIP1 promoter. EMSA was performed as described previously (7, 10) but using p32-labeled oligonucleotide containing the ETS binding sites (EBS) and invitro translated full length ELK-1 protein or using nuclear extract obtained from Hela cells. The bound complexes as well as free probes are indicated by arrows.
p21WAF1/CIP1 has dual functionality in tumor development and progression depending on the cellular context and its subcellular localization. Cytoplasmic expression of p21 plays a role as an oncogene, whereas nuclear p21 works as a tumor suppressor. Cytoplasmic localization of p21 was associated with advanced disease stage and overall survival in various types of cancers [32,33]. Therefore, identifying specific inhibitors that selectively block the cytoplasmic shuttling of p21 or promote cytoplasmic degeneration could be promising targeting strategies. Small molecule inhibitors of p21 such as sorafenib, a tyrosine kinase inhibitor, was shown to induce proteasomal degradation of p21 independent of p53 in renal cell carcinoma [34]. Challenges lie in devising therapeutic agents that inhibits the oncogenic activities of p21 without interfering with tumor suppressing activities. Further work is needed to elucidate the role of p21 under distinct cellular context independent of p53 and to explore is its potential as therapeutic target. Our results suggest for the first time that ELK-1, unlike Δ ELK-1, to function as a growth /tumor suppressor of androgen-independent human prostate cancer cells and breast cancer cells. We have found p21 WAF1/CIP1 to be one of the potential downstream targets in this pathway. ELK-1 protein, by binding to the ETS binding site in the p21 WAF1/CIP1 promoter, induces the expression of the protein in a p53-independent manner. These studies will help us in the future to develop an effective mechanism-based therapeutic paradigm for patients with advanced-stage androgen-independent prostate and breast cancers. It may also help in developing potential biomarkers for the early detection of these cancers.
Conflict of Interest
Authors have no conflict of interest to disclose.
Acknowledgements
Work supported in part by Georgia Cancer Coalition Distinguished Cancer Scholar award, NIH-NCRR-RCMI grant G-12- RR003034, U54 RR02613, 5P20RR11104 and NIHMD under NIH award 2S21MD000101, U54 CA118638. V.N. Rao’s lab was also supported in part by private funds from the VOYA foundation and It’s the Journey Inc, A Cure in Our Lifetime and Georgia CORE.
References
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020.
- Taitt HE (2018) Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am J Men’s Health 12(6): 1807-1823.
- Saraon P, Drabovich AP, Jarvi KA, Diamandis EP (2014) Mechanisms of Androgen-Independent Prostate Cancer. EJIFCC 25(1): 42-54.
- DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A (2017) Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 67(6): 439-448.
- American Cancer Society. Breast Cancer Facts & Figures 2019-2020. Atlanta: American Cancer Society, Inc. 2019.
- Rao VN, Huebner K, Isobe M, ar-Rushdi A, et al. (1989) elk, tissue-specific ets-related genes on chromosomes X and 14 near translocation breakpoints. Science 244(4900): 66-70.
- Hipskind RA, Rao VN, Mueller CG, Reddy ES, Nordheim A (1991) Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature 354: 531-534.
- Dalton S, Treisman R (1992) Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell 68(2): 597-612.
- Price MA, Rogers AE, Treisman R (1995) Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET). EMBO J 14(11): 2589-601.
- Rao VN, Reddy ES (1992) A divergent ets-related protein, elk-1, recognizes similar c-ets-1 proto-oncogene target sequences and acts as a transcriptional activator. Oncogene 1(1): 65-70.
- Rao VN, Reddy ES (1993) Elk-1 proteins are phosphoproteins and activators of mitogen-activated protein kinase. Cancer Res 53(15): 3449-3454.
- Bhattacharya G, Lee L, Reddy ES, Rao VN (1993) Transcriptional activation domains of elk-1, delta elk-1 and SAP-1 proteins. Oncogene 8(12): 3459-3464.
- Rao VN, Reddy ES (1994) elk-1 proteins interact with MAP kinases. Oncogene 9(7): 1855-1860.
- Shao N, Chai Y, Cui JQ, Wang N, Aysola K, et al. (1998) Induction of apoptosis by ELK-1 and deltaELK-1 proteins. Oncogene 1998; 17(4): 527-532.
- Rao VN, Reddy ES (1993) Delta elk-1, a variant of elk-1, fails to interact with the serum response factor and binds to DNA with modulated specificity. Cancer Res 53(2): 215-220.
- Chai Y, Chipitsyna G, Cui J, Liao B, Liu S, et al. (2001) c-Fos oncogene regulator Elk-1 interacts with BRCA1 splice variants BRCA1a/1b and enhances BRCA1a/1b-mediated growth suppression in breast cancer cells. Oncogene p. 20: 1357-1367.
- Maniccia AW, Lewis C, Begum N, Xu J, Cui J, et al. (2009) Mitochondrial localization, ELK-1 transcriptional regulation and growth inhibitory functions of BRCA1, BRCA1a, and BRCA1b proteins. J Cell Physiol 219(3): 634-641.
- Rosati R, Polin L, Ducker C, Li J, Bao X, et al. (2018) Strategy for Tumor-Selective Disruption of Androgen Receptor Function in the Spectrum of Prostate Cancer. Clin Cancer Res 24(24): 6509-6522.
- Roy S, Singh RP, Agarwal C, Siriwardana S, Sclafani R (2008) Downregulation of both p21/Cip1 and p27/Kip1 produces a more aggressive prostate cancer phenotype. Cell Cycle 7(12): 1828-35.
- Bayrak A and Oktay K (2003) The expression of cyclin-dependent kinase inhibitors p15, p16, p21, and p27 during ovarian follicle growth initiation in the mouse. Reprod Biol Endocrinol p. 1: 41.
- Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer p. 9: 400-414.
- Shin SY, Kim CG, Lim Y, Lee YH (2011) The ETS family transcription factor ELK-1 regulates induction of the cell cycle-regulatory gene p21(Waf1/Cip1) and the BAX gene in sodium arsenite-exposed human keratinocyte HaCaT cells. J Biol Chem 286(30): 26860-26872.
- Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, et al. (1995) p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev 9(8): 935-944.
- Lee YH, Bedford MT, Stallcup MR (2011) Regulated recruitment of tumor suppressor BRCA1 to the p21 gene by coactivator methylation. Genes Dev 25(2): 176-188.
- Mullan PB, Quinn JE, Harkin DP (2006) The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene p. 25: 5854-5863.
- Chai YL, Cui J, Shao N, Shyam E, Reddy P et al. (1999) The second BRCT domain of BRCA1 proteins interacts with p53 and stimulates transcription from the p21WAF1/CIP1 promoter. Oncogene p. 18: 263-268.
- Cui JQ, Shao N, Chai Y, Wang H, Reddy ES (1998) BRCA1 splice variants BRCA1a and BRCA1b associate with CBP co-activator. Oncol Rep 5(3): 591-595.
- Tai S, Sun Y, Squires JM, Zhang H, Oh WK, et al. (2011) PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 71(15): 1668-1679.
- Funaoka K, Shindoh M, Yoshida K, Hanzawa M, Hida K, (1997) Activation of the p21(Waf1/Cip1) promoter by the ets oncogene family transcription factor E1AF. Biochem Biophys Res Commun 236(1): 79-82.
- Jarrard, DF, Modder, J, Fadden, P, Fu V, Sebree, L, et al. (2002) Alterations in the p16/pRb cell cycle checkpoint occur commonly in primary and metastatic human prostate cancer. Cancer Lett 185(2): 191-199.
- Yuli C, Shao N, Rao R, Aysola P, Reddy V, et al. (2007) BRCA1a has antitumor activity in TN breast, ovarian and prostate cancers. Oncogene p. 26(41): 6031-6037.
- Huang Y, Wang W, Chen Y, Huang Y, Zhang J, et al. (2014) The opposite prognostic significance of nuclear and cytoplasmic p21 expression in resectable gastric cancer patients. J Gastroenterol 49(11): 1441-1452.
- Kreis NN, Louwen F, Yuan J (2017) The Multifaceted p21 (Cip1/Waf1/CDKN1A) in Cell Differentiation, Migration and Cancer Therapy. Cancers (Basel) 11(9): 1220.
- Liu R, Wettersten HI, Park SH, Weiss RH (2013) Small-molecule inhibitors of p21 as novel therapeutics for chemotherapy-resistant kidney cancer. Future Med Chem 5(9): 991-994.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




