Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Research Article(ISSN: 2638-5945) 
Ipilimumab Prognostic Score in Progressive Metastatic Melanoma Patients. A Retrospective Analysis on Behalf of Italian Melanoma Intergroup Volume 4 - Issue 2
Marconcini Riccardo1, Nuzzo Amedeo1, Manacorda Simona1, De Rosa Francesco2, Palla Marco3, Fava Paolo4, Astrua Chiara4, Di Guardo Lorenza Alessia5, Raimondi Alessandra5, Tucci Marco6, Todisco Annalisa6, Cortellini Alessio7, Bersanelli Melissa8, Nigro Olga9, Stucci Luigia Stefania6, Crucitta Stefania10, Palmieri Giuseppe11, Falcone Alfredo1
- 1Medical Oncology Unit, Azienda Ospedaliero Universitaria Pisana, University of Pisa, Pisa (Italy)
- 2Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) – IRCCS – Meldola (Italy)
- 3Melanoma Unit, Cancer Immunotherapy and Innovative Therapies, Istituto Nazionale Tumori IRCCS Fondazione Pascale, Naples, Italy
- 4SC Dermatologia U. AOU Città della salute e della Scienza, Torino (Italy)
- 5S.S. Oncologia Medica Melanomi, Dipartimento Oncologia Medica ed Ematologia, Fondazione IRCCS Istituto Nazionale dei Tumori di Milano (Italy)
- 6Medical Oncology Unit, Department of Biomedical Sciences and Clinical Oncology, University of Bari, (Italy)
- 7Department of Biotechnology and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy
- 8Unità Operativa di Oncologia Medica, Azienda Ospedaliero-Universitaria di Parma
- 9U.O. Oncologia Medica, ASST-Settelaghi, Ospedale di Circolo di Varese, Varese (Italy)
- 10Department of Clinical and experimental Medicine- University of Pisa (italy)
- 11Unit of Cancer Genetics, Head Institute of Biomolecular Chemistry (ICB) National Research Council (CNR) Sassari, Italy
Received: March 8, 2021 Published: March 26, 2021
Corresponding author: Riccardo Marconcini, Medical oncologist Azienda ospedaliero universitaria Pisana, Pisa-Unità Operativa Oncologia Medica 2 universitaria, Roma 67, 56100 Pisa Italy
DOI: 10.32474/OAJOM.2021.04.000187
Abstract
Background: Ipilimumab is an option in Metastatic Melanoma patients in case of disease progression after antiPD1 treatment and BRAF+MEK inhibitors administration (for BRAF mutated melanoma). We evaluate the prognostic role of some relevant clinical or laboratories parameters for Ipilimumab used in late line after AntiPD1 progression to define patients that benefit most from Ipilimumab monotherapy in this setting.
Methods: A retrospective multicenter study was conducted in 8 Italian Oncology Centers, evaluating metastatic melanoma patients treated with Ipilimumab after AntiPD1 and/or BRAF plus Mek inhibitors progression. Endpoints were overall survival (OS) and Progression free survival (PFS), Kaplan Mayer and Cox regression were applied for survival analysis.
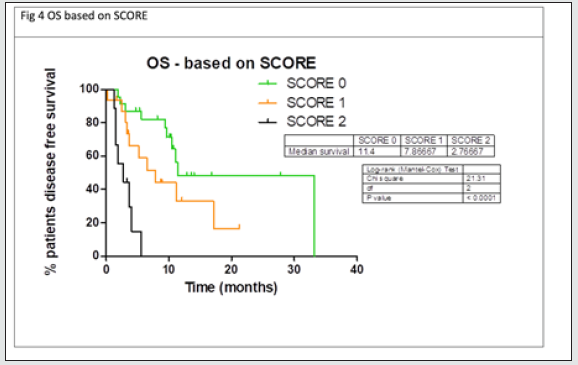
Results: Among patients that received AntiPD1 and-or Bramber inhibitors, 54 were treated with Ipilimumab monotherapy in 2nd/3rd line. Before Ipilimumab treatment, Number of metastatic sites were equal or less than 3 in 22 (40,7%) patients, ECOG Performance status was 0 in 32 (59%) patients, baseline LDH levels were within normal range in 25 (46,3%) patients, NLR (neutrophile/lymphocyte ratio) was equal or less than 0.7 in 33 (59%) patients. In Univariate analysis, ECOG PS 0 and NLR<0.7 resulted statistically significant good prognostic. In multivariate analysis for PFS, only NLR maintained statistically significance, while in multivariate analysis for OS both ECOG PS and NLR maintained a statistical significance. A score was counted for each patient considering the sum of number of negative factors associated with OS worse prognosis (ECOG PS>0, NLR more than 0.7). For patients with SCORE 0,1,2 median OS was respectively 11.4, 7.87 and 2.77 (p value<0.0001).
Conclusions: ECOG PS 0 and NLR<0.7 resulted prognostic factors associated with favorable OS of metastatic melanoma patients treated with Ipilimumab after AntiPD1 progression. Subgroup with all these factors has a better prognosis. These data can help treatment choice and should be evaluated prospectively.
Keywords: Metastatic Melanoma; Ipilimumab; prognostic score; anti-PD1; NLR ratio; immunotherapy
Introduction
Metastatic melanoma is one of the most aggressive solid tumors [1], and its management has been improved over the past few years thanks to new immune checkpoint inhibitors and targeted therapies [2-6]. Standard first line treatment of metastatic melanoma includes antibody targeting Programmed cell death protein 1 (PD1) Nivolumab or Pembrolizumab [2, 3], associated with Anti-Cytotoxic T-Lymphocyte Antigen 4 (CTLA4) Ipilimumab, and, in case of BRAF v600 mutation, the use of BRAF and MEK inhibitors [4-6]. In case of progression to first line treatments, one of the approved therapies that can be used is Anti-CTLA4 Ipilimumab, if not administered in first line in combination with other drugs. Ipilimumab is a recombinant human monoclonal antibody against CTLA-4 [7]. It affects the immune system by inhibiting the suppression of T-cell function. As a result, activated T-cells remain stimulated and can exert antitumor effects. In many trials, when it is used as monotherapy, Ipilimumab seems to maintain a durable clinical benefit in only about 15% of treated patients either if administered in first line [2] or second line [8], after progression to previous systemic treatments. It could be interesting to characterize the patients belonging to this 15% to optimize the use of available treatments and guarantee a long survival in this group of patients. Some patients treated with Anti- PD1 in first line show primary or acquired resistance to Anti-PD-1 therapy, and biologic modality of resistance; best treatments after progression are arguments of research [9, 10]. Several studies are currently underway to evaluate the use of multiple combinations of experimental immunomodulating drugs in case of progression to Anti-PD1, and many of these trials involve the use of drug combinations including Anti-CTLA4, investigating whether these combinations can achieve greater efficacy [11-17]. Only limited evidence is available regarding the efficacy of Ipilimumab in patients who progressed after Anti-PD-1 therapy [18-20]: it would be optimal to know in advance which patients are most likely to achieve maximum efficacy with Ipilimumab monotherapy alone. The present study is a multicenter retrospective analysis conducted in multiple Italian Centers, in which we collected data related to patients with metastatic melanoma treated with Ipilimumab after treatment with Anti-PD1 and with BRAF and MEK inhibitors if BRAF mutated: our goal is to identify clinical characteristics or routine laboratory facilities tests which may have a prognostic connotation regarding the efficacy of Ipilimumab; this analysis may potentially help clinicians in choosing Ipilimumab monotherapy in this setting instead of other experimental combinations in specific subgroups of patients.
Patients and Methods
Patient Population
We conducted an institutional review board-approved, multicenter retrospective study regarding patients treated with at least one dose of Ipilimumab after prior Anti-PD-1 therapy failure, between July 2017 and January 2020. Patients were accrued in 10 referral centers in Italy, in collaboration with Italian Melanoma Intergroup scientific society. Each participating center identified patients via pharmacy databases or medical records. Patients were enrolled in data collection if the following criteria were identified: histologically-proven unresectable stage IV melanoma following American Joint Committee on Cancer (AJCC) 8th edition criteria, previous treatment with Anti-PD1 used as systemic treatment for metastatic setting, in case of patients affected by BRAF v600 mutated melanoma BRAF+MEK inhibition treatment could be administered, documented disease progression on prior anti-PD-1 therapy (and BRAF+MEK inhibition if administered) as per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, received at least one dose of Ipilimumab as systemic treatment after Anti-PD1 progression (and after BRAF+MEK inhibition progression, if administered).
Study Design
We collected data from enrolled patient regarding the following items: baseline characteristics (demographics, age, sex, AJCC stage, BRAF mutational status, date of metastasizing, extent of metastasizing), previous systemic therapies administered in adjuvant setting, previous Anti-PD1 therapy administered in first line (date of first cycle, best response assessed, date of disease progression). The following clinical and laboratories potential prognostic factors were documented before Anti-PD1 therapy and more importantly before Ipilimumab treatment: Eastern Cooperative Oncology Group (ECOG) performance status, Neutrophile Lymphocyte Ratio (NLR), serum lactate dehydrogenase level (LDH) and presence of more than 3 metastatic sites. Ipilimumab treatment was administered at the approved dosing of 3 mg/kg every 3 weeks for four intravenous infusions. Efficacy endpoints of Ipilimumab therapy were RECIST 1.1 response, Progression Free survival (PFS) and Overall Survival (OS).
Statistical Methods
Ipilimumab response was evaluated using RECIST criteria and
categorized as complete response [CR], partial response [PR] and
stable disease [SD] to progression. The overall response rate (ORR)
was defined as the proportion of patients with PR and CR, whereas
the disease control rate (DCR) was defined as the proportion of
patients with CR, PR, and SD. PFS was defined as the time from the
first dose of ipilimumab to the first date of documented progression
as per RECIST, or date of death, whichever came first. Patients last
known to be alive and progression-free were censored at the date
of last contact (all in January 2020). OS was defined as the time
from the first administration of ipilimumab to death from any
cause. Patients last known to be alive were censored at the date
of last contact (all in January 2020). PFS and OS were estimated
by the Kaplan Meier method. Log-rank tests were used to compare
PFS and OS between several subgroups based on characteristics
collected prior to the first cycle of Ipilimumab: ECOG (0 versus
>0), LDH level (
Results
Patients and efficacy of prior Anti-PD-1 therapy
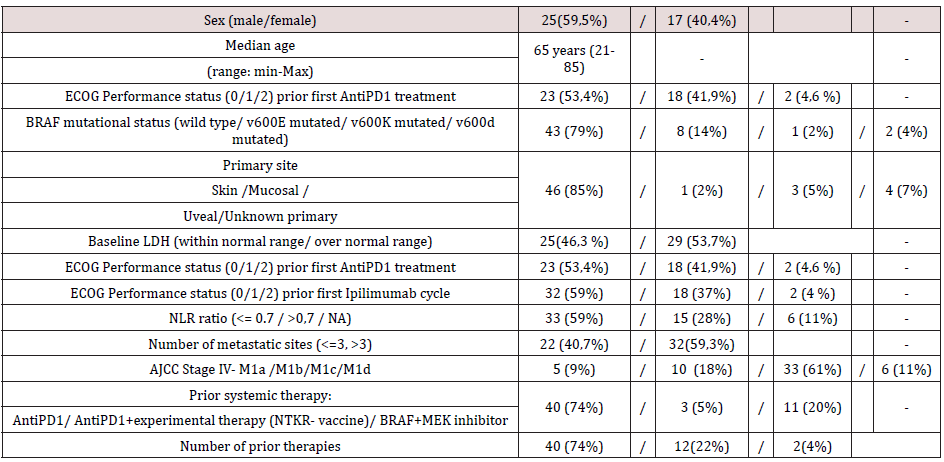
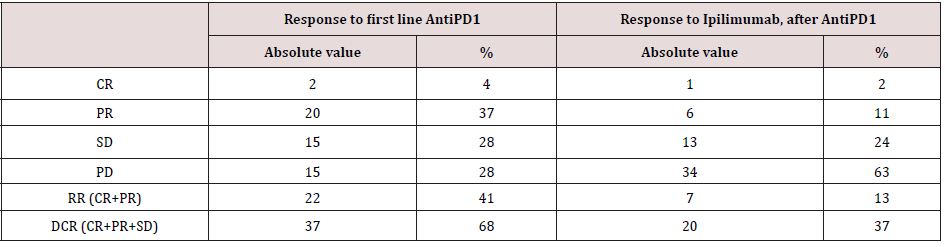
Between July 2017 and January 2010, among patients progressed after AntiPD1, 54 patients were treated with at least one dose of Ipilimumab after treatment failure to prior Anti- PD-1 therapy. Baseline patients’ characteristics before the first administration of Ipilimumab are reported in (Table 1). The median age at the time of Ipilimumab first cycle was 65 years (range 21- 85); most patients were males with cutaneous primary sites. BRAF mutational status was wild type in 43 (79%) patients. Most of the patients had AJCC stage M1c; brain metastasis (M1d AJCC stage) was present in 6 (11%) patients, M1a and M1b AJCC stage was present in 5 (9%) and 10 (18%) respectively. Number of metastatic sites were equal or less than 3 in 22 (40,7%) patients. ECOG Performance status prior first Ipilimumab cycle was 0 in 32 (59%) patients. Baseline LDH levels were within normal range in 25 (46,3 %) patients. NLR ratio was equal or less than 0.7 in 33 (59%) patients, and not analyzable (data not detected in medical records) in 6 (11%). All patients received Anti-PD1 prior to Ipilimumab. As described in (Table 2), the response rate of Anti-PD1 administered in first line was 41 % (with 4% as Complete response) and the disease control rate was 68%.
Efficacy of Ipilimumab
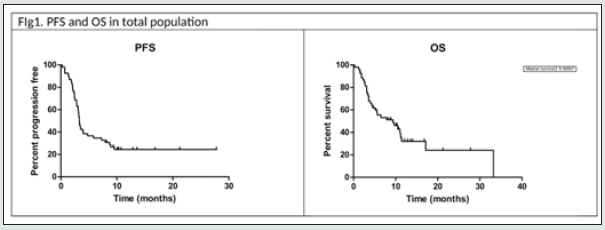
After Anti-PD-1 failure, the median follow-up for all patients after commencement of Ipilimumab was 12.1 months (range 1 to 33,2 months) (median follow-up was calculated with reverse Kaplan Meier estimation).
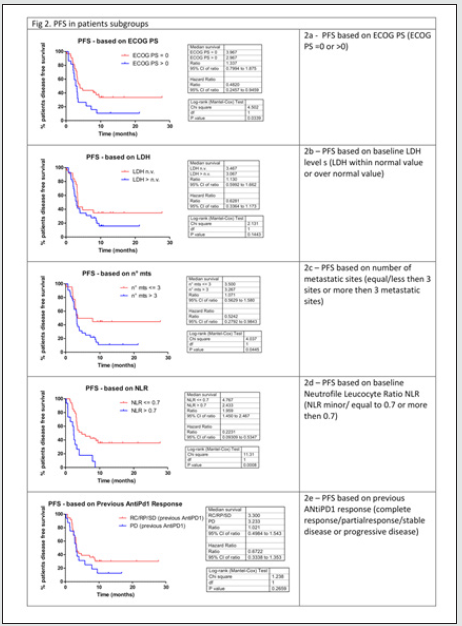
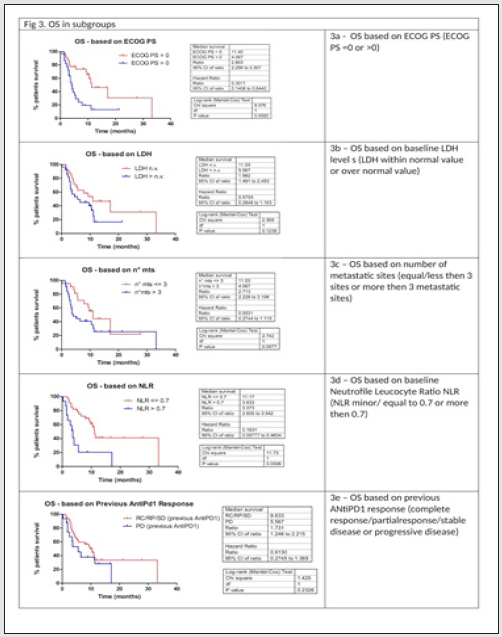
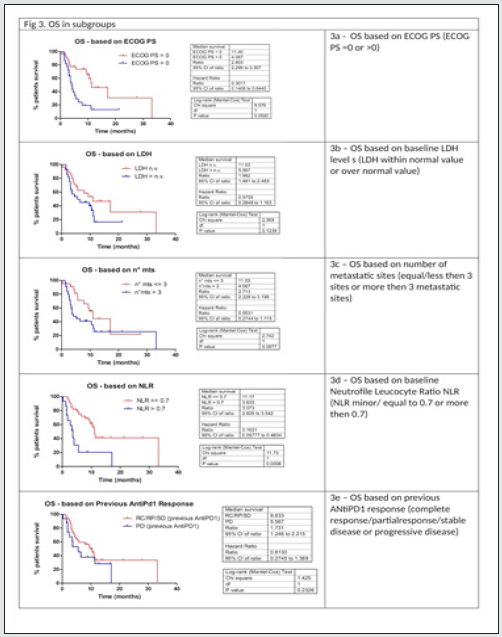
As described in (Table 2), with Ipilimumab treatment DCR was 37%, ORR was 13%. Thirty-three patients (61%) in the Ipilimumabgroup received all four doses of ipilimumab, 16 patients (29%) stopped treatment before third cycle due to tumor progression and/ or clinical deterioration. None of the patient is still on treatment. Median PFS in total population of patients treated with ipilimumab was 3,3 months, with 1- and 2-year PFS rate of 24% (95%CI 14- 38%) and 24% (95%CI 14-38%) respectively. Median OS in total population was 9.4 months, with 1- and 2-year OS rate of 32.2 % (95%CI 17.9-47.2%) and 24.2 % (95%CI 9.2-42.9%). Considering subgroups, median PFS was higher in ECOG 0 patients compared to patients with ECOG 1 and 2 (3.97 months versus 2.97 months, HR 0.482, 95% CI of ratio 0.246 to 0.946, p 0.0339, (Figure2a); in patients with less than three metastatic sites compared to patients with more than three metastatic sites (3.5 months versus 3.27 months, HR 0.524 95% CI of ratio 0.279 to 0.964, p 0.0445, (Figure 2c); in NLR <0.7 patients compared to patients with NLR > 0.7 (4.77 months versus 2.43 months, HR 0.223 95% CI of ratio 0.093 to 0.535, p 0.0008, (Figure 2d). LDH prior to Ipilimumab therapy, and best response to prior anti-PD-1 therapy were not associated with PFS (figure 2b, 2e). Median OS was higher in ECOG 0 patients compared to patients with ECOG 1 or 2 (11.4 months versus 4.07 months, HR 0.301 95% CI of ratio 0.141 to 0.644, p 0.002 (Figure 3a), in NLR <0.7 patients compared to patients with NLR > 0.7 (11.17 months versus 3.63 months, HR 0.161 95%CI of ratio 0.058 to 0.460, p 0.0006, (Figure 3d). LDH prior to ipilimumab therapy, best response to prior anti-PD-1 therapy and number of metastatic sites were not associated with OS (figure 3b, 3c, 3e)
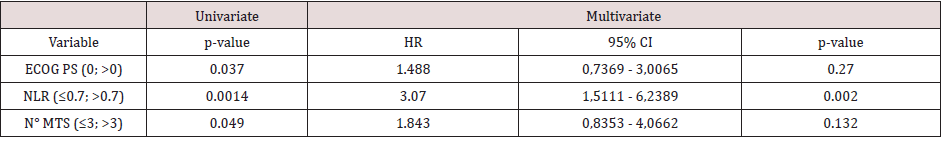
Multivariate analyses were conducted considering factors that resulted statistically significant in previous univariate analysis. In table 3a multivariate analysis for PFS is reported: between all factor that resulted statistically significant in univariate analysis only NLR maintained statistically significance in multivariate analysis. In table 3b multivariate analysis for OS is reported: both ECOG PS and NLR maintained a statistical significance in multivariate analysis. Considering the two factors that impacted in a statistically significant way on OS (ECOG PS and NLR), for each patient a score was composed, based by the sum of number of negative factors associated with worse prognosis (ECOG PS > 0, NLR more than 0.7). As illustrated in figure 4, OS was significantly higher in patient with score 0 then patient with score 1 or 2. Median OS for patient with score 0, 1 or 2 were respectively 11.4, 7.87 and 2.77 respectively (p value < 0.0001) (Figure 4)
Discussion
The treatment algorithm for patients suffering from affected
by metastatic melanoma is constantly evolving. Although much
debate has been focused on the optimal choice of first-line
systemic treatment, many options are also available regarding
subsequent lines of treatment and clinicians must choose which
drug to administer in case of progression after using Anti-PD1 in
the metastatic setting [2-6]. Our study investigates patients with
metastatic melanoma who progressed to Anti-PD1 and eventually
to BRAF and MEK inhibitors if BRAF v600 mutated: in this setting
the standard treatment is Ipilimumab at a dose of 3 mg/kg every 21
days for 4 total cycles; further alternatives are chemotherapeutic
treatments; finally, experimental drugs are investigated within
ongoing clinical trials. Among these options, chemotherapy
has limited activity. Experimental treatments often consist in
combination regimens of several drugs; often these regimens
are associated with a higher incidence of toxicity compared to
monotherapies: now, however, these combinations are the topic of
ongoing trials and they can be only administered among enrollment
in experimental protocols [11-17]. In this setting it is therefore
further useful to be able to identify those patients with a high
probability of obtaining good disease control using Ipilimumab.
Treatment with Ipilimumab is associated with several advantages:
its use is now well established, and clinicians are experienced in
the management of its toxicity, in case of efficacy after a total of 4
cycles the patients continue with only follow-up. In patients who
get disease control for 3 years, we can reasonably expect to have
disease control that will last over time. Unfortunately, in each study
reported in literature only about 15% of the patients treated with
Ipilimumab shows good disease control: the great difficulty for
the scientific community is to identify these patients. Even in the
setting of AntiPD1 progressed patients, the knowledge of clinical
or laboratory prognostic factors connoting Ipilimumab responders
could help clinicians to choose more easily the treatment to use
among the available drugs.
From our retrospective case series, it is highlighted that some
clinical and laboratory characteristics can compose a score capable
to give a prognostic value to patients treated with Ipilimumab and
previously progressed to antiPD1: ECOG PS equal to 0, NLR<0.7
at the time of Ipilimumab initiation appear to be associated with
a better prognosis. The complete absence of these parameters
seems to be associated with a poor prognosis: patients in whom
these factors are all absent have shown a very poor survival, in such
patients an inability of Ipilimumab to control the disease seems
evident and, in these cases, therefore the use of Ipilimumab seems
inadvisable. In such patients it would therefore seem justified to
undertake alternative treatments. On the other hand, if all these
factors are present together, the best prognosis of the series is
highlighted: these patients are associated with the best prognosis
of Ipilimumab treatment; in these patients the use of Ipilimumab
shows the highest probability of efficacy and long survival. We
would like to recommend the evaluation of a possible treatment
with Ipilimumab in patients with these characteristics. Other
authors had investigated the efficacy of Ipilimumab in the second
or third line of treatment, but in our work for the first time we try to
identify prognostic parameters useful for characterizing the efficacy
of Ipilimumab in this setting. The prognostic parameters that we
have identified are reminiscent of the parameters that have shown
a prognostic value both for Ipilimumab used in the first line and for
Anti-PD1 in the first line [21-23]: this concept underlines how the
current characteristics of an immunotherapy treatment undergo to
connote the prognosis of patients. From our analyzes, efficacy of
previous treatment with Anti-PD1 does not seem to influence the
efficacy of the subsequent treatment with Ipilimumab: from this
series, therefore, a sure efficacy of Ipilimumab does not seem to
be evident in those who have had a good efficacy of Anti-PD1, or conversely an inactivity of Ipilimumab in those who have had a low
efficacy of AntiPD1 does not seem obvious. On the other hand, it
is the current characteristics at the time of undertaking the next
treatment line with Ipilimumab that prognostically characterize
patient’s prognosis.
Recently, some studies have tried to investigate the efficacy
and activity of the combination of Ipilimumab with Anti-PD1 on
progression after Anti-PD1. In the retrospective study by Zimmer
et al, this combination was compared with the use of Ipilimumab
alone and no data of efficacy of the combination emerged compared
to ipilimumab monotherapy, in the face of greater toxicity of the
combination [8]. Other trials were recently presented at the
American Society of Clinical Oncology 2020 conference evaluating
the use of antiPD1 plus Ipilimumab in progression after antiPD1. In
the phase II study of Olson et al, Pembrolizumab at 200 mg combined
with Ipilimumab 1 mg / kg every 21 days was administered
[11]. Data show a median PFS of 5 months and a median OS of
approximately 24.7 months comparable to what was obtained in
our study in the subgroup at score 0. Da Silva presented the results
of a study in which patients who progressed to Anti-PD1 received
Ipilimumab alone or in combination with Anti-PD1 [12]; from the
comparison it would seem to highlight an advantage in terms of OS
and PFS for the combination, but the population investigated in this
study also included patients progressing to AntiPD1 used in the
adjuvant setting, and it is not possible to evaluate how much this
component of the population affected the results.
In any case, if we compare the results of these trial to our
casuistic, in the 0-score population of our study, we observed a
relationship between efficacy and toxicity profile that is competitive
when compared to the much more toxic AntiCTLA4 plus AntiPD1
combination regimen. Further studies to identify which subgroups
benefit most from Anti-CTLA4 monotherapy or the combination
of Anti-CTLA4 and Anti-PD1 are required. Our study has some
limitations. First, it is a retrospective study, and therefore it
presents the typical biases of all retrospective studies. Follow-up
is not comparable to that of first-line Ipilimumab studies, but we
believe that the setting (second line after Anti-PD1 and BRAF-MEK
inhibitors) may justify shorter follow-up times for conducting
analyzes. The use of Ipilimumab in combination with Anti-PD1 in
the first line is becoming increasingly widespread, therefore the
population investigated (treated with Anti-PD1 or BRAF and MEK
inhibitors in sequence) in the current population of patients with
metastatic melanoma and progressed to previous lines will be a
minority, as more and more patients will have already been treated
with Ipilimumab in combination with other immunotherapy:
however we believe that in the near future there will always be a
space for the use of Ipilimumab in the second line, because there
will still be patients treated in first line with Anti-PD1 monotherapy
or AntiPD1 in combination with drugs other than Ipilimumab:
for such patients the disquisitions of this study may possibly be
applicable and useful for the choice of undertaking Ipilimumab
monotherapy or not. In conclusion, in our multicenter retrospective
study, metastatic melanoma patients previously treated with Anti-
PD1 inhibitors and treated with Ipilimumab have a better prognosis
in the presence of ECOG PS 0, NLR <0.7 at the time of Ipilimumab
first cycle; we do not recommend its use in the total absence of these
parameters, preferring in such cases any therapeutic alternatives.
References
- Garbe C, Amaral T, Peris K, Axel Hauschild, Petr Arenberger, et al. (2020) European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics-Update 2019. Eur J Cancer 126: 141-158.
- Ascierto PA, Long GV, Robert C (2019) Survival Outcomes in Patients with Previously Untreated BRAF Wild-Type Advanced Melanoma Treated with Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial. JAMA Oncol 5(2): 187-194.
- Robert C, Ribas A, Schachter J, Ana Arance, Jean Jacques Grob, et al. (2019) Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 20(9): 1239-1251.
- Robert C, Grob JJ, Stroyakovskiy D, Axel Hauschild, Evgeny Levchenko, et al. (2019) Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med 381(7): 626-636.
- Dummer R, Ascierto PA, Gogas HJ (2018) Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 19(5): 603-615.
- Ascierto PA, McArthur GA, Dréno B, Victoria Atkinson, Gabrielle Liszkay, et al. (2016) Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 17(9): 1248-1260.
- European Medicines Agency, product information about Yervoy.
- Zimmer L, Apuri S, Eroglu Z, Lisa A, Forschner A, et al. (2017) Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer 75: 47-55.
- Zaretsky JM, Garcia-Diaz A, Shin DS, Helena Escuin-Ordinas, Willy Hugo, et al. (2016) Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 375: 819-829.
- Koyama S, Akbay EA, Li YY, Kevin A, William G, et al. (2016) Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 7: 10501.
- Olson D, Luke JJ, Poklepovic AS, Madhuri Bajaj, Emily Higgs, et al. (2020) Significant antitumor activity for low dose ipilimumab (IPI) with pembrolizumab (PEMBRO) immediately following progression on PD1 Ab in melanoma (MEL) in a phase II trial. JCO 38(15): 10004-10004.
- Pires Da Silva I, Ahmed T, Lo S (2020) Ipilimumab (IPI) alone or in combination with anti-PD-1 (IPI+PD1) in patients (pts) with metastatic melanoma (MM) resistant to PD1 monotherapy. JCO 38(15): 10005-10005.
- Arance A, O Day SJ, de la Cruz Merino L (2020) Lenvatinib (len) plus pembrolizumab (pembro) for advanced melanoma (MEL) that progressed on a PD-1 or PD-L1 inhibitor: Initial results of LEAP-004. Ann Oncol 31(4): 1142-1215.
- Haymaker C, Andtbacka RHI, Johnson DB, Shaheen MF, Rahimian S, et al. (2020) Final results from ILLUMINATE-204, a phase I/II trial of intratumoral tilsotolimod in combination with ipilimumab in PD-1 inhibitor refractory advanced melanoma. Ann Oncol 31(4): 672-710.
- Johnson ML, Gonzalez R, Opyrchal M, Dmitry Gabrilovich, Peter Ordentlich, et al. (2017) ENCORE 601: A phase II study of entinostat (ENT) in combination with pembrolizumab (PEMBRO) in patients with melanoma. JCO 35(15): 9529-9529.
- Davar D, Boasberg P, Eroglu Z (2018) A Phase 1 Study of TSR-022, an Anti-TIM-3 Monoclonal Antibody, in Combination with TSR-042 (Anti-PD-1) in Patients with Colorectal Cancer and Post-PD-1 NSCLC and Melanoma. SITC 2018 Annual Conference Proceedings pp. 21.
- Ascierto PA, Melero I, Bhatia S, Petri Bono, Rachel E Sanborn et al. (2017) Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. JCO 35(15): 9520-9520.
- Bowyer S, Prithviraj P, Lorigan P, Larkin J, McArthur G, et al. (2016) Efficacy and toxicity of treatment with the anti-CTLA-4 antibody ipilimumab in patients with metastatic melanoma after prior anti-PD-1 therapy. Br J Cancer 114(10): 1084-1089.
- Jacobsoone Ulrich A, Jamme P, Alkeraye S, Emmanuel Faure, Carole Templier, et al. (2016) Ipilimumab in anti-PD1 refractory metastatic melanoma: a report of eight cases. Melanoma Res 26(2): 153-156.
- Aya F, Gaba L, Victoria I, Aranzazu Fernández Martínez, Mónica Tosca, et al. (2016) Ipilimumab after progression on anti-PD-1 treatment in advanced melanoma. Future Oncol 12(23): 2683-2688.
- Long GV, Robert C, Blank CU (2017) Outcomes in patients (pts) treated with ipilimumab (ipi) after pembrolizumab (pembro) in KEYNOTE-006. Pigment Cell Melanoma Res 30(1): 118.
- Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, et al. (2016) Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 27(4): 732-738.
- Diem S, Kasenda B, Martin-Liberal J, Alexander Lee 1, Dharmisha Chauhan, et al. (2015) Prognostic score for patients with advanced melanoma treated with ipilimumab. Eur J Cancer 51(18): 2785-2791.
- Weide B, Martens A, Hassel JC, Michael A Postow, Kees Bisschop, et al. (2016) Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin Cancer Res 2016;22(22): 5487-5496.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...