Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Case Report(ISSN: 2638-5945) 
Identification of Stage 5/6 Blastocyst Retention: A Case Report Volume 5 - Issue 3
Yan Jiang, Cai-Ping Geng, Jing-Chuan Yuan, Ge Song and Xiao Hua Wu*
- The Center for Reproductive Medicine and Infertility, The Fourth Hospital of Shijiazhuang, Shijiazhuang Obstetrics and Gynecology Hospital affiliated to Hebei Medical University, Hebei Key Laboratory of Maternal and Fetal Medicine. Shijiazhuang Hebei 050011, PR China
Received: August 18, 2023 Published: August 29, 2023
Corresponding author: Xiao Hua Wu, The Center for Reproductive Medicine and Infertility, The Fourth Hospital of Shijiazhuang, Shijiazhuang Obstetrics and Gynecology Hospital affiliated to Hebei Medical University, Hebei Key Laboratory of Maternal and Fetal Medicine. 206 Zhong-Shan-Dong, Shijiazhuang, Hebei 050011, P.R. China
DOI: 10.32474/OAJOM.2023.05.000214
Abstract
Embryo retention (ER) in the catheter is a common phenomenon in Embryo Transfer (ET). However, it is difficult to distinguish 5-stage or 6-stage collapsed blastocysts without Zona Pellucida (ZP) in ER. This case reported a patient transferred a thawed 5-stage blastocyst with residue in the catheter in frozen ET cycles. The residue was similar with collapsed blastocysts without ZP. But when we examined it under an inverted microscope, it seemed to have more cells. And it remained no change after cultured 24 h. The residue and the blood of patient were checked for preimplantation genetic testing of aneuploidy heteroploidy (PGT-AH). The results showed: The number of Single Nucleotide Polymorphism (SNP) detected was 367942, proportion of SNPs with double heterozygous (HetHet) was 0.1509, proportion of SNPs with zero identical-by-state (IBS0) was zero. Estimated kinship coefficient form the SNP data was 0.4904 ( kinship value>0.354 supports the same sample or identical twins). Conclusion: the residue came from the patient instead of the transferred blastocyst. Considering the patient had endometrial hyperplasia and endometritis, we suspected that the residue maybe came from hyper-plastic endometrium.
Keywords: Embryo Retention; Stage 5/6 Blastocyst; Frozen Embryo Transfer
Introduction
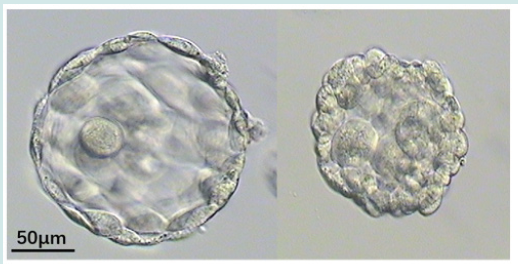
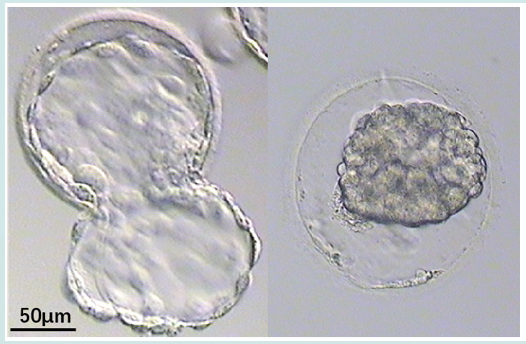
Embryo retention (ER) was common in catheters after embryo transfer (ET) [1]. The total incidence of ER was 0.27-2.8% [2-5]. There was no difference in ER rate between blastocyst and cleavage transfer cycles [2,4]. The incidence of ER in fresh and frozen transfer cycles remains controversial [2,4]. The number of embryos affects the ER rate. A lower number of embryos transferred has the advantage of decreasing the incidence of ER in catheters [5]. It’s easy to find the embryo retention of cleavage and blastocyst with zona pellucida (ZP). However, stage 5 or 6 collapsed blastocysts without ZP was difficult to distinguish (Figures 1 & 2). In the present study, a case of the residue was reported after thawing 5-stage blastocyst and it was attempted to find common characteristics through reviewing the literature.
Case report
Patient history
A 30-year-old female patient and her 28-year-old husband presented at the Center for Reproductive Medicine and Infertility of our hospital in February 2021 due to primary infertility for 3 years. The patient had a regular menstrual cycle.
After two IUI cycles failure, an antagonist protocol in vitro fertilization (IVF) treatment was adopted. The patient was stimulated with urinary FSH 225 IU on day two. The antagonist was given from day seven. The trigger was administered on day 12 of stimulation with recombinant human chorionic gonadotropin, 0.25 μg subcutaneously. The patient had seven follicles of 18 mm and ten follicles of 15-17 mm in size on the day of the trigger under ultrasound. Egg retrieval was performed 36 h after the trigger. A total of 17 oocytes were recovered by ultrasound-guided transvaginal aspiration. Short-term IVF fertilization was performed and 11 normal fertilized oocytes were observed. All embryos (2 cleavage embryos and 7 blastocysts) were frozen without fresh transferred. In June 2021, hysteroscopy was checked in our hospital showed endometrial hyperplasia with scattered spotty congestion. Pathology showed chronic inflammatory cell infiltration in the endometrial interstitium with CD138(+ + +). After intrauterine medicine therapy, the patient adopted pituitary down-regulation and hormone replacement protocol for frozen-thawed embryo transfer (FET) cycles.
Blastocyst vitrification and warming procedures [6].
The procedure was always performed using one blastocyst for each straw. An artificial shrinkage (AS), using a laser pulse was performed before vitrification. The blastocyst was then moved at room temperature (22 25˚C) to Kitazato (Japan) Equilibration Solution (ES). After 6 8 min, the blastocyst was quickly washed in vitrification solution (VS) for 45 60 sec and transferred onto the straw (Kitazato Japan) using a micropipette and immersed vertically into liquid nitrogen. An Kitazato (Japan) Taw Kit was used for warming. The carrier containing the embryo was removed from the straw and placed quickly into the dish containing the thawing medium (thawing solution) preheated at 37˚C. The blastocysts immediately fell from the device and could be easily identified in the medium. After 1 min, the blastocysts were transferred to the DS medium (dilution solution) for 3 min at room temperature (22 25˚C). In the last two step, the blastocysts were placed for 5 min, in the WS1 medium and WS2 (washing solution). The embryo was then returned to G 2 medium for zona pellucida assisted hatching (AH) using a laser pulse and culture until transfer. Embryo transfer was normally performed within 2 or 3 h.
Blastocyst Transfered
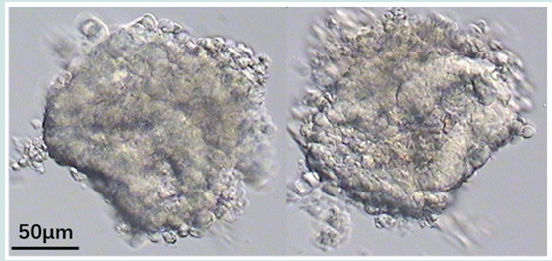
In April 2022, A 5-stage blastocyst was warming and transfered. A Wallace catheter (Smith Medical International Ltd.) was used, this catheter system consists of an outer firm and an inner soft catheter. An outer transfer catheter was passed through the external cervical os to the level of the internal cervical os. The embryos were loaded into an inner catheter which was advanced until the desired intrauterine location was reached. The operator gently expelled the embryos from the catheter. The catheter was withdrawn slightly and retrieved by the embryologist to check for retained embryos by microscopy. The laboratory operator observe the tip of the catheter without blood, and immediately check for any remaining embryos in the catheter under microscope and found residues after flushing (Figure 3). The residue was similar with collapsed blastocysts without ZP. But it seems had more cells when we check it under the inverted microscope. And it remained no change after cultured 24 h. The patient was biochemical pregnancy with a temporary increase in the serum hCG level but without clinical prengancy.
Preimplantation genetic testing of aneuploidy heteroploidy (PGT-AH).
The residue and the blood of patient were checked for preimplantation genetic testing of aneuploidy heteroploidy (PGTAH). The results showed: number of single nucleotide polymorphism (SNP) detected were 367942, proportion of SNPs with double heterozygous (HetHet) was 0.1509, proportion of SNPs with zero identical-by-state (IBS0) was zero. Estimated kinship cofficient form the SNP data was 0.4904 (kinship value>0.354 supports the same sample or identical twins). The participant provided written informed consent and the Fourth Hospital of Shijiazhuang ethics committee approved this study (ethical approval number: 20220139).
Discussion
ET is a crucial step in IVF performed under transabdominal ultrasound guidance. After the embryo was transferred, the laboratory operator immediately check for any remaining in the catheter under microscope. Except blood and mucus, embryo retention occurs occasionally. Whereas it’s seldom reported that other residues were found in the catheter through reviewing the literature. Finding ER in the transfer catheter is a clinically worrisome event [4]. As standardized ET technique under ultrasound guidance and the skill of the operator performing ET, the occurrence of ER were decrease. The recently reported incidence of ER varies between 0.33% (95/29,160) [4] and 1.59% (97/6,089) [2]. The occurrence of ER were associated with contamination of the transfer catheter with mucus or blood, the number of embryos transferred, the embryonic stage and the technical difficulties at the time of ET [4,5,7-9].
The incidence of ER in blastocyst transfer vs. cleavagestage embryos
Theoretically, the larger, less dense, expanded blastocyst compared to the dense, compact character of cleavage-stage embryos might result in an increased risk of blastocyst retention in the transfer catheter [5,10]. Considering these blastocyst features, it seems reasonable to postulate that blastocyst transfer itself might be a risk factor for ER [5]. However, except Silberstein, et al. [7] reported the incidence of ER in blastocyst transfer cycles was increased than that of cleavage-stage transfer cycles (4.4% vs. 2.3%), but there was no statistically significant difference. Majority studies showed the incidence of ER in blastocyst transfer was similar to the incidence of ER with cleavage-stage embryos [2,4- 5]. The blastocyst and cleavage ER rate in corresponding transfer cycles was 1.56% (12/960) and 1.66% (85/5,129), respectively (P=0.35) [2]. Another study showed that the overall incidence of ER in Day 5 blastocyst transfer ((32/1,131=2.8%) was similar to the incidence of ER in ET with cleavage-stage embryos [5]. The bias between theories and reports may be due to uncertainty about the number of embryos transferred.
ER rate in fresh ET cycles vs. frozen transfer cycles
In general, the ‘freeze-all’ strategy implies transfer of frozenthawed embryos only, with no fresh embryo transfers [11]. The incidence of ER in fresh and frozen transfer cycles remains debatable. Some studies reported a signifificantly increased ER rate were found in fresh ET cycles (2.71%, 52/ 1,920) compared with frozen transfer cycles (1.08%, 45/4,169) (P<.01) [2]. In fresh transfer cycles, the rate of mucus in or on the catheter after ET in ER group was signifificantly higher than in the non-ER group (48.09% vs. 13.65%) [2].
However other studies concluded there was no difference in the rate of ER rate in fresh ET cycles compared with frozen transfer cycles [3]. Transfer of fresh/frozen embryos were not associated with the frequency of ER [4].
Does embryo number influence ER rate?
At the beginning of IVF, transfer number of embryos were not limited, so comparing of multiple embryos were studied. When transfer of one or two embryos was compared to three or more embryos, the likelihood of retained embryos increased from 1.2% (4/321) to 3.2% (37/1,133) [12]. ETs of > or =4 embryos were more likely to be associated with retained embryos than ETs of <4 embryos (3.7% vs. 2.2%) [7]. As pregnancy rate increased, transfer number of embryos were limited, single embryo transfer was preferred. Because single cleavage-stage embryo transfer is few, so reports focusd on blastocyst transfer. No ER was observed in single blastocyst transfer (SBT), All ER occured in double blastocyst transfer (DBT) cycles and the incidence of ER in DBT was 3.5% [5]. The number of transferred blastocysts might be a factor that leads to an increased incidence of ER. A lower number of blastocysts transferred has the advantage of decreasing the incidence of retained embryos in catheters [5].
When ER occurs, when is better time to retransfer of the embryo.
Currently, immediate retransfer is universally adopted as a rescue method when embryos are retained in transfer catheter [4]. If any embryo was found to have been retained in the catheter, the retained embryos were immediately reloaded by the embryologist, and a second transfer was performed [2]. It’s simple and convenient for patient and operator. However Visser DS et al recommended a 1-day delay before re-transferring ER to increase the pregnancy rate of ER [13].
Does ER affect the reproductive outcomes? Do multiple attempts at embryo transfer affect clinical pregnancy rates [14]?
There is no consensus on whether retransferring retained embryos has an impact on reproductive outcomes [2]. Some studies have found that there was no signifificant difference in the clinical pregnancy rate between transfer procedures in which all embryos were transferred at the first attempt and procedures that required multiple attempts [5,7,8,14]. Pregnancy outcomes were similar between the ER and the non-ER cycles in cases of no blood in the catheter [3]. Retained embryos in the transfer catheter and immediate retransfer of them have no adverse impact on clinical pregnancy and implantation rates unless other previously reported signs of difficult transfer are also observed [10,12]. Patients and physicians should not be concerned about the retention of embryos during transfer since there is no effect on pregnancy outcome [3]. On the contrary, Visser et al. And Alvero et al. concluded that retention of embryos during transfer signifificantly reduced the pregnancy rate (20.3% vs. 3.0%) [13,15]. When matched the factors of maternal age, embryo conditions, and causes of infertility, the ER group also showed a signifificantly lower clinical pregnancy rate, implantation rate, and live birth rate (all P<.01), and a signifificantly higher ectopic pregnancy rate (P<.05) [2].
Overall hatching out from the zona pellucida (ZP) is a crucial step for blastocyst implantation and development [16]. However, the ZP of the blastocyst is more fragile near the time of hatching, and ET may cause some trauma to the blastocyst [17]. In addition, stage 5 and 6 blastocysts without ZP were easily collapsed. There was seldom report about ER of blastocysts without ZP through reviewing the literature.
In this case report, suspected ER was found in SBT frozen transfer cycle. Considering blastocyst was performed an Artificial Shrinkage (AS), using a laser pulse, before vitrification. And ZP of 5-stage blastocyst was easily disengaged after transfer. So when the residue which was similar like collapsed blastocysts without ZP was found, it was checked under the inverted microscope and cultured. Because we are not sure whether the residue is ER, and the report recommended a 1-day delay before retransferring Res [13]. Therefore, we cultured it for 24 h and expansion could be observed if it was collapsed blastocyst. However, the residue had not any change. Therefor the residue and the blood of patient were checked and showed that the residue came from the patient instead of the embryo. Considering the patient had endometrial hyperplasia and chronic inflammatory cell infiltration in the endometrial interstitium, so we suspected that the residue was tissue of endometrial. The study about mechanism of embryo retention showed that pressure changes in the uterine cavity during ET can influence the distribution of the transferred fluid [1]. Under certain conditions, hyperplasia of the endometrium may flow backward in the catheter, which may lead to retention in the catheter. In conclusion, SBT has become common in order to reduce ER rates and to enhance IVF outcomes. Furthermore, the present study reported how to deal with suspected ER after ET stage 5 and 6 SBT.
Acknowledgements
Not applicable.
Funding
Hebei Province Medical Science Research Key Project (20231650).
Availability of Data and Materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
XIAO-HUA WU designed the study and revised the paper. YAN JIANG and CAI-PING GENG wrote the main manuscript text. JINGCHUAN YUAN, and GE SONG prepared Figures 1-3. All authors reviewed the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics Approval and Consent to Participate
The Fourth Hospital of Shijiazhuang ethics committee approved this study (ethical approval number: 20220139). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Patient Consent for Publication
Not applicable.
Competing Interests
There are no competing interests that could be perceived as prejudicing the impartiality of the research reported.
References
- Kozikowska M, Grusza M, Mrugacz G, Jerzy Gagan, Monika Zbucka-Krętowska, et al. (2019) The influence of intrauterine pressure on embryo retention in a catheter after embryo transfer. Sci Rep 9(1): 11969.
- Xu J, Yin MN, Chen ZH, Li Yang, De-Sheng Ye, et al. (2020) Embryo retention significantly decreases clinical pregnancy rate and live birth rate: a matched retrospective cohort study. Fertil Steril 114(4): 787-791.
- Kadour-Peero E, Tulandi T, Feferkorn I, Ranit Hiszkiahu, William Buckett, et al. (2022) Effects of embryo retention during embryo transfer on IVF outcomes. J Assist Reprod Genet 39(5): 1065-1068.
- Zhang HX, Li F, Jin H, Wen yan Song, Yingchun Su, et al. (2023) Effect of retained embryos on pregnancy outcomes of in vitro fertilization: a matched retrospective cohort study. BMC Pregnancy Childbirth 23(1): 5.
- Yi HJ, Koo HS, Cha SH, Hye Ok Kim, Chan Woo Park, et al. (2016) Reproductive outcomes of retransferring retained embryos in blastocyst transfer cycles. Clin Exp Reprod Med 43(2):133-138.
- Jiang Y, Song G, Zhang XH (2022) Frozen blastocysts: Assessing the importance of day 5/day 6 blastocysts or blastocyst quality. Exp Ther Med 23(5): 333.
- Silberstein T, Trimarchi JR, Shackelton R, Sherry Weitzen, David Frankfurter, et al. Ultrasound-guided miduterine cavity embryo transfer is associated with a decreased incidence of retained embryos in the transfer catheter. Fertil Steril 84(5): 1510-2.
- Nabi A, Awonuga A, Birch H (1997) Multiple attempts at embryo transfer: does this afect in-vitro fertilization treatment outcome? Hum Reprod 12:118890.
- Ghazzawi IM, Al-Hasani S, Karaki R (1999) Transfer technique and catheter choice infuence the incidence of transcervical embryo expulsion and the outcome of IVF. Hum Reprod 14(3): 677-682.
- Lee HC, Seifer DB, Shelden RM (2004) Impact of retained embryos on the outcome of assisted reproductive technologies. Fertil Steril 82(2): 334-337.
- Zaat T, Zagers M, Mol F, Mariëtte Goddijn, Madelon van Wely, et al. (2021) Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev 2(2): CD011184.
- Vicdan K, Işik AZ, Akarsu C, Eran Sözen, Gamze Cağlar, et al. (2007) The effect of retained embryos on pregnancy outcome in an in vitro fertilization and embryo transfer program. Eur J Obstet Gynecol Reprod Biol 134(1):79-82.
- Visser DS, Fourie FL, Kruger HF (1993) Multiple attempts at embryo transfer: efect on pregnancy outcome in an in vitro fertilization and embryo transfer program. J Assist Reprod Genet 10(1):37-43.
- Oraif A, Hollet-Caines J, Feyles V, Maggie Rebel 1, Hanin Abduljabar, et al. (2014) Do multiple attempts at embryo transfer affect clinical pregnancy rates? J Obstet Gynaecol Can 36(5): 406-407.
- Alvero R, Hearns-Stokes RM, Catherino WH (2003) The presence of blood in the transfer catheter negatively inflfluences outcome at embryo transfer. Hum Reprod 18(9): 1848-1852.
- Liu SJ, Sun JB, Hao X, Zhe Han, Xin Wen, et al. (2020) Blastocyst hatching site is regularly distributed and does not influence foetal development in mice. Sci Rep 10(1): 2475.
- Sheiner E, Har-Vardi I, Potashnik G (2001) The potential association between blastocyst transfer and monozygotic twinning. Fertil Steril 75(1): 217-218.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...