Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Case Report(ISSN: 2638-5945) 
High Dose Cladribine in Combination with High Dose Cytarabine as A Salvage Therapy for Adults with Refractory Relapsed Langerhans Cell Histiocytosis Volume 4 - Issue 4
Naif I AlJohani*, Nada AlHarbi, Jalil Ur Rehman, Binyam Usman
- Adult Hematology and Bone marrow transplant section, Oncology Department, King Faisal Specialist Hospital & Research Center, Jeddah, Saudi Arabia
Received:June 11, 2021 Published: June 22, 2021
Corresponding author: Naif I AlJohani, Section Head & Consultant, Adult Hematology and Bone Marrow Transplant. Oncology Department, King Faisal Specialist Hospital & Research Center, Jeddah, Saudi Arabia
DOI: 10.32474/OAJOM.2021.04.000194
Abstract
Introduction
Langerhans cell histiocytosis (LCH) is a rare condition with extremely variable manifestations ranging from single-organ involvement to multisystemic dysfunction that can be refractory or relapse. Currently, no international standard care guidelines exist for treating adult patients with refractory, multisystem LCH. This case series describes three patients with refractory multisystem LCH who failed to respond to multiple lines of chemotherapy or radiotherapy but remarkably achieved sustained complete metabolic remission after treatment with a modified “pediatric-inspired” cladribine-based intensive induction (CLAG) protocol.
Presentation of Cases
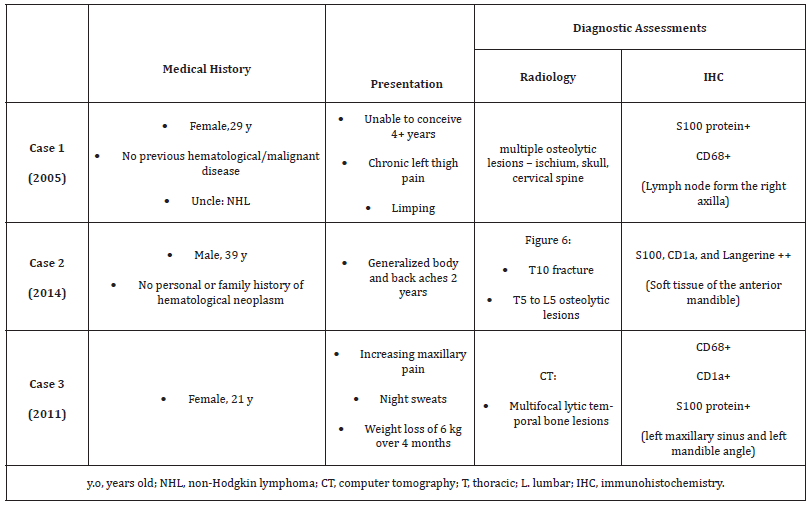
Case 1 was a 29-year-old female who presented with chronic left thigh pain and an abnormal gait. Case 2 was a 39-year-old male who presented with a 2-year history of generalized body and back pain. Case 3 was a 21-year-old female who presented with increasing maxillary pain, night sweats, and weight loss. In all three cases, the initial radiological imaging showed osteolytic lesions and tissue biopsies confirmed the diagnosis of LCH. Case 1 received standard induction chemotherapy and two lines of salvage therapy before the CLAG protocol was initiated. Case 2 was treated with standard induction chemotherapy, and the CLAG protocol was used as first line salvage therapy. Case 3 was treated with standard induction chemotherapy and the CLAG protocol used as second line salvage therapy. In Case 1, maintenance therapy was stopped at 6 months due to recurrent infection and prolonged cytopenia. In Case 2, significant changes were made to the maintenance phase protocol because of side effects. The CLAG protocol resulted in complete metabolic remission in all patients based on follow-up PET/CT examinations.
Conclusion
We report 3 cases of refractory/relapsed LCH in adult patients who experienced complete metabolic remission following salvage chemotherapy using a modified CLAG protocol after failure of at least one line of standard induction therapy. Keywords: Cladribine; Cytarabine; Langerhans Cell Histiocytosis; Osteolytic Lesions
Introduction
Histiocytosis X was renamed Langerhans cell histiocytosis (LCH) in 1987 and is a rare disorder with an unknown true incidence that is caused by abnormal proliferation of Langerhans cells resulting in the formation of granulomas [1,2]. The clinical manifestation of LCH is variable and ranges from infiltration of a single organ to multisystem infiltration; risk-organ involvement, which includes the hematopoietic system, liver, and/or spleen, carries a poor prognosis [1,2]. The most common site involved is the bone, with central nervous system (CNS) involvement being less common. The skull (predominantly the posterior jaw) followed by the femur, pelvis, and spine are the most frequent sites of skeletal involvement [3,4]. To the best of our knowledge, no international guidelines or studies have been published on the treatment of adult patients with refractory or relapsed LCH using a combination of cladribine and cytarabine (CLAG). This case series describes three patients with refractory multisystem LCH in whom we achieved sustained metabolic remission after treatment with a pediatricinspired, dose-modified, CLAG-based therapy [5].
Clag Protocol
The pediatric CLAG protocol was first described in a Phase II clinical study and included at least two 5-day courses of cytarabine (1 g/m2 per day) plus cladribine (9 mg/m2 per day). In addition, maintenance therapy consisted of two 3-day courses of cladribine (5 mg/m2 per day) followed by vinblastine (6 mg/m2) every 2 weeks for 6 months, combined with oral prednisolone (40 mg/ m2) for 5 days every other week, oral 6-mercaptopurine (6MP) (50 mg/m2) every day, and oral methotrexate (MTX) (20 mg/m2) once per week. Oral 6MP and MTX were continued for an additional 12 months.5
Case Reports
Case 1
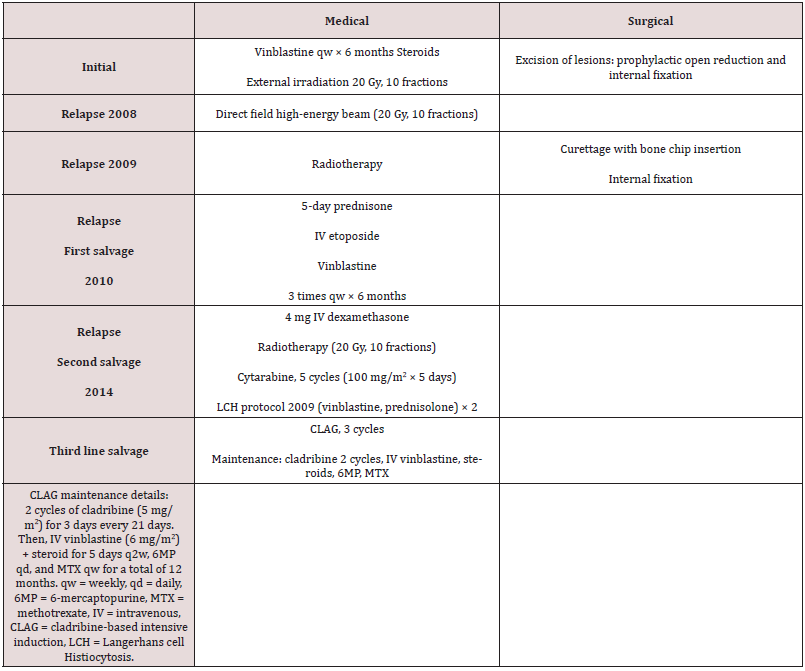
Case 1 was a 29-year-old female patient who initially presented to another facility with complains of persistent left thigh pain and walking with a limp in April 2005. She was seen by an orthopedic team, and a plain radiograph of the left femur showed the presence of multicentric multifocal osteolytic lesions in the shaft of the left femur with significant cortical destruction; however, other clinical examination findings were unremarkable. Laboratory workup revealed mild anemia. Skeletal survey & bone scanning noted multiple osteolytic lesions in the ischial bones, both parietal bones, and the C4 cervical vertebra. On the 20th of April 2005, she underwent surgical revision and excision of the bony lesions and prophylactic open reduction with internal fixation using plates & screws. Histopathological examination of the tissue biopsy confirmed LCH. Initial induction therapy and surgical intervention were selected based on the multifocal nature of the patient’s disease upon presentation as described in Table 1. Following this treatment regimen, the patient achieved first remission and became pregnant 2.5 years after the end of her treatment. In 2008, she developed severe persistent low back pain. Computed tomography (CT) showed localized disease with destruction of the T11 vertebra that was treated with direct field high-energy beam radiotherapy [Table 2]. Afterwards, her pain significantly improved. In 2009, she presented with pain in the right arm. A bone scan revealed a destructive lesion in the right humerus. Contrast-enhanced PAN-CT showed multiple osteolytic lesions in the dorsal, lumbar, and pelvic regions. Radiotherapy and surgery were initiated as described in Table 2. The patient remained progression-free until 2010 when positron emission tomography (PET)-CT imaging revealed widespread increased uptake in the right frontal-parietal and occipital bones, right humerus, right iliac crest, left femur, and several vertebrae. First line salvage chemotherapy according to Histiocytosis Society Protocol type-II was initiated, as outlined in Table 2. Upon radiological evaluation, the patient showed a promising response. During a clinic visit in 2014, the patient complained of a progressive swelling in her right neck. A whole spine MRI was conducted, and this revealed a soft tissue infiltrating lesion extending from the left side of the neck into the neural foramina and spinal canal at C2–C4 vertebral levels. A biopsy indicated that the mass was consistent with LCH. Owing to impending cord compression, the patient was placed on intravenous (IV) dexamethasone [Table 2] and subsequently became stable and asymptomatic. Additional chemo- and radiotherapy were initiated [Table 2], but a PET-CT scan showed interval disease progression with multiple new bone lesions and intense muscular uptake in both rotator cuff muscles. The patient was started on chemotherapy according to the LCH 2009 protocol [Table 2]. The follow-up PET/CT after two courses of the LCH 2009 protocol revealed no significant changes, and only one bone lesion involving the left ischium showed significant reduction in its maximum standardized uptake value (SUV) from 9.4 to 3.6 [Figure 1]. Additionally, a right axillary nodal mass biopsy was performed, and immunohistochemistry was positive for CD1a, S100, and langerin, all of which are consistent with LCH [Figure 2]. As the patient did not show a meaningful response to cytarabine therapy and the LCH 2009 protocol, the new pediatric-inspired CLAG protocol was initiated as third-line salvage therapy. The patient received the first and second cycles of the CLAG protocol including cladribine at a dose of 5 mg/m2 along with 500 mg/m2 of cytarabine [6]. Follow-up PET/CT showed findings suggestive of a good partial response [Figure 3A]. After receiving a third cycle of the CLAG protocol, the patient attained complete remission. The maintenance phase was initiated as described in Table 2. The patient was not able to tolerate this phase for long due to frequent episodes of febrile neutropenia and hepatotoxicity. Follow-up PET/ CT at the end of therapy showed findings suggestive of a complete metabolic response [Figure 3B]. Follow-up PET in September 2018 revealed complete metabolic remission. A follow-up CT scan in March 2020 still indicated complete metabolic remission [Figures 3C, 3D].
Figure 1: Case 1: Multiple lytic lesions involving the left pelvic bone, bilateral rotator cuff muscles, and left ischium. The left ischial lesion demonstrates a significant reduction in SUVmax from 9.4 to 3.6.
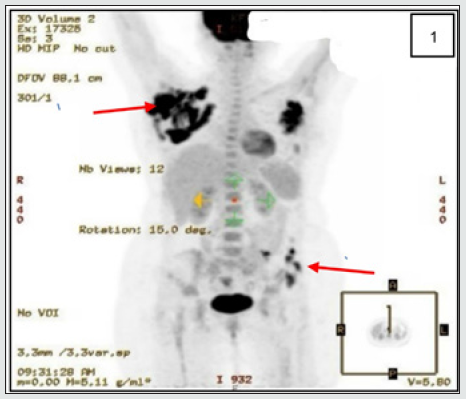
Figure 2: Case 1: Results from the histopathological examination. (A): Neoplastic tissue shows infiltrative polygonal cells with bright, abundant eosinophilic cytoplasm mixed with multiple eosinophils, lymphocytes, and neutrophils (×20). (B): Target cells containing longitudinal grooves with well-formed multinucleated giant cells (×40). (C): Immunohistochemistry shows diffuse positive staining for CD1a (×20) and diffuse positive staining for S100 protein (D, ×20).
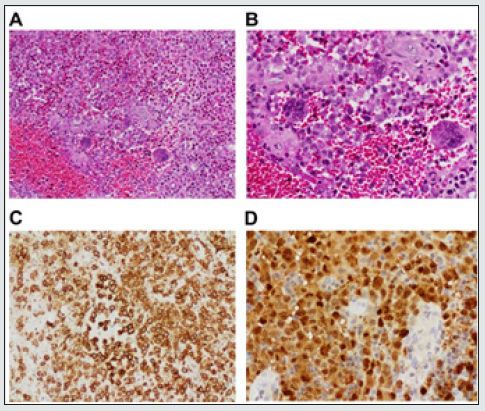
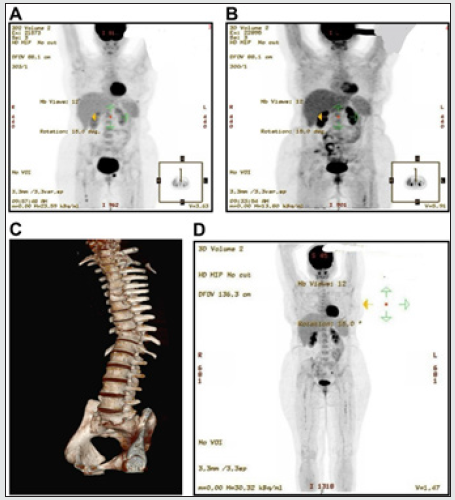
Figure 3: (A): Appearances are suggestive of a particularly good partial response to treatment in Case 1. (B): Scan appearances suggestive of a complete metabolic response. (C): Findings are consistent with no signs of disease relapse.
Case 2
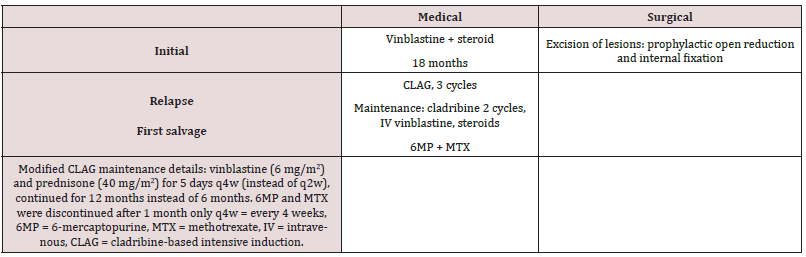
Case 2 was a 39-year-old male patient who presented with bone pain and difficulties in walking for 2 years prior to presentation. On radiological imaging, we found a pathological fracture of T10 vertebra and osteolytic lesions in the thoracolumbar vertebrae involving T5 to L5. Tissue biopsy confirmed the lesion to be LCH. He initially received vinblastine and steroids at the referring hospital for approximately 1.5 years. The patient’s characteristics at presentation and diagnosis are described in Tables 2, 3. He was refractory to initial treatment and was referred to us for further evaluation and management in 2015. We performed a spinal MRI which revealed multilevel lytic vertebral and bone lesions with complete loss of the T10 vertebra, as well as wedge fractures and vertebral body fusions at T5, T6, T10, and T11 vertebrae without of features suggestive of significant spinal canal stenosis or cord compression [Figures 4A, 4B and 4C]. A CT scan also revealed mandibular lesions. Histopathological examination of tissue biopsy specimens revealed Actinomyces infection in the soft tissue of the anterior mandible, along with LCH [Figures 5A-D]. BRAF mutation testing was negative. The patient was evaluated by the infectious disease team and was started on a prolonged course of oral and intravenous antibiotics. An urgent PET/CT revealed diffuse fluorodeoxyglucose (FDG)-avid bone involvement [Figure 6A]. Therefore, in March 2017, the patient was treated with the pediatricinspired CLAG protocol as first line salvage chemotherapy. Both the first and second cycles of the induction phase were complicated by mild transaminitis and culture-negative febrile neutropenia. Following antibiotic treatment, the patient underwent a PET/ CT scan, which demonstrated a favorable complete response to treatment [Figure 6B]. The patient was admitted for the third cycle of the CLAG protocol, which was complicated by febrile neutropenia secondary to bacteremia. Eventually, the patient was started on the maintenance phase of the protocol. During maintenance, he developed disseminated herpes zoster infection. Per patient preference, the 6 MP and MTX treatments were discontinued after 1 month, vinblastine and steroids was continued. Subsequently, a modified maintenance regimen was initiated, lasting for a total of 12 months [Table 3]. The last PET/CT scan was conducted in September 2020, and this showed no evidence of FDG-avid bone disease [Figure 6C].
Figure 4: (A): Pathological fracture of the T10 vertebra and osteolytic lesions in thoracolumbar vertebrae T5–L5 in the Case 2 patient. (B): Multilevel vertebral body lytic and sclerotic bony lesions with complete loss of the T10 vertebral body without evidence of significant canal stenosis or cord compression (C).
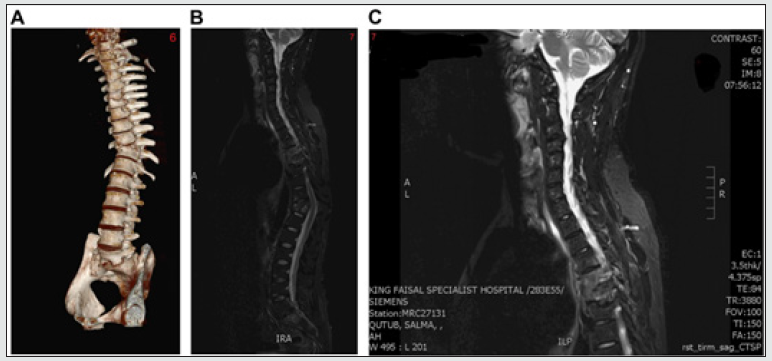
Figure 5: Case 2: Results from the histopathological examination. (A): Neoplastic tissue shows infiltrative polygonal cells with eosinophilic cytoplasm mixed with lymphocytes and neutrophils (×20). (B): Target cells contain oval nuclei with longitudinal grooves that resemble coffee beans (yellow arrow) (×40). (C): Immunohistochemistry showing diffuse positive staining for CD1a (×20) and diffuse positive staining for S100 (D, 20x).
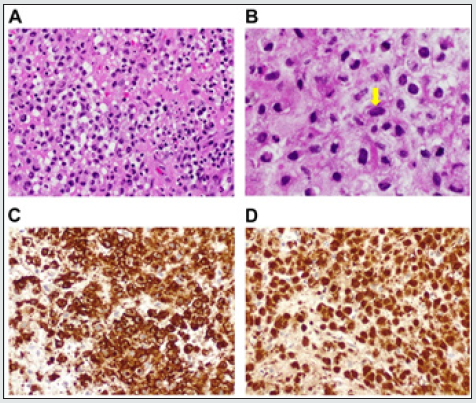
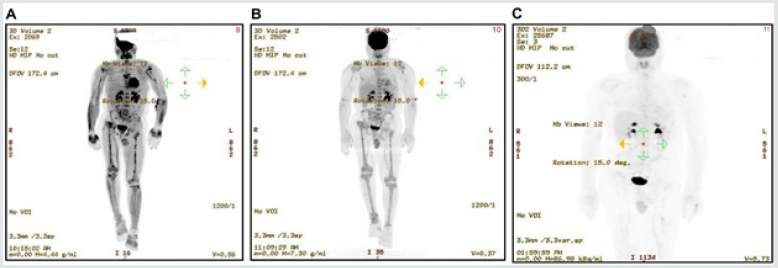
Figure 6: (A): Diffuse FDG-avid bony disease involvement, indicating active disease in Case 2. (B): Results indicating a favorable response to treatment. (C): No evidence of FDG-avid lesions in keeping with disease remission.
Case 3
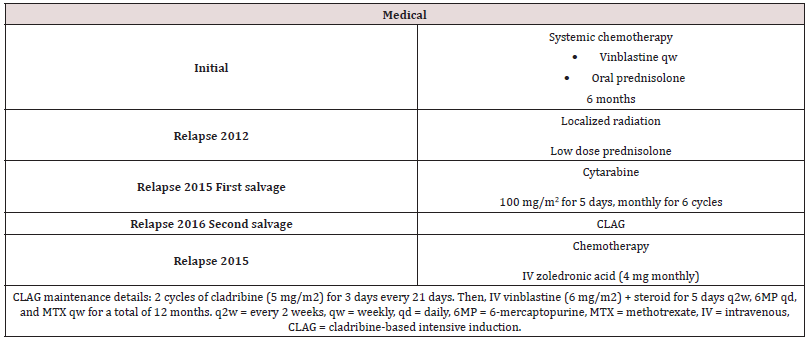
Case 3 was a 21-year-old female patient who presented in May 2011 with complains of increasing maxillary pain and night sweats associated with g weight loss of approximately 6 kg within the previous four months. A skeletal survey showed multiple lytic lesions involving the skull and the right femur. CT scans of the chest, abdomen, and pelvis were normal, but a maxillofacial CT performed on the 28th of March 2011 revealed multifocal osteolytic lesions within the facial bones. LCH was confirmed based on histological examination findings of biopsy specimen taken from the left maxillary sinus and the angle of the left mandible on the 17th of April 2011. The biopsy specimen was also positive for CD68, CD1a, and S100 protein, and showed a focal area with marked fibrosis and chronic inflammation that was suggestive of LCH. A brain MRI conducted on the 11th of May 2011 showed temporal bone lesions suggestive of histiocytosis with no evidence of brain parenchymal involvement. The patient was diagnosed with LCH in 2011, and systemic induction therapy was started as described in Table 4. Following induction therapy, the patient reported complete pain relief and her overall clinical condition was satisfactory. Radiology confirmed improvement of the lesions in the right temporal and left maxillary bones. At a follow-up visit in 2012, she complained of right leg pain. A nuclear study confirmed recurrence of the disease in the right femur, which was treated with local radiotherapy along with low doses of prednisolone. The patient remained well until 2015 when she started to complain of recurrent jaw pain and was found to have a new lytic lesion in the temporomandibular joint and right femur. Following a CT scan, the first line of salvage therapy was started as described in Table 4. The patient suffered an LCH relapse that was confirmed by tissue biopsy [Figures 7A, 7B, 7C, and 7D] in 2016. Due to the refractory nature and disseminated involvement of her disease [mandibular alveolar ridge lesion and two ribs] [Figure 8A], she was not considered a candidate for radiotherapy and the CLAG protocol was initiated as second line salvage therapy [Table 4]. A PET-CT was conducted after the completion of the second cycle, which confirmed a complete response [Figure 8B]. The patient continued maintenance chemotherapy as per the CLAG protocol for 12 months. During the follow-up PET/CT scan in 2018, the patient showed possible new lytic lesions involving the left iliac region and the right acetabulum [Figure 8C]. The patient was clinically asymptomatic and was heavily pretreated with chemotherapy and zoledronic acid [Table 4]. The tumor board decided to do a followup PET scan. Fortunately, a repeated PET/CT in September 2019 and another done after 6 months showed no evidence of residual disease [Figure 8D].
Figure 7: Case 3: Results from the histopathological examination. (A): Infiltrative polygonal cells with abundant eosinophilic cytoplasm mixed with eosinophils, lymphocytes, and neutrophils (×20). (B): Immunohistochemistry showing diffuse positive staining for CD1a (×20), diffuse positive staining for S100 protein (C, ×20), and CD68 highlights adjacent reactive histiocytic cells (D, ×20).
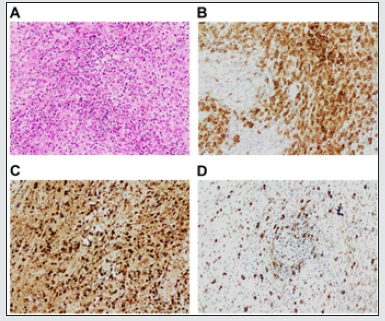
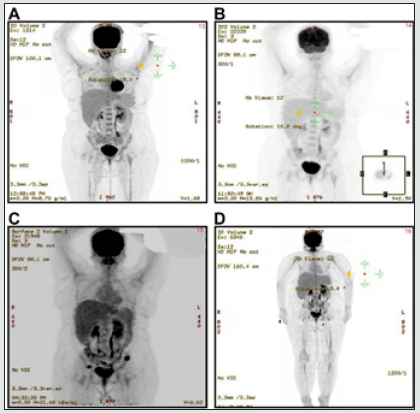
Figure 8: (A): Focal uptake (SUVmax 1.3) in the right anterior third and left anterior eight ribs of the patient in Case 3. (B): No evidence of FDG uptake suggestive of a complete response. (C): New lytic lesions involving the left iliac region and the right acetabulum. (D): No evidence of FDG-avid lesions, in keeping with disease remission.
Discussion
To the best of our knowledge, this is the largest reported case series of refractory LCH in adult patients that was treated with a modified CLAG regimen after failure of at least one line of standard chemotherapy. The patients in this series were from Saudi Arabia. The age at diagnosis ranged from 21 to 39 years. Two patients had multisystem LCH involving the musculoskeletal system with CNS involvement, and all patients had refractory disease or experienced relapse after standard induction chemotherapy. Our patients had no lesions in “risk organs” including the bone marrow, spleen, or liver. The time between the initial diagnosis and commencement of the CLAG protocol was at least 3 years in Cases 2 and 3 and more than 10 years in Case 1. In all cases, the patients experienced febrile neutropenia with septicemia and prolonged pancytopenia (grades 3-4 as per the World Health Organization grading) after administration of the CLAG protocol and required supportive care with transfusion of blood product along with granulocyte-colony stimulating factor, and prolonged hospitalization. None of the patients required admission into the intensive care unit. Nevertheless, our patients achieved complete remission based on their clinical and radiological response. The use of cladribine as a single agent has been reported since the 1990s. It has been successfully used in many patients and is considered the standard therapy for patients with multifocal bone disease [7,8]. A handful of studies have reported the use of high dose cladribine and cytarabine in pediatric patients. Rosso et al presented a retrospective study of nine pediatric patients, of which seven achieved complete remission after treatment [9]. Pant et al reported on a single-case study of an 8-month-old infant who had relapsed while receiving vinblastine and prednisolone but similarly had responded well to the CLAG protocol [10].Side effects, such as febrile neutropenia, anemia, and thrombocytopenia have also been documented in these studies and should also be anticipated after using the CLAG protocol in adults [9-12]. Severe toxicity should be taken seriously to avoid premature discontinuation of the protocol, and it is recommended that intense chemotherapy should only be administered in centers that are experienced in the management of severe toxicity, capable of providing adequate supportive care, and are trained in providing AML chemotherapy [12]. Many pediatric trials have shown that using cladribine as a monotherapy is effective for low-risk recurrent disease, and as per the current Histiocyte Society LCH IV protocol, it is used as second- or third-line salvage therapy [13-15]. treatment outcomes were also particularly unfavorable in patients who had risk-organ involvement based on the LCH-S-98 protocol for refractory cases [16-20]. The CLAG protocol used in this study was first presented in an international Phase II pediatric study published in 2015.6 The study showed promising results for refractory patients with an overall response rate of 92% and an overall survival rate of 85% [6-21]. Here we report a case series of three adult patients with refractory multisystem LCH who were treated with the pediatric-inspired intensive CLAG protocol. However, in some patients, changes to the protocol, including dose modification, were required. In Case 1, the maintenance therapy was stopped at 6 months instead of at 12 months because of repeated hospitalization for recurrent infection and prolonged cytopenia. In Case 2, a significant change to the protocol was made during the maintenance phase because of the side effects of MTX and 6MP, both of which were discontinued. Treatment with vinblastine and a steroid continued for 12 months instead of 6 months. Case 3 received a full course of treatment as per the CLAG protocol. These changes further illustrate the toxicity issues arising from this type of intense chemotherapy and the importance of establishing adequate dose modification and toxicity management guidelines. While it would be beneficial for both patients and clinical staff to avoid treatments with widespread toxicity, the options for patients with refractory multisystem LCH are extremely limited. For 30 years, several different therapies for multisystem LCH have been tested, ranging from light to intensive chemotherapy resulting in only minor advances [22]. The predominant treatment strategy includes prednisone as a single agent or in combination with vinblastine. The LCH-III trial showed that prolonging therapy with vinblastine and prednisone in patients with multisystem LCH for at least 12 months was effective and associated with improvement in the survival rate of up to 84% and a decrease in the 5-year relapse rate to 27% [23]. The LCH-I randomized study was performed in patients with multisystem LCH without risk-organ involvement who were treated with either vinblastine or etoposide. While this combination proved efficacious, vinblastine and prednisolone was preferred because of the increased risk of secondary leukemia associated with using etoposide [24]. As an alternative, Abla et al. suggested the use of cytarabine [100-170 mg/m2 daily over 3–5 days every 3–4 weeks] as a single agent salvage chemotherapy [25]. A number of studies found that switching to salvage therapy early in patients with a slow response to initial induction therapy and providing more efficient salvage regimens resulted in better outcomes [22-27]. Although most patients will attain a complete response, approximately 50% of patients will either be unresponsive to treatment or relapse. Patients without risk-organ involvement tend to have favorable outcomes, whereas those with multisystemic disease and risk-organ involvement tend to be more difficult to treat and require intensified treatment that still results in poor outcomes [28]. For the cases presented here, the initial induction therapy was vinblastine and a steroid, with the duration ranging from 6 months in two patients [Case 1 and 3] to 18 months in one patient [Case 2]. Cladribine is a deoxyadenosine nucleoside analog that is converted to its triphosphate moiety following intracellular phosphorylation. The triphosphate moieties can be incorporated into both DNA and RNA during synthesis, and this results in the inhibition of enzymes, such as ribonucleotide reductase and DNA polymerase, ultimately resulting in the inhibition of proliferation and promotion of apoptosis [29-31]. In combination with cytarabine, the activating effect of cladribine on deoxycytidine kinase results in increased phosphorylation, activation, and accumulation of cytarabine [32]. Other nucleoside analogs, such as clofarabine, have also been assessed for their efficacy in the treatment of refractory pediatric LCH with results that are comparable to those of cladribine [33,34]. A Phase II clinical trial for the evaluation of clofarabine as a treatment for adult patients with recurrent or refractory LCH is currently recruiting subjects in the United States and in Canada [35]. In the last decade, our understanding of the histopathology of LCH has improved, and after the discovery of somatic mutations of the BRAF V600E gene in 65% of LCH cases, analysis of this gene has become part of the standard care. Although BRAF assessment is not yet included as a part of risk stratification, almost 50% of LCH patients with BRAF mutation become refractory or experience relapse to initial treatment and experience more aggressive disease, with a majority relapsing within the first 2 years after diagnosis [26,27]. Unfortunately, the BRAF V600E gene was only analyzed in Case 2, who was negative for mutations. While BRAF inhibitors, such as vemurafenib have been examined as a treatment option for LCH, the high rates of resistance and potential risk of cutaneous and pancreatic cancers seen in patients with BRAF-V600E-mutated melanoma following monotherapy with a BRAF inhibitor are a cause of concern [36]. Combined approaches with BRAF and MEK inhibitors have been evaluated in sporadic studies,12 and based on the results presented here, assessment of a combination of cladribine or CLAG and a BRAF inhibitor could be another useful avenue of investigation.
Conclusion
Despite some limitations in the treatment protocol and number of patients, we found that an AML-type CLAG-based intensive regimen can be effective and generally safe in adult patients with refractory or relapsed LCH, especially if introduced early, and resulted in complete metabolic remission in all our patients based on follow-up PET/CT examinations. Caution is warranted as this type of intensive chemotherapy is associated with recurrent febrile neutropenia, hepatotoxicity, and other infectious complications as shown in our patients. Our study indicates that the combination of high dose cytarabine and cladribine could be an effective salvage therapy in the treatment of adult patients with relapsed or refractory LCH and warrants continued examination of the CLAG protocol in expanded cohorts of adult patients including those with high-risk lesions.
Acknowledgment
We would like to acknowledge Dr. Haneen Al-Maghrabi and Dr. Jaudah Al-Maghrabi for providing the histopathological specimens.
Disclosure
The author reports no conflicts of interest in this work.
Ethical Approval and Informed Consent
The institutional review board of our institution approved this study, and written informed consent was obtained from all the patients.
Consent for Publication
Written informed consent was obtained from the patients for the publication of their case details and accompanying images.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Authors’ contributions
All authors made a significant contribution to the work reported, whether that is in the conception, medical records review, data collection and entry, data analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
References
References
- Allen CE, Merad M, McClain KL (2018) Langerhans-cell histiocytosis. N Engl J Med 379(9): 856-868.
- El Demellawy D, Young JL, de Nanassy J, Chernetsova E, Nasr A (2015) Langerhans cell histiocytosis: a comprehensive review. Pathology 47(4): 294-301.
- Tella SH, Kommalapati A, Rech KL, Go RS, Goyal G (2019) Incidence, clinical features, and outcomes of Langerhans cell sarcoma in the United States. Clin Lymphoma Myeloma Leuk 19(7): 441-446.
- Grois N, Pötschger U, Prosch H (2006) Risk factors for diabetes insipidus in Langerhans cell histiocytosis. Pediatr Blood Cancer 46(2): 228-233.
- Egeler RM, Katewa S, Leenen PJ (2016) Langerhans cell histiocytosis is a neoplasm and consequently its recurrence is a relapse: in memory of Bob Arceci. Pediatr Blood Cancer 63(10): 1704-1712.
- Donadieu J, Bernard F, Van Noesel M (2015) Cladribine and cytarabine in refractory multisystem Langerhans cell histiocytosis: results of an international phase 2 study. Blood 126(12): 1415-1423.
- Gadner H, Minkov M, Grois N (2013) Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood 121(25): 5006-5014.
- Allen CE, Ladisch S, McClain KL (2015) How I treat Langerhans cell histiocytosis. Blood 126(1): 26-35.
- Rosso DA, Amaral D, Latella A, Chantada G, Braier JL (2016) Reduced doses of cladribine and cytarabine regimen was effective and well tolerated in patients with refractory-risk multisystem Langerhans cell histiocytosis. Br J Haematol 172(2): 287-290.
- Pant C, Madonia P, Bahna SL, Bass PF, Jeroudi M (2009) Langerhans cell histiocytosis, a case of Letterer Siwe disease. J La State Med Soc 161(4): 211-212.
- Apollonsky N, Lipton JM (2009) Treatment of refractory Langerhans cell histiocytosis (LCH) with a combination of 2-chlorodeoxyadenosine and cytosine arabinoside. J Pediatr Hematol Oncol 31(1): 53-56.
- Awada G, Seremet T, Fostier K, Everaert H, Neyns B (2018) Long-term disease control of Langerhans cell histiocytosis using combined BRAF and MEK inhibition. Blood Adv 2(16): 2156-2158.
- Adam Z, Szturz, P, Vaníček, J (2013) Cladribine (2-chlorodeoxyadenosine) in frontline chemotherapy for adult Langerhans cell histiocytosis: a single-center study of seven cases. Acta Oncol 52(5): 994-1001.
- Hu T, Liu R, Li J (2014) Cladribine for 13 cases refractory high-risk children Langerhans cell histiocytosis (Chinese). Zhonghua xue ye xue za zhi 35(11): 985-989.
- Mottl H, Starý J, Cháňová M, Nekolná M, Drahokoupilová E, et al. (2006) Treatment of recurrent Langerhans cell histiocytosis in children with 2-chlorodeoxyadenosine. Leuk Lymphoma 47(9): 1881-1884.
- Gadner H, Grois N, Arico M (2001) A randomized trial of treatment for multisystem Langerhans’ cell histiocytosis. J Pediatr 138(5): 728-734.
- Gadner H, Minkov M, Grois N (2013) Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood 121(25): 5006-5014.
- Simko SJ, McClain KL, Allen CE (2015) Up-front therapy for LCH: is it time to test an alternative to vinblastine/prednisone? Br J Haematol 169(2): 299-301.
- Abraham A, Alsultan A, Jeng M, Rodriguez Galindo C, Campbell PK (2013) Clofarabine salvage therapy for refractory high‐risk langerhans cell histiocytosis. Pediatr Blood Cancer 60(6): E19-E22.
- Krooks J, Minkov M, Weatherall AG (2018) Langerhans cell histiocytosis in children: Diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol 78(6): 1047-1056.
- Adam Z, Szturz P, Vaníček J (2013) Cladribine (2-chlorodeoxyadenosine) in frontline chemotherapy for adult Langerhans cell histiocytosis: a single-center study of seven cases. Acta Oncol 52(5): 994-1001.
- Kilpatrick SE, Wenger DE, Gilchrist GS, Shives TC, Wollan PC, et al. (1995) Langerhans’ cell histiocytosis (histiocytosis X) of bone a clinicopathologic analysis of 263 pediatric and adult cases. Cancer 76(12): 2471-2484.
- Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F (1997) Langerhans cell histiocytosis in adults. Med Pediatr Oncol 28(1): 9-14.
- Gardner H, Grois N, Arico M (2001) A randomized trial of treatment for multisystem Langerhans’ cell histiocytosis. J Pediatr 138(5): 728-734.
- Abla O, Rodriguez Galindo C, Veys P (2018) Treatment of relapsed and refractory Langerhans cell histiocytosis in children. In: Abla O, Janka G, (eds.) Histiocytic Disorders. Springer, New York: 119-137.
- Harmon CM, Brown N (2015) Langerhans cell histiocytosis: a clinicopathologic review and molecular pathogenetic update. Arch Pathol Lab Med 139(10): 1211-1214.
- Walker PD, Rosai J, Dorfman RF (1981) The osseous manifestations of sinus histiocytosis with massive lymphadenopathy. Am J Clin Pathol 75(2): 131-139.
- Héritier S, Emile JF, Barkaoui MA (2016) BRAF mutation correlates with high-risk Langerhans cell histiocytosis and increased resistance to first-line therapy. J Clin Oncol 34(25): 3023-3030.
- Hentosh P, Peffley DM (2010) The cladribine conundrum: deciphering the drug's mechanism of action. Expert Opin Drug Metab Toxicol 6(1): 75-81.
- Vasova I, Penka M, Hajek R, Mayer J, Krahulcova E (1997) A new purine analog in the treatment of hematologic malignancy I. Fludarabine. Vnitr Lek 43(1): 45-50.
- Zhenchuk A, Lotfi K, Juliusson G, Albertioni F (2009) Mechanisms of anti-cancer action and pharmacology of clofarabine. Biochem Pharmacol 78(11): 1351-1359.
- Gandhi V, Estey E, Keating MJ, Chucrallah A, Plunkett W (1996) Chlorodeoxyadenosine and arabinosylcytosine in patients with acute myelogenous leukemia: pharmacokinetic, pharmacodynamic, and molecular interactions. Blood 87(1): 256-264.
- Simko SJ, Tran HD, Jones J (2014) Clofarabine salvage therapy in refractory multifocal histiocytic disorders, including Langerhans cell histiocytosis, juvenile xanthogranuloma and Rosai-Dorfman disease. Pediatr Blood Cancer 61(3): 479-487.
- Allen CE, Ladisch S, McClain KL (2015) How I treat Langerhans cell histiocytosis. Blood 126(1): 26-35.
- gov. Study of clofarabine in patients with recurrent or refractory Langerhans cell histiocytosis and LCH-related disorders. 2019. Identifier: NCT02425904.
- Abla O, Weitzman S (2015) Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program 1: 565-570

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...