Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Clinical Case(ISSN: 2638-5945) 
Head Cancer and Metastasic Neck. What Have We Advanced in the Last Years? Volume 2 - Issue 1
Beatrz Losada Vila1*, David Gutiérrez Abad1, Beatriz Anton Pascual1, Maria Carmen Pantin1, Begoña Caballero Perea2 and Juan Antonio Guerra1
- 1Oncology Department, Universitary Hospital Fuenlabrada, Madrid, Spain
- 2Radiation Oncology, Universitary Hospital Fuenlabrada, Madrid, Spain
Received: May 26, 2018; Published: June 15, 2018
Corresponding author: Beatrz Losada Vila, Oncology Department. University Hospital Fuenlabrada, Madrid, Spain
DOI: 10.32474/OAJOM.2018.02.000129
Abstract
In 2012, 5.210 new cases of head and neck tumors were estimated in the USA, with an increasing incidence due to tobacco and alcohol habits in the population. A large percentage of the cases debut as a locally advanced disease, so control of the disease is key and we look for the best therapeutic strategy to achieve good survival rates while maintaining quality of life. We present the case of a 60-year-old patient in which our objectives to be presented are the assessment of comorbidity, toxicity and survival.
Clinical Case
A 60-year-old man without medical illnesses to be highlighted. He came to the emergency room in July 2016 due to injury at the cervical level of 1 month of evolution, with progressive growth and breathness, with also difficulty for eating. Also asthenia, anorexia and loss of weight not quantified in the last month.
Physical Examination: Weight 42 kg Head and Neck: mass of hard consistency of approximately 10 cm in diameter in the cervical left region that seems to deflect trachea. Pulmonary auscultation: generalized hypoventilation with some expiratory wheeze.
Additional Tests
a. Fibroscopy (August 2016) ulcerated lesion from right vallecula to mouth of Killian. Paresis of both vocal cords.
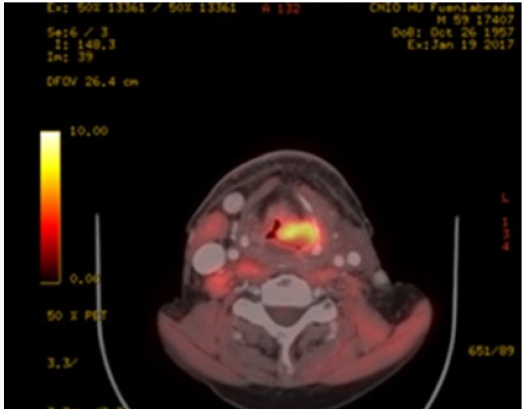
b. PET-CT (August 2016): Extensive tumor in pharynxlarynx- esophagus (> 6 cm) Lymph nodes in left IB-II spaces (> 6 cm). If confirmed, carcinoma would correspond to T3 N3 (stage IVB) (Figure 1).
c. Laryngeal Biopsy: Moderately differentiated and keratinizing squamous cell carcinoma.
So, confirmed Squamous cell carcinoma stage cT4N3 Mx.The case is presented in the Tumor Committee, deciding treatment with Chemoradiotherapy and 1 cycle of Docetaxel+ Cisplatin (60% dose reduction because of frailty/ malnutrition) previous to induction due to the large tumor volume and waiting for start radiotherapy. Tracheotomy is performed prior to starting because of the risk of airway obstruction and also gastrostomy is placed for nutritional support.
On 31th August 2016 the patient starts on RT concomitantly to Cisplain ( receiving 2 cycles on 12.09.2016 and 10.10.2016 and 70 Gy) During the treatment, he achieves a good general condition until January 2017, when he goes to Otolaryngology Clinics referring dysphagia again although he maintains weight in 51 kg. PET-CT is performed: disease progression with soft tissue increase in the pharyngoesophageal junction despite the good response of cervical adenopathies and the partial response of the primary tumor. New bilateral subpleural pulmonary nodules suggestive of metastasis. Figure 2 Due to progression, he restarts treatment with chemotherapy (palliative intention) with ERBITAX scheme (Paclitaxel 80 mg / m2 weekly (3 / 4s) + Cetuximab 400 mg / m2 followed by 250 mg / m2) with good tolerance,only highlighting secondary rash to cetuximab (predictive factor). After 3 cycles he presents significant partial response (Figure 3) and continues until 6 cycles. After 10 cycles it is considered whether to stop paclitaxel and follow on with cetuximab, but given the good tolerance they remain both of them. However, in January 2018, he presented a new pulmonary progression, so we decided to start a new strategy with inmunotherapy (Nivolumab), receiving 2 cycles to date.
Discussion
a. The concept of fragility is evaluated incorrectly through the Performance Status (PS). The ACE scale 27 assesses comorbidities and compares survival to having an advanced stage, so that is a fact to take into account more than the ECOG at the time of choosing the treatment.
b. When a patient progress to chemoradiotherapy, we have two good “palliative chemo” options: phase III EXTREME study(5-FU + Cisplatin + Cetuximab) or phase II of Hitt (Paclitaxel-Cetuximab) with fewer side effects, which is an alternative to cisplatin, achieving good response rates (20.43%) [1].
c. After 6 cycles of Paclitaxel + Cetuximab can be considered to keep Cetuximab as monotherapy, continue both or suspension until progression.
d. If pulmonary metastatic disease is controlled for> 1 year, we can consider surgical intervention.
e. Support treatment improves tolerance to chemotherapy. The indication of prophylactic enteral nutrition is a controversial issue, so we have to individualize.
f. We need predictive factors for each tumor type that we can know about before hand the prognosis and guide the treatment according to it. We must raise multidisciplinary strategies with the aim of achieving the best treatment sequence to improve survival in a population with few therapeutic options [2].
References
- Hitt R, Irigoyen A, Cortes Funes H (2012) Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamou. Ann Oncol 23(4): 1016-1022.
- Rivera F, García Castaño A, Vega N (2009) Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther 9(10): 1421-1428.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...