Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Review Article(ISSN: 2638-5945) 
Epidemiology of malignant lymphomas in Italy Volume 5 - Issue 2
Pier Paolo Piccaluga1-4, Chiara Cascianelli1-2, Clara Beruzzi2-5, Stefano Ascani6, Giulio Fraternali-Orcioni7, Stefano Lazzi8, Lorenzo Leoncini8, Giuseppe Visani9.
- 1Biobank of Research and Institute of Hematology and Medical Oncology “L. and A. Seràgnoli”, IRCCS S. Orsola-Malpighi Academic Hospital
- 2Department of Experimental, Diagnostic, and Specialty Medicine Bologna University Medical School; Bologna, Italy.
- 3Euro-Mediterranean Institute of Science and Technology (IEMEST); Palermo, Italy
- 4Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya
- 5Hematopathology Unit, IRCCS S. Orsola-Malpighi Academic Hospital, Bologna, Italy.
- 6Pathology Unit, S. Maria Hospital and University of Perugia, Terni, Italy.
- 7Pathology Unit, Santa Croce e Carle Hospital, Cuneo, Italy.
- 8Section of Pathology, Department of Medical Biotechnology, University of Siena, Italy
- 9Hematology & Transplant Center, AORMN, Pesaro, Italy
Received: September 5, 2022 Published: September 28, 2022
Corresponding author: Pier Paolo Piccaluga, Biobank of Research, IRCCS S. Orsola-Malpighi Academic Hospital, Institute of Hematology and Medical Oncology “L. and A. Seràgnoli”, Department of Experimental, Diagnostic, and Specialty Medicine Bologna University Medical School, Bologna, Italy, Via Massarenti, 9 - 40138 Bologna, Italy
Disclosure: The Authors have no conflicting financial interests to declare.
Sources of support: This work was supported by Ail Pesaro Onlus (Dr. Visani), BolognAIL, RFO (Prof. Piccaluga), AIRC (Prof. Piccaluga IG 2013 N.14355), FIRB Futura 2011 RBFR12D1CB (Prof. Piccaluga).
DOI: 10.32474/OAJOM.2021.05.000209
Abstract
Malignant lymphomas are the commonest hematological cancers, accounting for about 50% of them. Despite being diagnosed worldwide, differences have been reported in terms of absolute incidence in different geographical areas, being more frequently seen in Europe and Northern America rather than Asia and Africa. Even more evident is the different distribution of lymphoma types, based on the presence of different causative agents (e.g., HTLV1), postulated genetic backgrounds (eg. EBV-associated T-cell neoplasms), or unknown reasons. In this study, we aimed to assess, for the first time, the specific incidence of lymphoma types in Italy. To this aim, we included in the analysis 2,952 cases, collected in different regions, covering North, Center, and South of Italy, over a three-year period. We found that diffuse large B-cell lymphomas and follicular lymphomas are the commonest types, while T-cell lymphomas are overall rare. Interestingly, chronic lymphocytic leukemia/small lymphocytic lymphoma was diagnosed more often in the South. Differently from current literature, we found angioimmunoblastic lymphoma to be commoner in women, while anaplastic large cell lymphoma was recorded more frequently in men. Our findings were then discussed in the light of other available regional reports from Asia, Africa, Europe, and Americas. In conclusion, we provided for the first time to the best of our knowledge a description of relative incidence of lymphoma types in Italy, posing the rational basis for ad hoc analyses on risk factors and possible clinical strategies.
Peroxisome Proliferator-Activated Receptor Gamma
Malignant lymphomas represent approximately 4-6% of new cancers each year worldwide. However, their incidence is quite variable across the Globe (Figure 1). In fact, they are more common in Western countries, especially USA and Europe. A survey run by the Surveillance Epidemiology and End Results (SEER) program indicates an incidence rate of 19.6 cases of non-Hodgkin lymphomas (NHL) and 2.6 cases of Hodgkin lymphoma (HL) per 100,000 persons per year, with a mortality rate of 5.3 and 0.3 per 100,000 persons per year, respectively, in United States [1]. Overall, the lifetime risk of developing these diseases is approximately 2.1% for NHL and 0.2% for HL, based on 2016-2018 data [1]. By contrast, the European Cancer Observatory (ECO), a project developed by the International Agency for Research on Cancer (IARC), estimates an incidence in Europe of 16.4 NHL and 2.7 HL cases/100,000/year in 2020 [2]. As far as Italy is concerned, 22.6 new NHL cases/100,000/year (corresponding to 14,032 new cases in 2020) and 3.4 HL cases/100,000/year (2,120 new cases in 2020) were diagnosed [2]. Overall, lymphomas constituted about 4% of all cancers in Italy. When all the other hematologic malignancies are considered (therefore also including multiple myeloma, leukemias, myelodysplastic syndromes and chronic myeloproliferative neoplasms), the whole group of hemato-lymphoid tumors constituted 8.16% of all cancers, with 31,256 new diagnoses in 2020 [2]. The lowest incidence of lymphomas worldwide is described in most Asian and Africa countries [3] (Figure 1). Detailed data on lymphoma incidence in USA, Europe and Italy are presented in Table 1 Of interest, differently from what observed in USA and northern Europe, where the incidence of NHL has progressively raised in the last two decades (HL remaining stable), the incidence of both HL and NHL did not significantly vary over time in Italy (Figure 2A-C). Beside general estimation on NHL and HL, more detailed reports on NHL subtypes in single geographical areas are definitely scant (4,5). Particularly, no systematic descriptive analysis has considered incidence of lymphoid neoplasm subtypes as defined by the WHO classification in Italy. Bearing this in mind, in the present study we aimed to assess the relative incidence of lymphoma subtypes as defined by the 2017 WHO classification in Italy.
Figure 1: A) Distribution of non-Hodgkin lymphoma cases worldwide. Source Global Cancer Observatory (IARC); LAC= Latin America and Caribbean. Adapted from IARC, Cancer Totay. B) Distribution of Hodgkinlymphoma cases worldwide. Source Global Cancer Observatory (IARC); LAC= Latin America and Caribbean. Adapted from IARC, Cancer Totay.
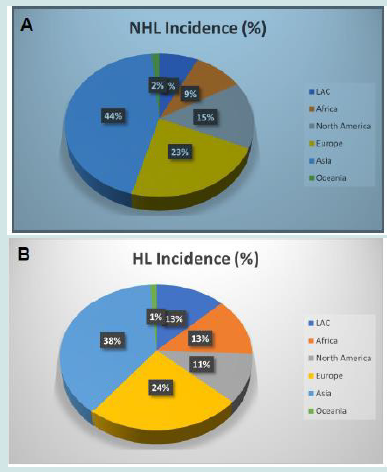
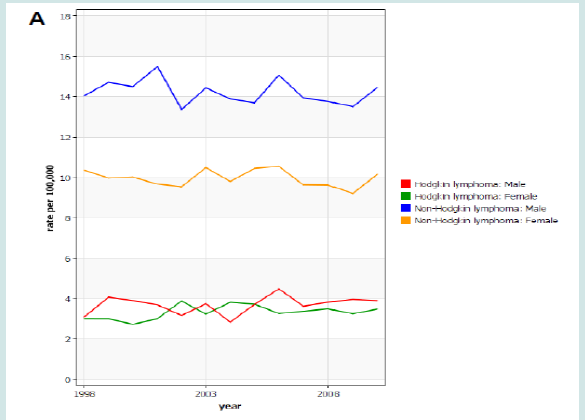
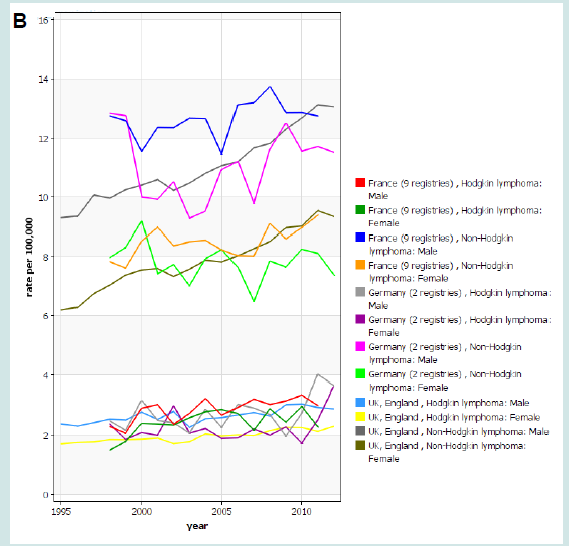
Figure 4: Age standardized incidence rate of malignant lymphomas in Italy between 1998 and 2010 in Italy (A), Europe (data from Germany, France and UK)(B), and USA (SEER, 9 registries) (C). Source Global Cancer Observatory (IARC).

Patients and Methods
Case Selection
We included in our analysis 2,952 consecutive cases for which pathological material (biopsies taken from bone marrow, lymphnode or other tissues) was referred to the Hematopathology Unit of the IRCCS S. Orsola- Malpighi Academic Hospital for diagnosis within a 36 months period of time. Cases referred for second opinion were excluded in order to avoid the selection bias favoring more difficult diagnoses (e.g., rarer subtypes). Similarly, relapses and staging samples (i.e. consecutive material for the same patient) were excluded. Non-lymphoma diagnoses were then excluded. Furthermore, in order to ensure a balanced ratio among cases from northern, central and southern Italy, cases from the three areas were included, roughly covering similar population amounts based on the population sizes of those areas. Finally, the availability of the basic demographic information including at least age, gender, and site of origin was requested for enrollment.
All cases were centrally diagnosed, all tissue samples being studied by histological examination by at least two well-experienced hematopathologists. The diagnoses were established according to the WHO classification.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics 20.0 and Prism (GraphPad software, USA). ANOVA and unpaired T-tests were used for continuous variables examination. When a sample size was less than 10 cases in at least 1 group a nonparametric (Mann-Whitney) test was used. Chi-square (or the non-parametric Fisher exact test when indicated) was used for dichotomic variables analyses [6,7].
Results
Lymphoma Incidence Rates Varies by Gender and Age
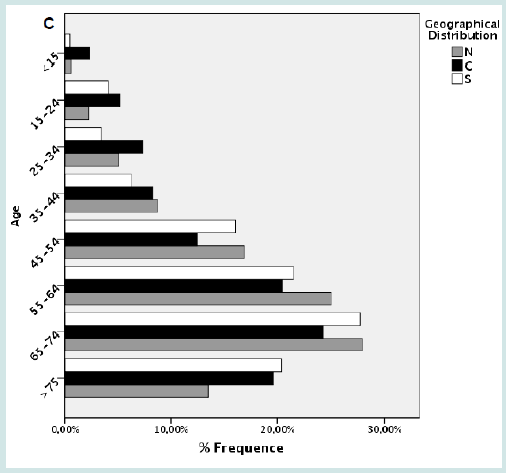
We analyzed 2,952 lymphoma cases diagnosed over a period of 36 months, corresponding to a mean of 983 cases per year and therefore to roughly 7% of all national cases. No significant differences were recorded in terms of number of cases during the study period. Despite the selection of Hospitals enrolling comparable populations in the three geographical areas, we observed a moderate imbalance among the different areas. Particularly, 40.52% of cases were from southern Italy, 38.82% from central, and only 20,66% from the north (Figure 3A). The mean age at diagnosis was 59.6 (range 5-90). Lymphomas were equally distributed in males and females (1,478 vs. 1,474 cases), with progressive increase of incidence by age (Figure 3B). The same trend was observed in the three geographical areas (Figure 3C).
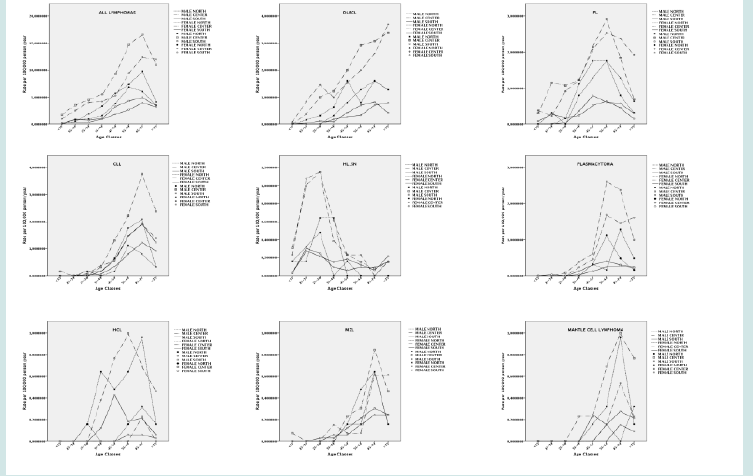
Figure 6: Age standardized incidence rate of malignant lymphomas in Italy between 1998 and 2010 in Italy (A), Europe (data from Germany, France and UK)(B), and USA (SEER, 9 registries) (C). Source Global Cancer Observatory (IARC).

Figure 7: A) Distribution of cases by geographical areas. B) Frequency of lymphomas by age and gender. C) Frequency of lymphomas by age and gender by geographical areas.
Incidence of Lymphomas by Subtype
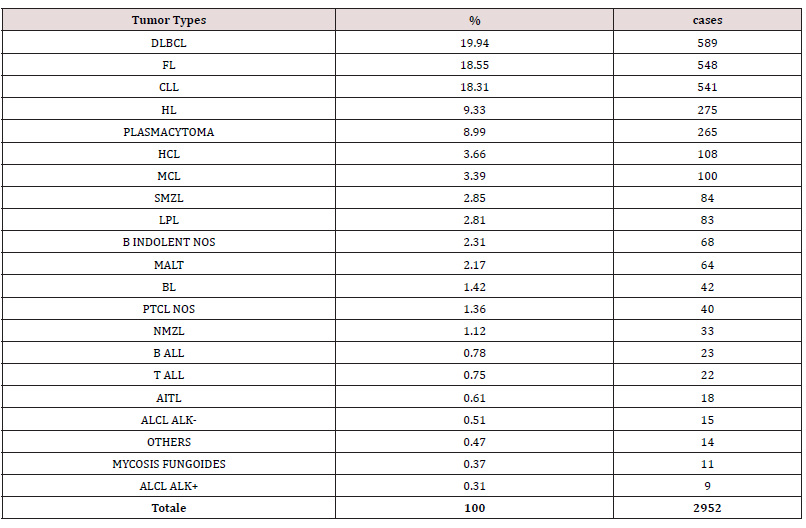
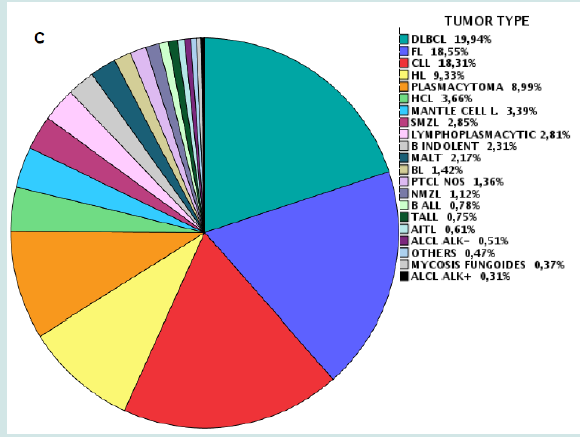
NHL represented 90,67% of cases, while 9.33% were HL (Figure 4A). As expected, B-cell malignancies were the commonest in all age groups, representing 96.09% of all cases (Figure 4B). Overall, the most frequent lymphoma was diffuse large B-cell lymphoma (DLBCL), representing 19.94% of cases, followed by follicular lymphoma (FL, 18.55%), chronic lymphocytic leukemia (CLL, 18.31%), and plasmacytoma (8.99%) (Figure 4C & Table 2). Males and females had overall a similar incidence of lymphoma (M/F ratio being about 1). However, some differences were observed across specific subtypes. Among B-cell neoplasm, male predominance was particularly increased for hairy cell leukemia (HCL), with a male/female (M/F) ratio of 5.35, mantle cell lymphoma (2.47) and lymphoblastic lymphoma (2.1). Classical Hodgkin lymphomas (cHL) were slightly more frequent in females (M/F=0.78), while the lymphocyte predominant type was not (M/F=1.47). Among T-cell neoplasm, the M/F ratio was increased in ALK- anaplastic large cell lymphoma (ALCL, 2.96) and peripheral T-cell lymphoma not otherwise specified (PTCL/NOS, 1.73), while angioimmunoblastic T-cell Lymphoma was more frequent in women (M/F=0.59).
Figure 8: A) Distribution of cases by geographical areas. B) Frequency of lymphomas by age and gender. C) Frequency of lymphomas by age and gender by geographical areas.
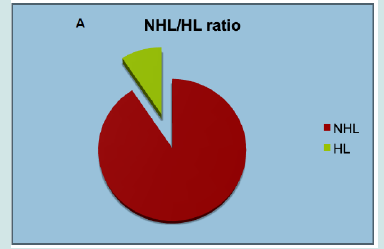
Figure 9: A) Distribution of cases by geographical areas. B) Frequency of lymphomas by age and gender. C) Frequency of lymphomas by age and gender by geographical areas.

Figure 10: A) Incidence of B-cell and T-cell lymphomas by age group. B) Relative frequency of HL and NHL. C) Relative frequency of lymphoma subtypes.
No Significant Differences Were Recorded Regarding Other Entities
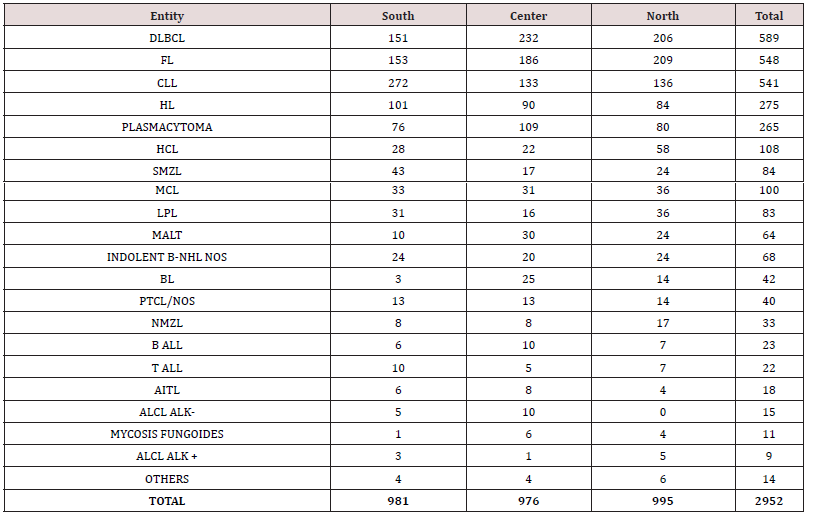
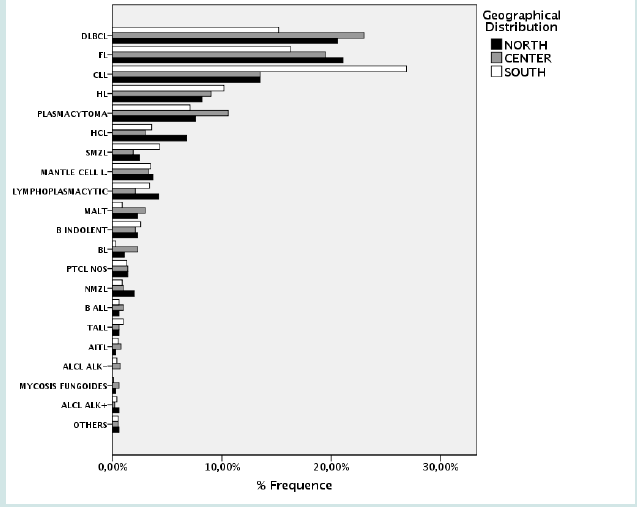
As mentioned, the incidence of lymphoma was overall higher in elderly; however, as expected, this trend was not observed for some entities. Specifically, Burkitt lymphoma (BL), HL and both B-cell and T-cell lymphoblastic lymphomas were almost exclusively diagnosed in children, while other lymphoid neoplasms like MCL, marginal zone lymphoma (MZL) and CLL were quite rare in younger patients (Figure 5). As far as the geographical distribution was concerned, although for most subtypes no major differences were observed, some entities showed an apparently different distribution (chi square= 144.546; p<0.001 – calculated for entities reaching at least 20 cases in each group). Specifically, FL, DLBCL, and MALT lymphomas were more frequently encountered in Northern and Central Italy, while CLL and splenic MZL were commoner in the South (Table 3; Figures 6 & 7). Furthermore, plasmacytomas were commoner in Central Italy, while LPL was rarer in this part of the country. Finally, HCL was more often observed in the North, cHL was only slightly more frequent in the South, and BL was absolutely infrequent in this same area (Table 3, Figures 6 & 7).
Figure 15: Relative frequency lymphoma subtypes in Northern (A), Central (B), and Southern (C) Italy.

Discussion
This is the first study addressing the epidemiology of malignant lymphomas in Italy, as classified by WHO classification, to the best of our knowledge. First, we showed that Italian patients are like other European as well as to Americans in terms of age at onset and gender. Specifically, a progressive increase in lymphoma incidence was noted according to age, most cases being diagnosed in adults and elderly. However, as expected, HL and LBL were indeed more common in younger patients. By contrast, in Africa several reports indicated a younger age at presentation for different lymphoproliferative disorders, including entities usually rare in young adults, such as CLL [8,9] Consistent with the WHO classification, we found a striking male predominance in HCL cases and, at lower extent, in MCLs [3-11]. Conversely, cHL was more frequent in women, as already described [12]. Of interest, AITL was also found to be slightly more common in female, differently from what reported so far in other Western Countries and Asia [13,14]. Furthermore, a significantly older age at diagnosis was recorded in female AITL patients (69 years vs. 60.87 years in males) [13,14]. In addition, we observed that ALCL ALK- was almost three time more frequent in men than in women, this being in contrast with most recent literature data on case collected in western counties [15]. Nonetheless, the mean age of presentation was in line with literature data, being significantly higher than what recorded for ALK+ cases (57.73 vs. 49.77 years) [14,15]. Concerning ALCL, no breast implant associated cases were diagnosed in the considered time frame.
Cases were collected at reference centers across the Country, including Northern, Central and Southern Italy, to ensure an adequate picture. Indeed, this is not trivial, since the climatic, environmental as well as nutritional difference might affect incidence of the diseases. In our analysis, we observed a difference in the relative frequency of some common entities. While DLBCL and FL were the commonest entities in Northern and Central Italy, CLL was largely the commonest in the Southern regions. This might of course reflect a real higher incidence of the disease or, alternatively, may be the consequence of a different approach to CLL diagnosis at different centers. In fact, CLL is more commonly diagnosed by hematologists based on peripheral blood immunophenotyping at most Italian centers. However, our data may indicate that CLL diagnosis is often achieved by bone marrow/lymph node histological examination in many Southern Italy hospitals. It should be noted, nonetheless, that the European cancer registry-based project on hematological malignancies (HAEMACARE), including data from all European countries, reported CLL as the most common lymphoid neoplasm, followed by DLBCL and FL [4]. Again, this data may reflect a more comprehensive analysis of diagnostic tools, including flow cytometric analysis of peripheral blood to identify patients affected by CLL.
According to the latest WHO classification, the most common NHL in Western countries is DLBCL, representing about 31% of cases in adults, followed by FL (22%), MZL (8%), MCL and CLL/ SLL (6% each), BL (2%), and lymphoplasmacytic lymphoma (LPL) (1%) (16–18)T-cell derived lymphomas account for only 10–15% of all NHL, PTCL/NOS, angioimmunoblastic T-cell lymphoma (AITL) and cutaneous forms being the commonest [19,20]. However, several works aiming to define the epidemiology of lymphomas in selected geographical areas have been produced in the past decade, challenged this scenario, indicating peculiar patterns. In general, a lower incidence of lymphoid malignancies was observed in Asian populations, particularly concerning FL CLL, and HL. On the other hand, a higher incidence of marginal zone lymphoma (MZL) and extra-nodal NK/T-cell lymphoma (ENKTL), nasal type was documented [21-23]. The Korea Central Cancer Registry (KCCR) reported that lymphoid malignancies represented about 3% of all cancers, with increased incidence between 1999 and 2012, DLBCL, MZL and peripheral T-cell lymphomas not otherwise specified being the commonest entities [24]. A large Japanese study showed that the incidence of FL and methotrexate-associated lymphoproliferative disorders increased during the analyzed time frame (2007- 2014) [25] Interestingly, the authors observed that the onset age appeared increased for both FL and DLBCL over time [25]. Further, a significantly higher incidence of T-cell lymphomas was recorded, ranging between 12% and 33% in the different considered provinces [25]. As far as Latin America was concerned, a national study from Colombia showed that most NHL were aggressive, mostly DLBCL [26]. Of note, HL appeared to be more common than FL, while T-cell neoplasms were definitely rare [26]. The International Non- Hodgkin Lymphoma Classification Project extensively investigated the epidemiology of lymphomas in Southern-Africa [27] that similarly to what described in Colombia, Southern Africa had a significantly higher prevalence of aggressive vs. of indolent NHL (51.5 vs. 34.3%) if compared to Europe and Northern-America. Particularly, high grade B-cell lymphomas (formerly Burkitt-like lymphomas) were significantly more common in Southern Africa (8.2%) than in Europe (2.4%) and Northern-America (2.5%), being frequently associated with HIV infection [27]. Of interest, whites presented with significantly higher frequency of indolent B-NHL (60.4%) and a lower frequency of aggressive B-NHL (32.7%) compared to blacks (22.5% vs. 62.6%) [27]. Specifically, whites had a significantly higher frequency of FL and a lower frequency of highgrade B-cell lymphomas compared to blacks [27]. Furthermore, the median age at onset was significantly higher in whites than in blacks, as far as indolent, aggressive B-NHL as well as T-NHL (64, 56 and 67 years vs. 55, 41 and 34 years, respectively). Similar patterns were observed in Eastern Africa, as reported by Dr. Rogena and Colleagues [28], and Egypt, where again indolent disorders like FL and CLL represented together only 15% of the total [24]. The major limitations of our analysis are certainly represented by the lack of many clinical as well as social information that would be useful to interpret the results. Indeed, different frequency of certain diseases in different geographical area might be due to different exposure to risk factors. Second, although collecting cases from a remarkable part of the country, covering North, Center, and South, this is not properly a national registry-based study. Certainly, a study covering these two issues is warranted. However, on the other hand, we provided a series of unprecedented information regarding lymphomas in Italy. In conclusion, our study described for the first time the basic information about lymphoma epidemiology in Italy after WHO 2017 was released. It underlined the importance of national registries and local epidemiological analyses; in fact, a better knowledge of regional peculiar situations can be the basis for local specific intervention and global health care improvement [29].
References
- National Cancer Institute. Surveillance Epidemiology and End Results (SEER) program. Cancer Stat Facts. 2020.
- International Agency for Research on Cancer. ECIS - European Cancer Information System. Cancer Factsheets. 2020.
- Huh J (2012) Epidemiologic overview of malignant lymphoma. The Korean Journal of Hematology 47(2).
- Sant M, Allemani C, Tereanu C, de Angelis R, Capocaccia R, Visser O, et al. (2010) Incidence of hematologic malignancies in Europe by morphologic subtype: Results of the HAEMACARE project. Blood 116(19): 3724-3734.
- Edlefsen KL, de Roos A, LaCroix A (2008) Application of the InterLymph Consortium’s Proposed Classification of Lymphoid Neoplasms for Epidemiologic Research to a Large, Nationwide Health Study: Experience in the Women’s Health Initiative. Blood 112(11): 4669.
- Piccaluga PP, Fuligni F, de Leo A, Bertuzzi C, Rossi M, Bacci F, et al. (2013) Molecular profiling improves classification and prognostication of nodal peripheral t-cell lymphomas: Results of a phase III diagnostic accuracy study. Journal of Clinical Oncology 31(24): 3019-3025.
- Navari M, Etebari M, Ricci F, Tazzari PL, Agostinelli C, Went P, et al. (2022) T-Cell Receptor Dependent and Independent NF-kappa B Activation is a Prognostic Marker and a Therapeutic Target in Peripheral T-cell Lymphoma Not Otherwise Specified. Digital Medicine and Health Technology 2022: 1-28.
- Amato T, Sall A, Dièye TN, Gozzetti A, Iacono M, Ambrosio MR, et al. (2017) Preferential usage of specific immunoglobulin heavy chain variable region genes with unmutated profile and advanced stage at presentation are common features in patients with chronic lymphocytic leukemia from Senegal. American Journal of Clinical Pathology 148(6): 545-554.
- Amato T, Abate F, Piccaluga P, Iacono M, Fallerini C, Renieri A, et al. (2016) Clonality analysis of immunoglobulin gene rearrangement by next-generation sequencing in endemic burkitt lymphoma suggests antigen drive activation of bcr as opposed to sporadic burkitt lymphoma. American Journal of Clinical Pathology 145(1): 116-127.
- Foucar K, Falini B (2017) Stein H Hairy cell leukaemia. In: Steven H. Swerdlow, Elias Campo, Nancy Lee Harris, Elaine S. Jaffe, Stefano A. Pileri, Harald Stein, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th Lyon: IARC pp. 226-268.
- Swerdlow SH, Campo E Seto M Muller-Hermelink HK (2017) Mantle cell lymphoma. In: Steven H. Swerdlow, Elias Campo, Nancy Lee Harris, Elaine S Jaffe, Stefano A Pileri, Harald Stein, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th Lyon: IARC pp. 285-290.
- Jaffe ES, Stein H, Swerdlow SH (2017) Classic Hodgkin lymphoma. In: Steven H Swerdlow, Elias Campo, Nancy Lee Harris, Elaine S Jaffe, Stefano A Pileri, Harald Stein, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC pp. 435-442.
- Federico M, Rudiger T, Bellei M, Nathwani BN, Luminari S, Coiffier B, et al. (2013) Clinicopathologic characteristics of angioimmunoblastic t-cell lymphoma: Analysis of the international peripheral t-cell lymphoma project. Journal of Clinical Oncology 31(2): 240-246.
- Phan A, Veldman R, Lechowicz MJ (2016) T-cell Lymphoma Epidemiology: the Known and Unknown. Vol. 11, Current Hematologic Malignancy Reports 11(6): 492-503.
- Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, et al. (2008) ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12): 5496-5504.
- Thandra KC, Barsouk A, Saginala K, Padala SA, Barsouk A (2021) Epidemiology of Non-Hodgkin’s Lymphoma. Medical Sciences 9(1): 5.
- Pileri SA, Agostinelli C, Sabattini E, Pileri A, Falini B, et al. (2013) Lymphoma classification: Past, present and future. Non-Hodgkin Lymphomas: Advanced Diagnostics & Personalized Therapies.
- Pileri SA, Agostinelli C, Sabattini E, Bacci F, Sagramoso C, Pileri A, et al. (2011) Lymphoma classification: The quiet after the storm. Seminars in Diagnostic Pathology 28(2): 113-123.
- Piccaluga PP, Agostinelli C, Tripodo C, Gazzola A, Bacci F, Sabattini E, et al. (2011) Peripheral T-cell lymphoma classification: The matter of cellular derivation. Expert Review of Hematology 4(4): 415-425.
- Paolo Piccaluga P (2019) Introductory Chapter: Updates and New Insights from WHO 2017 Peripheral T-Cell Lymphoma Classification. In: Peripheral T-cell Lymphomas.
- Bassig BA, Au WY, Mang O, Ngan R, Morton LM, et al. (2016) Subtype-specific incidence rates of lymphoid malignancies in Hong Kong compared to the United States, 2001-2010. Cancer Epidemiology 42: 15-23.
- Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, et al. (2014) Differences in incidence and trends of haematological malignancies in Japan and the United States. British Journal of Haematology 164(4): 536-545.
- Müller AMS, Ihorst G, Mertelsmann R, Engelhardt M (2005) Epidemiology of non-Hodgkin’s lymphoma (NHL): Trends, geographic distribution, and etiology Annals of Hematology 84(1): 1-12.
- Yoo KH, Lee H, Suh C (2018) Lymphoma epidemiology in Korea and the real clinical field including the Consortium for Improving Survival of Lymphoma (CISL) trial International Journal of Hematology 107(4): 395-404.
- Muto R, Miyoshi H, Sato K, Furuta T, Muta H, et al. (2018) Epidemiology and secular trends of malignant lymphoma in Japan: Analysis of 9426 cases according to the World Health Organization classification. Cancer Medicine 7(11): 5843-5858.
- Combariza JF, Lombana M, Torres AM, Castellanos AM, Arango M (2015) General features and epidemiology of lymphoma in Colombia. A multicentric study. Annals of Hematology 94(6): 975-980.
- Perry AM, Perner Y, Diebold J, Nathwani BN, Maclennan KA, et al. (2016) Non-Hodgkin lymphoma in Southern Africa: Review of 487 cases from The International Non-Hodgkin Lymphoma Classification Project. British Journal of Haematology 172(5): 716-723.
- Rogena EA, de Falco G, Schurfeld K, Leoncini L (2011) A review of the trends of lymphomas in the equatorial belt of Africa Hematological Oncology.
- Chihara D, Nastoupil LJ, Williams JN, Lee P, Koff JL, et al. (2015) New insights into the epidemiology of non-Hodgkin lymphoma and implications for therapy. Vol. 15, Expert Review of Anticancer Therapy 15(5): 531-544.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...