Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Research Article(ISSN: 2638-5945) 
Characteristics of Pure Familial Pancreatic Cancer Families and Those with Additional Breast Cancer Volume 4 - Issue 1
Detlef K Bartsch1*, Elvira Matthäi1, Ioannis Mintziras1, Lutz Benedikt Böhm1, Norman Gercke1, Christian Bauer2, Jens Figiel3 and Emily P Slater1
- 1Department of Visceral, Thoracic and Vascular Surgery
- 2Gastroenterology and Endocrinology
- 3Radiology, Philipps University Marburg, Germany
Received: August 17, 2020 Published: September 10, 2020
Corresponding author: Detlef K Bartsch, Philipps University of Marburg, Department of Visceral, Thoracic and Vascular Surgery, Germany
DOI: 10.32474/OAJOM.2020.04.000178
Abstract
Familial pancreatic cancer (FPC) is a rare hereditary tumor syndrome. The large phenotypic and genotypic heterogeneity is not yet well-established. FPC families without (pure FPC) or with additional occurrence of breast cancer (FPC-breast) were analyzed regarding phenotype, genotype and the diagnostic yield of prospective screening for pancreatic ductal adenocarcinoma (PDAC). The total cohort of 227 FPC families included 85 (38%) pure FPC and 70 (30.7%) FPC-breast families. The proportions of affected family members with PDAC (27.2%, 197/724 vs. 18.3%, 177/962, p<0.0001) and of 2 or more affected generations (84.2% vs. 62.9%, p<0.05) were significantly higher in pure FPC families. In 48 (68.6%) FPC-breast families additional tumor types occurred, most frequently colorectal cancer (n=16, 22.8%). Deleterious germline mutations (2 BRCA2, 1 PALB2) were detected in 3 of 53 (5.6%) analyzed pure FPC and in 17 of 56 (30.4%) analyzed FPC-breast families (3 BRCA1, 6 BRCA2, 3 PALB2, 3 CDKN2A, 2 ATM; p=0.001). Individuals at risk (IAR) from FPC-breast families participated significantly more often in a prospective PDAC screening program (54.4% vs. 33.5%; p=0.0001) resulting in a diagnostic yield of 3.7% and 0% in FPC-breast and pure FPC families, respectively. The phenotypic and genotypic characteristics of pure FPC and FPC-breast families should be considered for the genetic counseling and management of these families.
Keywords: Familial Pancreatic Cancer; Mutations; Phenotype; Genotype
Abbreviations: FPC: Familial Pancreatic Cancer; PDAC: Pancreatic Ductal Adenocarcinoma; HBOC: Hereditary Breast and Ovarian Cancer; PanIN: Pancreatic Intraepithelial Neoplasia; IPMN: Intraductal Papillary Mucinous Neoplasia; AFL: Atypical Flat Lesions; IAR: Individual at Risk
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a challenging tumor entity with an increasing incidence and a dismal prognosis. The overall five-year-survival rate is less than 5%, attributed to late clinical symptoms, low resection rates and poor response to radio- and chemotherapy [1]. One of the greatest risk factors for developing PDAC is a positive family history. A familial aggregation of PDAC is present in 1.9% to 6% of cases depending on the confirmation of the PDAC diagnosis by either only family history or by histopathology and medical reports [2, 3]. An inherited predisposition to PDAC is potentially given in the so-called familial pancreatic cancer (FPC) families, which are characterized by two or more first-degree relatives with PDAC that do not fulfill the criteria for another inherited tumor syndrome [4, 5]. On the other hand several hereditary tumor predisposition syndromes such as Peutz- Jeghers-Syndrome (PJS) and hereditary breast and ovarian cancer (HBOC) might predispose to PDAC, although these syndromes are characterized by another clinical phenotype than PDAC [4, 5]. Tumor registries such as the North American National Familial Pancreatic Tumor Registry (NFPTR), the German National Case Collection of Familial Pancreatic Cancer (FaPaCa) and the European Registry of Hereditary Pancreatitis and Familial Pancreatic Cancer (EUROPAC) were established to investigate the phenotype and genotype of FPC families [6-8]. In the last years, studies focused mainly on the discovery of the underlying gene defects and the evaluation of diagnostic yield of prospective PDAC screening programs in individuals at risk (IAR) of such families [9-17]. Current sequencing and WES data suggest that FPC is genetically highly heterogeneous with no major predisposing gene [17]. The phenotypic variance of FPC families, however, is still not well-established. The current analysis was performed to analyze the phenotype and genotype and the diagnostic yield of prospective PDAC screening in pure FPC and FPC-breast cancer families.
Materials and Methods
The FaPaCa registry is a national case collection for familial pancreatic cancer families in Germany funded by the Deutsche Krebshilfe in 1999 [7-18]. Families with two or more first-degree relatives with a confirmed diagnosis of PDAC and without evidence of any other inherited tumor syndrome were collected. The current report analyzed the genotype and phenotype of FPC families with the occurrence of only PDAC (pure FPC families) and FPC families with the additional occurrence of breast cancer (FPC-breast) families. Members from FPC families were recruited between July 1999 and July 2019 by direct referral via their physicians or by personal contact to the FaPaCa study-office based on information about the study, e.g. via the internet (http://www.fapaca.de). All eligible persons and families were initially genetically counseled and a three-generation family pedigree was constructed. All PDAC diagnoses were confirmed by review of medical records, deaths certificates and by examination of the pathology slides when available. All patients with PDAC of FPC families with available blood samples who gave their informed consent were subjected to mutation analysis of the potential predisposing PDAC genes ATM, BRCA1/2, CDKN2A, PALB2, PLLD and CHEK2 as described previously [12-18]. In addition, whole exome sequencing (WES) was performed in 7 of those families (Slater et al., submitted). If a deleterious germline mutation was identified in the index patient, predictive genetic testing of this mutation was offered to all family members after genetic counseling. The result of the predictive testing was explained to the family members during another interdisciplinary counseling, involving a geneticist, a psychologist, a surgeon and gastroenterologist. Individuals at risk (IAR) older than 18 years were encouraged to participate in a prospective screening program that was conducted at our institution. Firstdegree relatives of an affected patient of a FPC family and members of a FPC-family carrying a predisposing mutation such as BRCA2, independent of the degree of relationship, were classified as IAR. The screening started at age 40 years until 2016 and thereafter at age 50 years or 10 years before the earliest age of onset of PDAC in the family, whichever was first [19]. The screening program included an annual physical examination, determination of serum HbA1c, amylase, GOT, GPT, bilirubin and CA19-9, and imaging with magnetic resonance imaging plus MRCP and endosonography as described previously [19]. The screening program was restricted to mutation carriers, if the underlying gene defect was known in the family. Resection specimens were analyzed by experienced pathologists with special regard to the presence of PDAC, pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasia (IPMN) and atypical flat lesions (AFL). Previous screening results of some IAR have been already published [10, 18-20]. The FaPaCa registry, including the genetic analyses and the screening program, was approved by the Ethics Committee of the Philipps- University of Marburg (36/1997, last amendment 9/2010) and all participants provided written informed consent. Descriptive statistics of the relatives who enrolled were compiled. Variables included age, gender, number of relatives with PDAC, earliest age of onset in the family and underlying germline mutations. The age of diagnosis of PDAC was retrieved from the 3-generation pedigrees and early age of onset was defined as the occurrence of PDAC prior to the age of 50 years in a family. Significant lesions were defined as the presence of histologically verified PDAC, PanIN3 or IPMN with high-grade dysplasia. Potentially relevant lesions were defined as histologically verified multifocal PanIN2 lesions with/without BDIPMN with low grade dysplasia and/or atypical flat lesions (AFL). The chi-square, Fisher’s exact test, t test and Wilcoxon rank sum test were performed for categorical and numerical variables, where appropriate, to compare patient characteristics. Two-tailed p values <0.05 were considered to be statistically significant. Analyses were performed using Prism 6 for Mac OS X from GraphPad Software, Inc.
Results
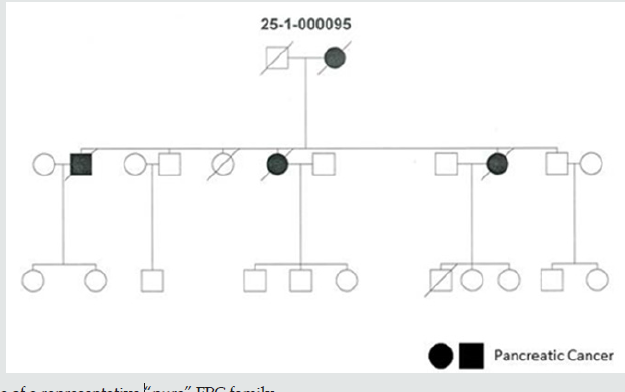
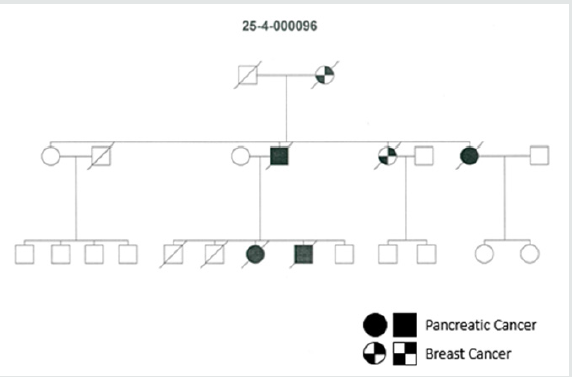
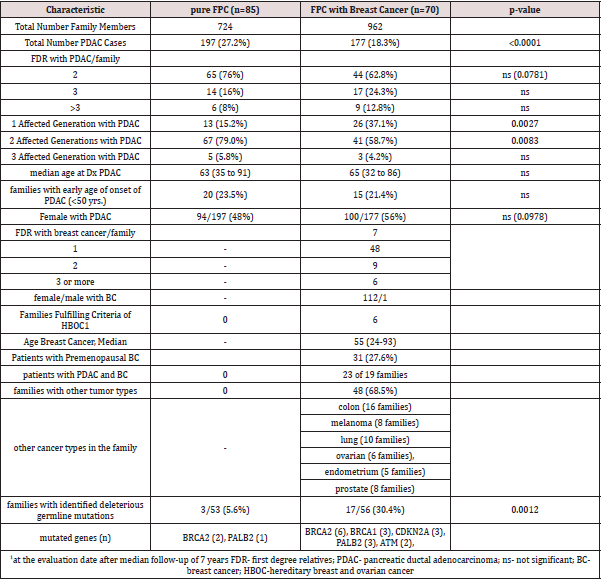
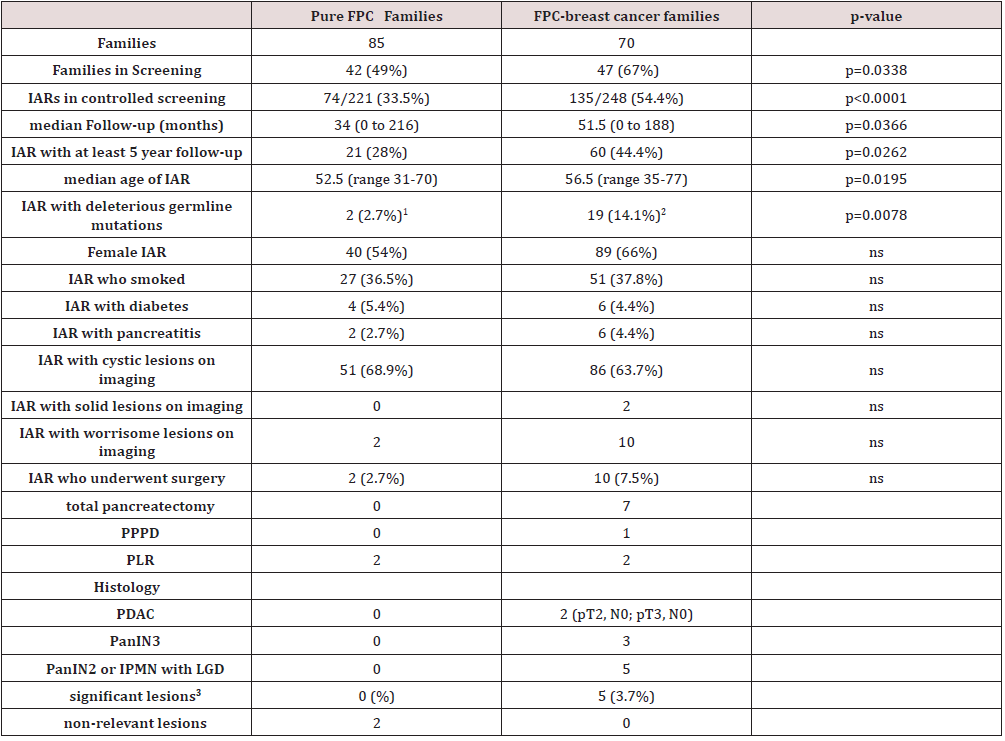
Of 227 verified FPC families, 85 (37.4%) revealed only PDAC and 70 (30.8%) families showed both PDAC and breast cancer. The proportion of affected family members with PDAC was significantly higher in pure FPC families (27.2%, 197/724) compared to all FPCbreast families (18.3%, 177/962, p<0.0001). This also holds true when comparing FPC-breast families with or without detected predisposing germline mutations. In the 17 FPC breast families with a germline mutation the PDAC rate was 18.3% (49 of 267 family members) compared to 16.4% (90 of 550 family members) in the 39 families without mutations. Twenty (24%) pure FPC families had 3 or more affected first-degree relatives with PDAC compared to 26 (37.1%) of the FPC-breast families (p=0.08). The proportion of 2 or more affected generations, however, was significantly higher in pure FPC families than in FPC-breast families (84.2% vs. 62.9%, p<0.05). The rate of females affected with PDAC tended to be lower in pure FPC (48%, 94/197) than in FPCbreast families (56%, 100/177, p=0.08). The median age of PDAC diagnosis (63 vs. 65 years) and the rate of families with early age of PDAC onset <50 years (23.5% vs 21.4%) were not significantly different between family groups (Table 1). In the 70 FPC-breast families a total of 113 (1-6 per family) breast cancers occurred, all but one in female patients. The mean rate of breast cancer cases per family was 1.6 (SD=1.207; SEM=0.1443). At inclusion none of these 70 families fulfilled the criteria for HBOC [21,22], but this was the case for six 6 (8.6%) families due to the additional occurrence of breast/ovarian cancer cases after a median follow-up of 7 years. In 19 families 23 (23%) female patients developed PDAC and breast cancer, synchronous and metachronous. The median age of breast cancer diagnosis was 55 (range 24-93) years, 31 (27.4%) patients had a premenopausal (<50 years) diagnosis. Five (3.6%) female patients developed bilateral breast cancer. In 48 (68.6%) FPCbreast family’s tumor types in addition to PDAC and breast cancer also occurred. The most frequent additional cancers were colon cancer in 16 (22.8%), lung cancer in 10 (14.3%), prostate cancer and malignant melanoma in 8 (11.4%) families each, respectively (Table 1). The characteristics of pure FPC families and FPC-breast families are summarized in (Table 1). Representative pure FPC and FPC-breast families are shown in (Figures 1, 2).
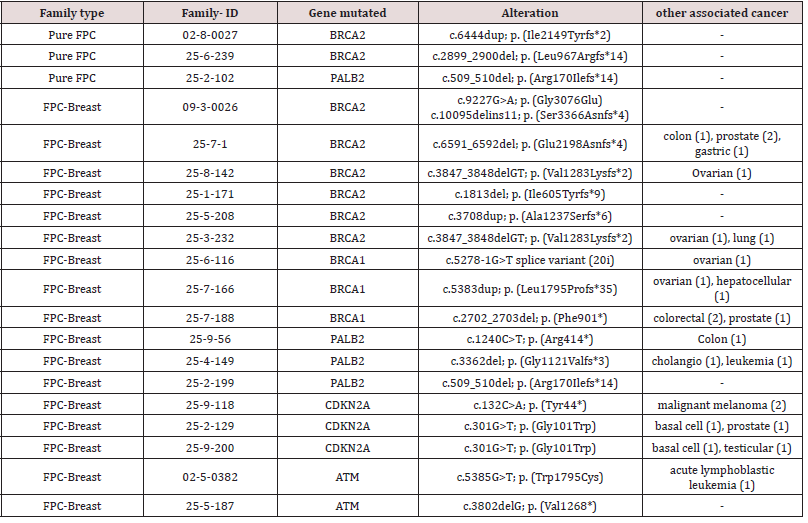
Mutation analysis complemented by WES sequencing of
7 families identified deleterious gene alterations in known
predisposing PDAC genes, such as BRCA2 and PALB2, in 3 of 53
(5.6%) analyzed pure FPC-families and 17 of 56 (30.4%) analyzed
FPC-breast families (p=0.001). In pure FPC families only BRCA2
and PALB2 were altered, whereas in the FPC-breast families several
genes, including BRCA1/2, CDKN2A, PALB2 and ATM were mutated
(Table 2). The most frequently mutated gene was BRCA2 in 8 of
20 (40%) families with mutations. In all pure FPC and FPC-breast
families the identified gene defect co-segregated with the disease.
Variants of unknown significance in several genes such as ACAD9,
KCNJ12, KMTC2, NOTCH2NL and SUFU were identified in 5 of 7
families investigated with WES. These gene alterations did not
always segregate with PDAC disease in the respective families and
are classified in ClinVar as variants of unknown significance.
Individuals at risk (IAR) from FPC-breast families (135 of 248,
54.4%) participated significantly more often in a recommended
screening program than IAR from pure FPC families (74 of 221,
33.5%; p<0.0001). The median follow-up was 34 months in the IAR
of pure FPC and 51 months in IAR of FPC-breast families (p=0.0366).
IAR of pure FPC and FPC-breast families were comparable regarding
gender distribution, smoking habits, the presence of diabetes mellitus and history of pancreatitis, respectively (Table 3). A total
of 904 examination visits (median 3 ranges 1-16) were performed
in the 209 IAR over the period. On imaging small (<10mm) cystic
lesions, most likely BD-IPMN, were detected in 68.9% and 63.7%
of IAR form pure FPC and FPC-breast families. Imaging detected
“worrisome” lesions in 2 (2.7%) IAR from pure FPC and 10 IAR
(7.5%) from FPC-breast families (p=0.21). These 12 IAR underwent
pancreatic resections and significant lesions such as PDAC or PanIN3
were detected in 5 (3.7%) of the IAR from FPC-breast families.
None of these IAR was a carrier of a PDAC-predisposing germline
mutation. The other 5 IAR from the FPC-breast families, including 2
germline mutation carriers, revealed potentially relevant multifocal
PanIN2 lesions and/or IPMN with moderate dysplasia, whereas
both operated IAR from pure FPC families had non-relevant lesions
such serous cystadenoma and focal fibrosis (Table 3). Thus, the
diagnostic yield of the prospective PDAC screening was 0% and
3.7% in pure FPC and FPC-breast families according to the strict
criteria of an international consensus conference [5, 22].
Discussion
Despite several new molecular insights into the pathogenesis of hereditary forms of cancer, its translation into the clinical setting remains still somehow problematic. A comprehensive history of cancer in a family is still one milestone to establish a potentially hereditary cancer syndrome. The family history, however, has been insufficiently recorded in many patients’ medical records, thereby compromising its clinical significance [24]. This is especially true for FPC families, since the phenotype is highly heterogeneous and affected patients die fast due to the aggressive disease. FPC can be mainly divided into two groups, namely pure PC families and those associated with other tumor types [4]. The most frequent other tumor type associated with FPC in the FaPaCa registry is breast cancer, in almost one third (30.7%) of FPC families. Therefore, we undertook a detailed analysis of the phenotype and genotype of pure FPC families and FPC with an additional occurrence of breast cancer. Initially, none of these families fulfilled the criteria for HBOC [21, 22], but after a median follow-up of 7 years this was the case for 6 (8.6%) families due to the new development of additional breast and/or ovarian cancer cases. It has been previously reported that some HBOC families are associated with an increased risk for PDAC. A retrospective analysis of 5143 Italian family trees with breast and/ or ovarian cancer, for example, showed that 392 (7.6%) families also had cases of PDAC [25]. It has also been postulated that the risk of PDAC is especially increased in those 25 to 30% of HBOC families who are associated with BRCA1 or BRCA2 germline mutations [25]. Lynch et al. reported that 213 (3.7%) of 5742 families with a BRCA1 mutation and 138 (6.1%) of 2269 families with a BRCA2 mutation had a case of PDAC [26]. A study by the Breast Cancer Linkage Consortium (BCLC) yielded for female BRCA1 mutation carriers a 2.3-fold risk and for female BRCA2 mutation carriers a 3.5-fold risk for the development of PDAC [27]. From another viewpoint, it has been reported that a familial accumulation of PDAC might be associated with the occurrence of breast and/or ovarian cancer. A mortality analysis of the National Familial Pancreatic Tumor Registry probands, which included over 200,000 person-years of follow-up from 8564 first-degree relatives of PDAC probands, found that relatives of FPC probands had a significantly increased risk of dying from breast (wSMR 1.66, 95% CI 1.15–2.34), ovarian (wSMR 2.05, 95% CI 1.10–3.49), and bile duct cancers (wSMR 2.89, 95% CI 1.04–6.39) [28]. Analysis of the familial aggregation of PDAC with other malignancies using the updated Swedish Family-Cancer Database with more than 11.5 million individuals disclosed that a significantly increased the risk of PDAC was associated with earlyonset breast cancer in siblings [29].
Based on the present analysis one can postulate some
phenotypic differences between pure FPC and FPC-breast families.
The proportion of affected family members with PDAC (27.2% vs.
18.3%, p<0.0001) and the involvement of 2 or more generations was
significantly higher in pure FPC families than in FPC-breast families
(84.2% vs. 62.9%, p<0.05). The rate of females affected with PDAC
tended to be higher in FPC-breast than in pure FPC families (56%
vs. 48%, p=0.0978). A previous analysis of 317 BRCA1/2 mutated
HBOC families with 351 cases of PDAC, however, revealed a slightly
higher risk in males (58% vs. 46%) [26]. The median age of PDAC
diagnosis (63 vs. 65 years) and the rate of families with early age
of PDAC onset <50 years (23.5% vs 21.4%) were not significantly
different between family groups (Table 1), which is in line with
previous reports on pure FPC families and BRCA1/BRCA2 families
with the occurrence of PDAC [26, 27].
Comparing the FPC-breast families with the reported
characteristics of HBOC families one can also note some phenotypical
differences. The PDAC rate in FPC-breast families (18%) appears
comparably high to HBOC families with 1.5 to 8% [24, 25]. In the
FPC-breast families the rate of breast cancer cases with mean 1.6
cases per family was lower than in German HBOC families [30, 31].
The median age of onset of breast cancer (55 vs. 49 years) was older and the proportion of women with premenopausal onset of
breast cancer (27.4% vs. 45%) was also lower than those reported
for German HBOC families [30]. It is of note, that 68.5% of analyzed
FPC-breast families showed additional cancers, including colorectal
cancer (22.8%) and ovarian cancer (8.6%).
Recent advances in sequencing technology provided an unbiased
way to search for the genes underlying disease susceptibility in
FPC. Using this approach BRCA1/2, CDKN2A, PALB2 and ATM
were identified as FPC susceptibility genes, together explaining
about 8%-15% of FPC cases [17, 18, 32]. A previous whole genome
sequencing study of 638 patients from 593 American FPC kindreds
demonstrated that the genetic underpinning of inherited pancreatic
cancer is highly heterogeneous [17]. This analysis supported the
role of the known FPC susceptibility BRCA2, CDKN2A, PALB2 and
ATM, and identified a few novel candidate genes such as BUB1B and
FANCC harboring rare, deleterious germline variants. Interestingly,
many of these candidate genes are involved in processes regulating
DNA repair or chromosomal stability.
The genetic basis underlying FPC susceptibility, however,
remains still unknown in 80% to 90% of patients with FPC. In
the present study a predisposing gene defect could be identified
in the susceptibility genes BRCA1, BRCA2, CDKN2A, ATM and
PALB2 in 17 (15.5%) of 109 analyzed FPC families. As in previous
studies BRCA2 was the most frequently mutated gene 8 (7.3%). It
is of note, however, that the prevalence of deleterious mutations
was significantly higher in FPC-breast (30.4%) than in pure FPCfamilies
(5.6%, p=0.001). ATM, BRCA1 and CDKN2A mutations
were only detected in FPC-breast families. A previous Italian study
postulated a BRCA2 cluster region within c.7180 and c.8248, which
might be associated with the occurrence of PDAC [25]. We cannot
confirm this observation, since all of the 8 BRCA2 mutations were
located outside of this region.
Knowledge of the genes responsible for FPC susceptibility is
important for a number of reasons. One is that early detection can
be targeted to mutation carriers and pancreatic neoplasms can
be detected at an earlier stage, when therapeutic interventions
with curative intent are still possible. A recent expert consensus
conference recommends pancreatic screening under boardapproved
protocols for IAR from FPC families [5, 23]. Various
screening programs reported a diagnostic yield of PDAC screening
between 1.9 and 7%, when defining high grade dysplasia or stage I
PDAC as true success of screening [9, 10, 19]. In the present study
it was obvious that IAR from FPC-breast families participated
significantly more often in the recommended screening than IAR
from pure FPC families (33.5% vs. 54.4%, p<0.0001). The reasons
for this remain speculative and are probably multifactorial. The
diagnostic yield of the screening program itself appears to be more
efficient in FPC-breast families compared to pure FPC families
(3.7% vs. 0%), although the prospective follow-up of less than 5 years is too short to draw any definitive conclusions. A recent North
American study [33] reported that the cumulative incidence of
PDAC and high grade dysplasia was significantly higher in the IAR
group with predisposing germline mutations compared to those
IAR without (RR 2.85, 95% CI 1.0-8.18, p=0.05). We can neither
confirm nor reject this observation due to the limited number of
mutation carriers (n=21) participating in screening.
Conclusion
In summary, there seems to be a distinct FPC-breast family phenotype, which might be associated with other solid cancers such as colon cancer and has at least a 30% prevalence of predisposing germline mutations in known susceptibility genes BRCA1/2, PALB2, CDKN2A and ATM. IAR of these families have a comparably high motivation to participate in PDAC screening, which in short term appears to be more effective than in pure FPC families. These data should be considered for the counseling and management of these families.
Acknowledgements
We are grateful to all FPC families for participating in the registry. We thank A. Ramaswamy, Marburg, and Günter Klöppel, Munich, for the pathological examination of the resection specimens. This study was supported by the Deutsche Krebshilfe, grant number 111092, and a generous donation from the GAUFF-Foundation.
Conflict of interest
None of the authors has competing financial interests nor other conflicts of interest.
References
- QuanteA,MingC,RottmannM,EngelJ,BoeckS,(2016)Projectionsofcancerincidenceandcancer‐relateddeathsinGermanyby2020and2030.CancerMed5(9):2649-2656.
- BartschDK,KressR,Sina-FreyM,GrützmannR,GerdesB,etal.(2004)PrevalenceoffamilialpancreaticcancerinGermany.IntJCancer110(6),902-906.
- HemminkiK,LiX(2003)Familialandsecondprimarypancreaticcancers:anationwideepidemiologicstudyfromSweden.IntJCancer103(4):525-530.
- BartschDK,GressTM,LangerP(2012)Familialpancreaticcancer–currentknowledge.NatRevGastroenterolHepatol9(8): 445-553.
- CantoMI,HarinckF,HrubanRH,GeorgeJohanOfferhaus,Jan-WernerPoley,etal.(2013)InternationalCancerofPancreasScreening(CAPS)ConsortiumInternationalCancerofthePancreasScreening(CAPS)Consortiumsummitonthemanagementofpatientswithincreasedriskforfamilialpancreaticcancer.Gut62(3):339-347.
- TersmetteAC,PetersenGM,OfferhausGJ,FalatkoFC,BruneKA,etal.(2001)Increasedriskofincidentpancreaticcanceramongfirst-degreerelativesofpatientswithfamilialpancreaticcancer.ClinCancerRes7(3):738-744.
- BartschDK,Sina-FreyM,ZieglerA,HahnSA,PrzypadloE,etal.(2001)Pancreatology1(5):510-516.
- ApplebaumSE,KantJA,WhitcombDC,EllisIH(2000)Genetictesting,counselling,laboratory,andregulatoryissuesandtheEUROPACprotocolforethicalresearchinmulticenterstudiesofinheritedpancreaticdiseases.MedClinNorthAm84(3):575-588.
- CantoMI,AlmarioJA,SchulickRD,YeoCJ,KleinA,etal.(2018)Riskofneoplasticprogressioninindividualsathighriskforpancreaticcancerundergoinglong-termsurveillance.Gastroenterology155(3):740-751.
- VasenH,IbrahimI,PonceCG,SlaterEP,MatthäiE,etal.(2016)Benefitofsurveillanceforpancreaticcancerinhigh-riskindividuals:outcomeoflong-termprospectivefollow-upstudiesfromthreeeuropeanexpertcenters.JClinOncol34(17):2010-2019.
- CorralJE,MarethKF,Riegert-JohnsonDL,DasA,WallaceMB,etal.(2019)Diagnosticyieldfromscreeningasymptomaticindividualsathighriskforpancreaticcancer:ameta-analysisofcohortstudies.ClinGastroenterolHepatol17(1):41-53.
- HahnSA,GreenhalfB,EllisI,Sina-FreyM,RiederH,etal.(2003)JNatlCancerInst95(3):214-221.
- MurphyKM,BruneKA,GriffinC,SollenbergerJE,PetersenGM,etal.(2002)EvaluationofcandidategenesMAP2K4,MADH4,ACVR1B,andBRCA2infamilialpancreaticcancer:deleteriousBRCA2mutationsin17%.CancerRes62(13):3789-3793.
- BartschDK,Sina-FreyM,LangS,WildA,GerdesB,etal.(2002)CDKN2Agermlinemutationsinfamilialpancreaticcancer.AnnSurg236(6):730-737.
- SlaterEP,LangerP,NiemczykE,StrauchK,ButlerJ,etal.(2010)PrevalenceofPALB2mutationsinfamilialpancreaticcancer.ClinGenet78(5):490-494.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...