
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Review Article(ISSN: 2638-5945) 
Advances in Treating Relapsed Diffuse Large B Cell Lymphoma Treatment Volume 4 - Issue 4
Dahlia Sano*
- Department of Oncology, Karmanos Cancer Institute, Wayne State University, USA
Received:April 19, 2021 Published: May 13, 2021
Corresponding author: Department of Oncology, Karmanos Cancer Institute, Wayne State University, USA
DOI: 10.32474/OAJOM.2021.04.000192
Abstract
Diffuse large B cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL), comprising about 25% of all mature NHL. First-line therapy cures about 40-60% of patients. High dose chemotherapy followed by autologous stem cell transplant can cure about 50% of patients at relapse. Transplant-ineligible patients have shorter survival with every line of subsequent therapies with a median overall survival (OS) of 10 months at second line and 4.7 months at fourth lines of therapy. There is unmet need to treat patients with relapsed DLBCL. Chimeric antigen receptor T-Cells (CAR-T), with now three FDA- approved products to treat relapsed DLBCL, provide a cure in about 40% of patients. Other recently approved agents include antibody-drug conjugate targeting CD79b (Polatuzumab Vedotin), Anti-CD19 antibodies (Tafasitamab-cxix), and nuclear export inhibitor XPO1 (Selinexor) have provided a hope to patients with relapsed DLBCL. We will discuss these new approvals with comparison of response rate and side effect profiles.
Keywords: Diffuse Large B Cell Lymphoma; CAR-T Cell Therapy; Tafasitamab; Relapsed Refractory Lymphoma; Polatuzumab
Introduction
Non-Hodgkin lymphoma (NHL) is the eighth cause of cancer death. With an estimated 81,560 new cases and 20,720 deaths in 2021 [1] DLBCL is the most common type of NHL comprising about 25% of all NHL cases [2].
The prognosis depends on different factors at diagnosis including histological subtypes (Germinal center (GC) type versus non-GC subtypes) [3], genetic subtypes (double HIT) [4], patient age, stage of the disease, extra nodal involvement, and elevated LDH (IPI scoring) 5. Other important prognostic factors can be assessed after starting therapy, includes response at interim PET scan (PET scan after 2-4 cycles of therapy)6. Initial therapy for DLBCL remained the same for all different prognostic factors and different risk group which is the famous combination of chemotherapy R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone) despite multiple attempts to try to find a better alternative [7-9]. Except for high grade DLBCL with c-Myc and BCL2 and/or BCL6 gene rearrangement which most centers comfortable with using R-EPOCH chemotherapy regimen [10]. With the stander of care R-CHOP, about 30% of diffuse large B cell lymphoma patients relapse within the first 5 years [11]. Poor response to initial therapy and, or very early relapse (within the first six months), or what is called refractory disease is one of the worse predictors of poor survival based on data from scholar-1 study [12].
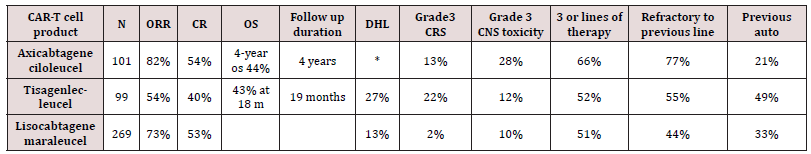
Current stander of care at relapse including high dose chemotherapy followed by autologous stem cell transplant [13]. In patient with very high-risk relapse (relapse within 1 year of therapy or refractory patients) there is a suggestion that early therapy with CAR-T cell may improve outcome. this question will be answered once we receive data from the now completed ZUMA7, and BELINDA and TRANSFORM trials comparing different CAR-T cell products (axicabtagene, Tisagenlecleucel, Lisocabtagene respectively) to the current stander of care i.e., high dose chemotherapy followed by autologous stem cell transplant in this very high-risk population. Treatment after relapse from second line of therapy inpatient who continue to maintain reasonable performance status is CAR-T cell therapy. Different CAR-T cell products are currently FDA approved to treat relapsed DLBCL after failure of at least two lines of therapy including Axicabtagene ciloleucel (Axi-Cel), Tisagenlecleucel and Lisocabtagene maraleucel(liso-cel). CAR-T provide about 40-55% complete response rate (CR). Most worrisome Toxicity including cytokine release syndrome (CRS) and neurological toxicity [14-16]. See Table 1 for comparing of different CAR-T cell products. Of note patient with DHL and DEL benefited from CAR-T cell therapy at relapse with Best overall response (ORR) was 56% (10/18) for DEL patients, 50% (5/10) for DHL patients which was not different from non-DHL/DEL in a retrospective data evaluation4. In patient who are intolerable to transplant or are ineligible for CAR-T therapy or had a relapse after CAR-T cell therapy there is no stander approach. Recent advances in newer agent gave some hope in managing these patients with improving survival.
Table 1: CAR-T cell that are FDA approved for DLBCL. *DHL status was not checked in Zuma1 trial, but the real-world data included patients with DHL. M: month.
Polatuzumab Vedotin
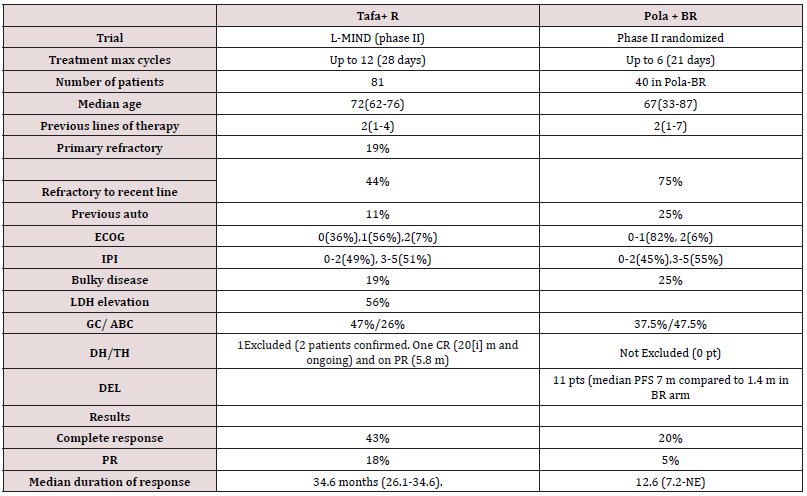
Polatuzumab Vedotin (Pola) is an antibody-drug conjugated that bind to CD79b which is present in all B-lymphocytes including mature malignant B-Cells delivering the microtubule inhibitor (MMAE) causing direct cytotoxicity. Polatuzumab alone or in combination with anti-CD20 antibody had a modest response with CR ranging from 0-13% [17-18]. The combination of Polatuzumab, Bendamustine and Rituximab was approved by FDA for patients with relapsed DLBCL after failure of at least 2 lines of therapy. In phase II randomized study comparing Pola BR to BR alone. The median age of patient on Pola BR arm was 67, 25% had prior stem cell transplant and 75% were refractory to prior line of therapy. ORR was 63% (25 of 40 patients) with CR rate of 40%. 48% of patients remained in CR at 1 year. Common side effects included fatigue, infusion reaction, neuropathy, and pancytopenia and increase risk of infection [19].
Tafasitamab
Tafasitamab (Tafa) is a Fc-enhanced, humanized, anti-CD19 monoclonal antibody. its FC portion is enhanced by modulating two amino acids that leads to increase affinity to Fcy receptors. As single agent Tafa has a response in DLBCL of 26% with responses lasting >12 months [20]. Phase II L-MIND study studied the combination of Tafa with lenalidomide as previous in vitro studies showed synergetic activity [21]. The study showed impressive CR rate of 43% with median duration of response of about 34 months. To compare the data from Pola-BR and Tafa-Lenalidomide combinations refer to (Table 2).
Table 2: comparsion between L-MIND trial (Tafa with lenalidomide) compared with Polatuzumab with Bendamustine and Rituximab).
Selinexor
Selinexor is an XPO1(exportin 1) inhibitor. XPO1 is responsible for removal of multiple tumor suppressor genes out of the nucleus like P53, P73 and IkBk outside nucleus and inhabiting their function. The FDA had approved Selinexor as a single agent for the treatment of relapsed or refractory DLBCL after at least 2 lines of therapy. The approval was based on SADAL study, which was a Phase II study that included 134 patients, 45% were 70 years or older, 4% with DHL,41% had more than 3 lines of previous therapy with 72% refractory to the last line, 13% had elevated LDH. Single agent Selinexor resulted in ORR of 29%(95%CI:22,38) with CR rate of 13% with response duration of 6 months. Most common adverse reaction was fatigue, gastrointestinal side effects and pancytopenia [22]. Preclinical data suggest that the inhibition of XPO1 can provide a therapeutic target for DHL [23-24].
Bispecific T cell engager
Bispecific T-Cell engager (BiTe) therapy provide a promising off the shelve immune therapy for multiple cancers including NHL. In the contrary to CAR-T cell therapy, BiTe does not require manufacturing time or pre-infusion conditioning chemotherapy. Side effect can be similar but potentially less severe than CAR-T including CRS and neurological toxicity [25]. At this time BiTE therapy is not FDA approved to treat NHL but multiple trials across the world are testing different BiTe as single agent or with different combination including combining BiTe with Polatuzumab, with lenalidomide or other combinations. Recently published phase I/ II trial with Glofitamab, Bivalent CD20-target T-cell engaging BiTe. The study included 171 patients, 90% refectory to prior lines of therapy with median of 3 previous lines of therapy, 74% had DLBCL. ORR was in the phase II dose was 65.7% with CR of 57.1%. 84% of Cr patients had a maximum of 27.4 duration of response. Grade ¾ CRS was seen in 3.4% and grade 3 CNS toxicity in 1.2% [26]. Other promising BiTes including Blinatumomab, Epicoritamab, Monsunetuzumab and others [27-30].
Immune-Checkpoint Inhibitors in lymphoma
Immune check point inhibitors including programmed cell death /ligand inhibitors PD1 and PD-L1 inhibitors and CTL4 inhibitors have so far limited role in the therapy of DLBCL in the absence of biomarker targeted therapy. Future use may include pretesting for PD1 expression or the use of checkpoint inhibitors with combination [31].
CD47 Inhibitors
CD47 is present in virtually all cancer cells and it over expression is associated with poor prognosis. It is an inhibitory signal to macrophages (Do not Eat me signal) and inhabitation of CD47 lead to potentially increasing macrophage activation and tumor destruction. In addition, macrophages will present more tumor antigen and increase T-cell mediated cytotoxicity [32]. In Phase I/II trial combining CD47 inhibitor 5F9 with rituximab, the study included 22 patients (15 had DLBCL). Among patients with DLBCL, objective response rate was 40% with CR rate of 33%. Most common side effects were anemia and infusion reaction [33].
Conclusion and opinion
The treatment of relapsed DLBCL remains a challenge. With the approval of now three CAR-T cell therapy product we do have a chance of curing at least a third of those patients. Other may not benefit or may not have access to CAR-T cell therapy. Recently approved regimens that provide a good promise are the Tafa and lenalidomide combination and polatuzumab with Rituximab and Bendamustine combination. Although we have some patients with prolonged responses these combination does not provide a cure as of now. And most patients eventually succumb to their disease. With sequencing different combination and possibly prolonged maintenance therapy as, possible with the use of BiTE or other targeted agents we may see in the future an improved survival of relapsed refractory lymphoma.
References
- NCIhscgshnhA, 2021.
- Teras LR, DeSantis CE, Cerhan JR, Lindsay MM, Ahmedin J, et al. (2016) US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 66: 443-459.
- Rosenwald A, Wright G, Chan WC, Joseph MC, Elias C, et al. (2002) The Use of Molecular Profiling to Predict Survival after Chemotherapy for Diffuse Large-B-Cell Lymphoma. New England Journal of Medicine 346: 1937-1947.
- Landsburg DJ, Falkiewicz MK, Maly J, Kristie AB, Christina H, et al. (2017) Outcomes of Patients with Double-Hit Lymphoma Who Achieve First Complete Remission. J Clin Oncol 35: 2260-2267.
- Ruppert AS, Dixon JG, Salles G, Anna W, David C, et al. (2020) International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood 135: 2041-2048.
- Le Gouill S, Casasnovas RO (2017) Interim PET-driven strategy in de novo diffuse large B-cell lymphoma: do we trust the driver? Blood 129: 3059-3070.
- Younes A, Sehn LH, Johnson P, Pier Luigi Z, Xiaonan H, et al. (2019) Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J Clin Oncol 37: 1285-1295.
- Nowakowski GS, Chiappella A, Gascoyne RD, David WS, Qingyuan Z, et al. (2021) ROBUST: A Phase III Study of Lenalidomide Plus R-CHOP Versus Placebo Plus R-CHOP in Previously Untreated Patients With ABC-Type Diffuse Large B-Cell Lymphoma. Journal of Clinical Oncology 39: 1317-1328.
- Bartlett NL, Wilson WH, Jung SH, Eric DH, Matthew JM, et al. (2019) Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol 37: 1790-1799.
- Friedberg JW (2017) How I treat double-hit lymphoma. Blood 130(5): 590-596.
- Maurer MJ, Ghesquières H, Jais JP, Thomas EW, Corinne H, et al. (2014) Event-Free Survival at 24 Months Is a Robust End Point for Disease-Related Outcome in Diffuse Large B-Cell Lymphoma Treated with Immunochemotherapy. Journal of Clinical Oncology 32: 1066-1073.
- Crump M, Neelapu SS, Farooq U, Eric Van DN, John K, et al. (2017) Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 130: 1800-1808.
- Crump M, Kuruvilla J, Couban S, David AM, Vishal K, et al. (2014) Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol 32: 3490-3496.
- Neelapu SS, Locke FL, Bartlett NL, Lazaros JL, David BM, et al. (2017) Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New England Journal of Medicine 377: 2531-2544.
- Schuster SJ, Bishop MR, Tam CS, Edmund KW, Peter B, et al. (2018) Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New England Journal of Medicine 380: 45-56.
- Abramson JS, Palomba ML, Gordon LI, Matthew AL, Michael W, et al. (2020) Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet 396: 839-852.
- Palanca WMC, Czuczman M, Salles G, Sarit A, Laurie HS, et al. (2015) Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol 16(6): 704-715.
- Morschhauser F, Flinn IW, Advani R, Laurie HS, Catherine D, et al. (2019) Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol 6: e254-e265.
- Sehn LH, Herrera AF, Flowers CR, Manali KK, Andrew MM, et al. (2020) Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 38 p. :155-165.
- Jurczak W, Zinzani PL, Gaidano G, A Goy, M Provencio, et al. (2018) Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin's lymphoma. Ann Oncol 29: 1266-1272.
- Salles G, Duell J, Eva González B, Olivier T, Wojciech J, et al. (2020) Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncology 21 p. : 978-988.
- Kalakonda N, Maerevoet M, Cavallo F, George F, Andre G, et al. (2020) Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 7(7): e511-e522.
- Liu Y, Azizian NG, Dou Y, Lan VP, Yulin L, et al. (2019) Simultaneous targeting of XPO1 and BCL2 as an effective treatment strategy for double-hit lymphoma. J Hematol Oncol 12(1): 119.
- Deng M, Zhang M, Xu-Monette ZY, Lan VP, Alexandar T, et al. (2020) XPO1 expression worsens the prognosis of unfavorable DLBCL that can be effectively targeted by selinexor in the absence of mutant p53. J Hematol Oncol 13: 148.
- Doraiswamy A, Shah MR, Bannerji R (2021) Immunotherapies Old and New: Hematopoietic Stem Cell Transplant, Chimeric Antigen Receptor T Cells, and Bispecific Antibodies for the Treatment of Relapsed/Refractory Diffuse Large B Cell Lymphoma. Curr Hematol Malig Rep 16(1): 72-81.
- Hutchings M, Morschhauser F, Iacoboni G (2021) Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J Clin Oncol Jco2003175.
- Viardot A, Hess G, Bargou RC, Nicholas JM, Giuseppe G, et al. (2020) Durability of complete response after blinatumomab therapy for relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma 61(11): 2767-2770.
- van der Horst HJ, de Jonge AV, Hiemstra IH, Anne TG, Daniella RB, et al. (2021) Epcoritamab induces potent anti-tumor activity against malignant B-cells from patients with DLBCL, FL and MCL, irrespective of prior CD20 monoclonal antibody treatment. Blood Cancer J 11: 38.
- Schuster SJ, Bartlett NL, Assouline S, Sung SY, Francesc B, et al. (2019) Mosunetuzumab Induces Complete Remissions in Poor Prognosis Non-Hodgkin Lymphoma Patients, Including Those Who Are Resistant to or Relapsing After Chimeric Antigen Receptor T-Cell (CAR-T) Therapies, and Is Active in Treatment through Multiple Lines. Blood 134: 6-6.
- Harris LJ, Patel K, Martin M (2020) Novel Therapies for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Int J Mol Sci 21(22): 8553.
- Kuzume A, Chi S, Yamauchi N, et al. (2020) Immune-Checkpoint Blockade Therapy in Lymphoma. International journal of molecular sciences 21: 5456.
- Tseng D, Volkmer JP, Willingham SB, Humberto CT, John WF, et al. (2013) Anti-CD47 antibody–mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proceedings of the National Academy of Sciences 110: 11103.
- Advani R, Flinn I, Popplewell L, Andres FM, Nancy LB, et al. (2018) CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. New England Journal of Medicine 379 p. : 1711-1721.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




