Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Case Report(ISSN: 2638-5945) 
A Novel Vascular Endothelial Growth Factor trap KP-VR2 with Enhanced Ligand Blocking Volume 4 - Issue 4
VM.Molina*1, J. Morales, MF1, Gutierrez2,3
- 1Boehringer Ingelheim, Pet Technical Service, Colombia
- 2Clinic veterinary Campomaskotas. Colombia
- 3University of La Salle, Colombia
Received:March 03, 2021 Published: April 26, 2021
Corresponding author: VM. Molina, Boehringer Ingelheim, Pet Technical Service, Colombia
DOI: 10.32474/OAJOM.2021.04.000191
Abstract
Antiangiogenic therapies targeting vascular endothelial growth factor (VEGF)-A have been commonly used to treat various cancers; however, their clinical efficacy remains limited due to resistance and activation of compensatory pathways resulting from elevated circulating VEGF-B and placental growth factor (PlGF) levels. Thus, we developed a novel VEGF-Trap, KP-VR2, which can neutralize VEGF-A, VEGF-B, and PlGF to mediate these problems. KP-VR2 consists of two consecutive second Ig-like domains (D2s) of VEGF receptor 1 (VEGFR-1) fused to human IgG1 Fc. KP-VR2 showed more potent decoy activity than the current VEGF-Trap against VEGF and PlGF. Most importantly, two consecutive D2s of VEGFR-1 can generate two putative binding sites, resulting in a significant improvement in binding capacity. These advances resulted in stronger antitumor efficacy in implanted tumor models than aflibercept and bevacizumab. Overall, the results of this study highlight KP-VR2 as a promising therapeutic candidate for further clinical drug development.
Introduction
Angiogenesis is defined as the neoformation of blood vessels through a multistep mechanism that provides nutrients and oxygen to tissues, allowing the discharge of waste products [1]. This process is involved in wound healing, embryogenesis, and inflammation, but is also crucial for pathological conditions like cancer [2]. The vascular endothelial growth factor (VEGF) is the most important growth factor to angiogenesis [3] and is most important and widely studied proangiogenic factor family comprising of five growth factors namely VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF) [4-6]. VEGFs are involved in angiogenesis by binding to three different receptors located on the cell membrane: VEGFR-1 (Flt-1), VEGFR-2 (Flk/KDR), and VEGFR-3 (Flt-4) [7]. VEGF-A is the most important regulator in human physiological and pathological angiogenesis, and it is associated with poor prognosis in several cancers [8]. VEGF-A may interact with both VEGFR-1 and VEGFR-2, but since the kinase activity of VEGFR-1 is limited, VEGFR-2 is considered the most important effector of VEGF-A downstream signaling [9]. VEGF-B, which shares a structural homology with VEGF-A and whose activity is mediated by interaction with VEGFR-1, plays a role in tumorigenesis and blood vessel survival under stress conditions [10]. VEGF-C and VEGF-D, which bind VEGFR-3, are involved in lymphangiogenesis [11]. PlGF is also important growth factor that regulates vessel growth and maturation by affecting endothelial cells and regulates these by recruiting proangiogenic cell types. PlGF shares structural homology with VEGF-A and stimulates angiogenesis through interaction with VEGFR-1 [12-15]. VEGF-A binds to both VEGFR-1 and VEGFR-2, whereas PlGF binds to VEGFR-1 but not to VEGFR-2 [7]. Aflibercept, also known as ziv-aflibercept or VEGF-Trap (Eylea® and Zaltrap®, Regeneron Pharmaceuticals, Tarrytown, NY, USA and Sanofi-Aventis, Bridgewater, NJ, USA, respectively), has been approved by the US Food and Drug Administration (FDA) for the treatment of macular degeneration and metastatic colorectal cancer [16]. Aflibercept is a recombinant fusion protein, which consists of the second immunoglobulin (Ig) domain of VEGFR-1 and the third Ig domain of VEGFR-2 fused to human IgG1 Fc. It exhibits affinity for VEGF-A, VEGF-B, and PlGF [3, 17]. The VEGF-Trap bevacizumab (Genentech, Inc., San Francisco, CA, USA) is a VEGF blocking monoclonal antibody and has two ligand binding sites. However, bevacizumab binds VEGF-A but not VEGF-B or PlGF. To address the limitations of the current VEGF-Trap, we designed a new structure of VEGF-Trap KP-VR2 to consist of two consecutive D2s of VEGFR-1 fused to the Fc region of human IgG1. Considering the main role of VEGFR-1 D2 and the minor role of VEGFR-2 D3 in the binding of VEGF [18], we generated KP-VR2 by replacing VEGFR-2 D3 with VEGFR-1 D2 in aflibercept. Thus, two consecutive D2s of VEGFR-1 create two putative ligand binding sites, resulting in improved decoy efficiency compared to aflibercept with one binding site. In addition, KP-VR2 exhibits a high affinity for PlGF, to which bevacizumab cannot bind. This improvement was demonstrated by binding enzyme-linked immunosorbent assay (ELISA), human umbilical vein endothelial cells (HUVECs) migration inhibition, and tumor xenograft models.
Results
Construction and Expression of KP-VR2
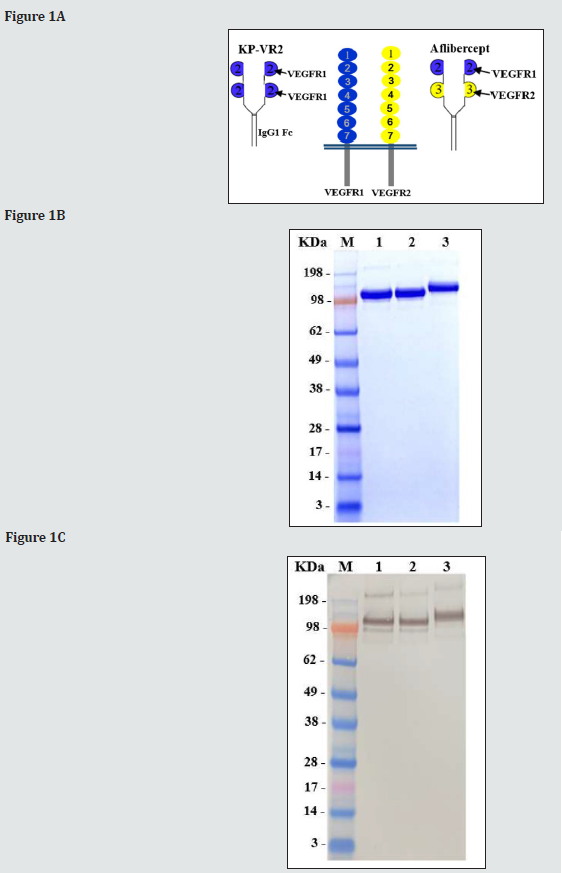
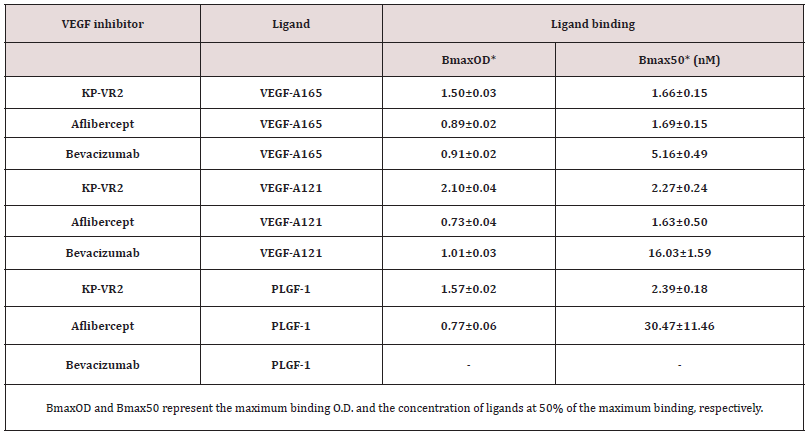
We designed a novel VEGF decoy receptor fusion protein that consists of the two consecutive D2s of VEGFR-1 to create the putative two binding sites for ligands, resulting in greatly improved decoy efficiency (Figure 1A). The KP-VR2 was produced from CHO cells and purified by protein A affinity chromatography with two additional steps of ion exchange chromatography. The purified KP-VR2 showed ~120 kDa band in a non-reduced SDS-PAGE (Fig. 1B). In western blotting, using an antibody specific to Fc (Fig. 1C), specific bands could be observed for each specified protein at 115 kDa (lane 1, aflibercept Eylea®), 115 kDa (lane 2, aflibercept Zaltrap®), and 120 kDa (lane 3, KP-VR2). Schematic diagram of KP-VR2 and aflibercept. KP-VR2 is composed of VEGFR1 D2-D2-Fc fusion (A). SDS-PAGE analysis of aflibercept and KP-VR2 in nonreduced conditions (B). Western blot analysis of aflibercept and KP-VR2 in non-reduced conditions (C). Lanes 1, 2, and 3 represent aflibercept Eylea®, aflibercept Zaltrap®, and KP-VR2, respectively. Western blot analysis of KP-VR2 and aflibercept was resolved on 4-12% gel under non-reducing conditions, transferred to a polyvinylidene difluoride membrane, and probed with Fc-specific antibody. For comparison, protein MW size marker is shown.
Comparison of the Binding Ability of KP-VR2, Aflibercept and Bevacizumab
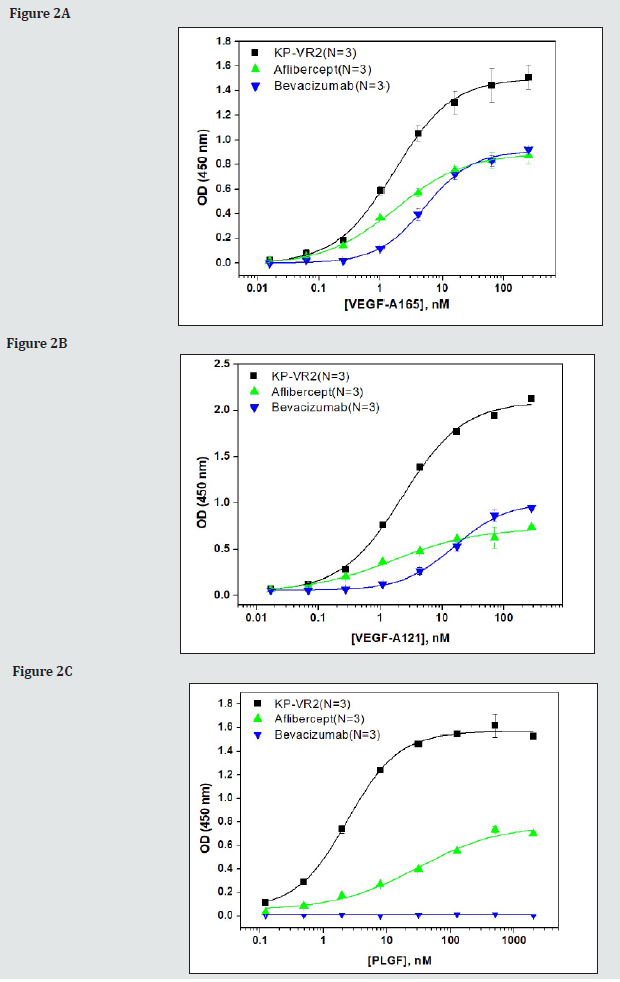
We compared KP-VR2 to aflibercept and bevacizumab to analyze its ability to bind their ligands in vitro. To determine the binding of KP-VR2, aflibercept, and bevacizumab for their ligands, such as VEGF-A165, VEGF-A121, and PLGF-1, sandwich ELISA assays were performed in which an increasing amount of VEGF-A165 and VEGF-A121 (from 0.0625 to 256 nM), and PLGF-1 (from 0.122 to 2,000 nM) were reacted to immobilized KP-VR2, aflibercept, and bevacizumab. The binding was measured through incubation with Goat anti-Human VEGF antibody and then with peroxidaseconjugated anti-Goat Ig antibody.
The concentration (nM) of 50% of the maximum binding (Bmax50) of KP-VR2, aflibercept, and bevacizumab for VEGF-A165 was 1.66 nM, 1.69 nM, and 5.16 nM, respectively (Figure 2A and Table 1). The maximum binding optical density (BmaxO.D.) of KP-VR2, aflibercept, and bevacizumab for VEGF-A165 was O.D. 1.50, 0.89, and 0.91, respectively (Figure 2A and Table 1). Binding abilities of KP-VR2, aflibercept, and bevacizumab to VEGF-A165 (A), VEGF-A121 (B), and PlGF-1 (C) are presented. An increasing amount of VEGF-A165 and VEGF-A121 (from 0.0625 to 256 nM), and PLGF-1 (from 0.1 to 2,000 nM) were reacted to immobilized KP-VR2, aflibercept, and bevacizumab. The binding was measured using Goat anti-Human VEGF antibody and then with peroxidaseconjugated anti-Goat Ig antibody. For each group, n = 3. Values are presented as mean ± SD. BmaxO.D. and Bmax50 represent the maximum binding O.D. and the concentration of ligands at 50% of the maximum binding, respectively. The Bmax50 of KP-VR2, aflibercept, and bevacizumab for VEGF-A121 was 2.27 nM, 1.63 nM, and 16.03 nM, respectively (Figure 2B and Table 1). The BmaxO.D of KP-VR2, aflibercept, and bevacizumab for VEGF-A121 was O.D 2.10, 0.73, and 1.01, respectively (Figure 2B and Table 1). The Bmax50 of KP-VR2 and aflibercept for PlGF-1 was 2.39 nM and 30.47 nM, respectively (Figure 2C and Table 1). The BmaxO.D of KP-VR2 and aflibercept for PlGF-1 was O.D 1.57 and 0.77, respectively (Figure 2C and Table 1). No binding and bevacizumab binding was detected for PlGF-1 and bevacizumab.
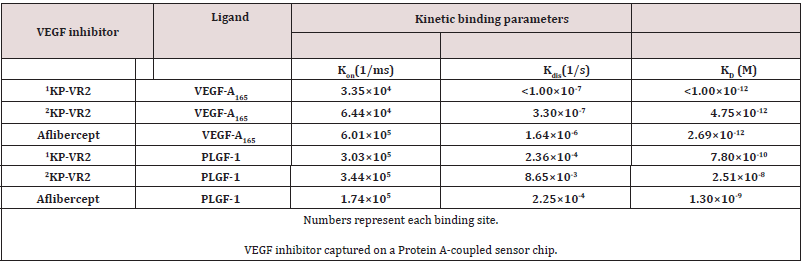
Comparison of Affinity of KP-VR2 and Aflibercept for VEGF A165 and PLGF 1 by Octet
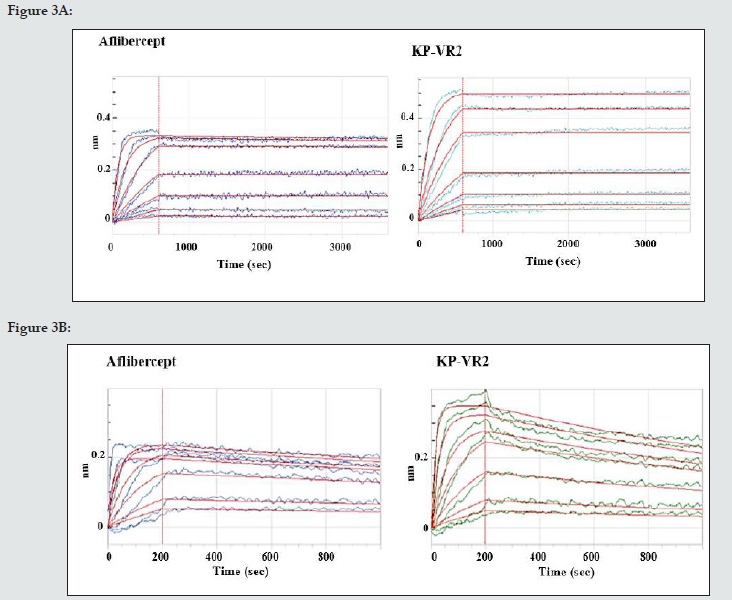
BLI analysis was performed to assess the binding kinetics of KP-VR2 to VEGF-A and PlGF using a ForteBio Octet System. KP-VR2 and aflibercept were immobilized onto biosensors, which were subsequently dipped into a dilution series of VEGF-A165 and PlGF- 1. The dynamic reactive process between analytes and ligands was automatically recorded by the Octet control software. The curve and results were analyzed according to the 1:1 binding model (aflibercept) and the 1:2 binding model (KP-VR2). The dynamic association and dissociation curves are shown in Figure 3A to 3B. The kinetics and affinity constants between analytes and ligands are listed in Table 2. Notably, the equilibrium dissociation constant (KD) of KP-VR2 for VEGF-A165 (< 1 pM and 4.75 pM) was similar to that of aflibercept (2.69 pM) (Table 2). KP-VR2 and aflibercept also bound human PlGF-1 with a KD of 0.78 nM and 25.1 nM, respectively. Aflibercept showed a binding affinity to PlGF (1.30 nM).
Table 2: Kinetic binding parameters of KP-VR2 and aflibercept to human VEGF family ligands by Octet.
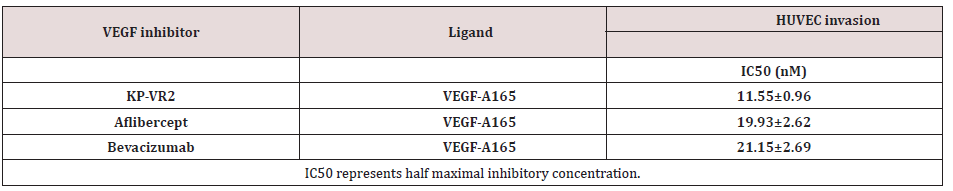
Comparison of KP-VR2, Aflibercept and Bevacizumab in migration inhibition
Figure 4: Inhibition of HUVEC migration by KP-VR2, aflibercept, and bevacizumab. Cell invasion assay with HUVEC in the presence of VEGF-A165 and VEGF-Traps is presented by image (A) and quantification (B). HUVECs were placed in the upper compartment of the Boyden chamber and allowed to migrate toward basal media containing 0.1% fetal bovine serum with or without VEGF-A165 and VEGF-A165 mixed with seven different concentrations of KP-VR2, aflibercept, and bevacizumab ranging from 3.27 to 209.44 nM. The percentage of total migration (y-axis) was calculated as described in the Experimental Procedures section.
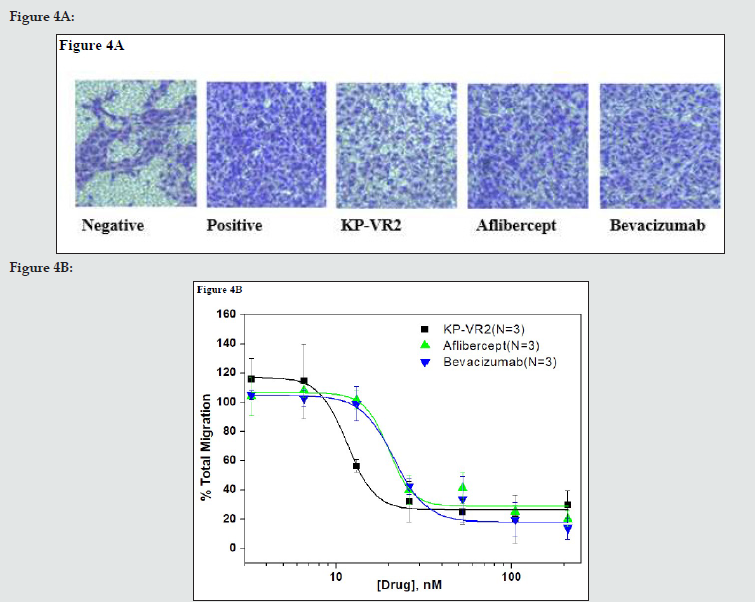
We determined whether KP-VR2 binding to VEGF could effectively block the ability of VEGF-induced endothelial cell migration. The inhibitory effect of KP-VR2 was evaluated by endothelial cell invasion and migration assay. KP-VR2, aflibercept, and bevacizumab could completely block VEGF-A165-induced HUVEC migration at the concentration of 100 ng/mL. KP-VR2 had a lower half maximal inhibitory concentration (IC50) value than aflibercept and bevacizumab (Figure 4B and Table 3) owing to a higher inhibition potency. VEGF induced cell invasion in HUVEC, whereas KP-VR2, aflibercept, and bevacizumab inhibited VEGFinduced cell invasion (Figure 4A). The blue-colored spot indicates VEGF-induced cell migration (Fig. 4A). KP-VR2 showed higher inhibitory activity than aflibercept and bevacizumab at the same concentration (Figure 4B). The IC50 values of KP-VR2, aflibercept, and bevacizumab were 11 nM, 20 nM, and 21 nM, respectively (Table 3), which reveals that the KP-VR2 has outstanding inhibitory activity against cell invasion. Cell invasion assay with HUVEC in the presence of VEGF-A165 and VEGF-Traps is presented by image (A) and quantification (B). HUVECs were placed in the upper compartment of the Boyden chamber and allowed to migrate toward basal media containing 0.1% fetal bovine serum with or without VEGF-A165 and VEGF-A165 mixed with four different concentrations of KP-VR2, aflibercept, and bevacizumab ranging from 3.27 to 209.44 nM. The percentage of total migration (y-axis) was calculated as described in the Experimental Procedures section.
Comparison of tumor growth inhibition by KP-VR2 and Aflibercept
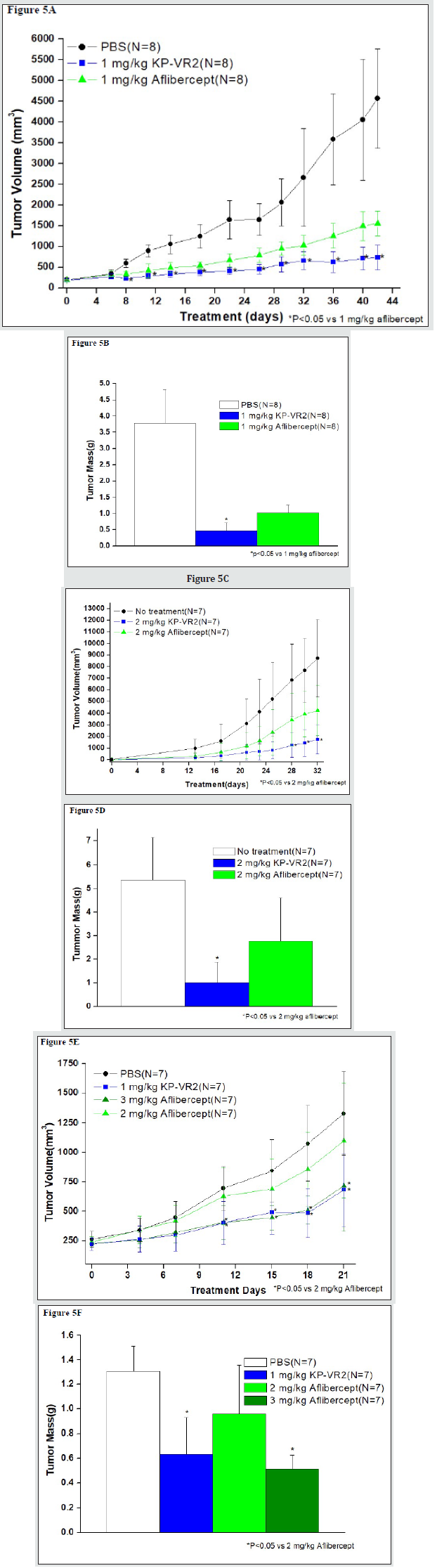
The inhibition of tumor growth by KP-VR2 and aflibercept was evaluated in a mouse xenograft model. Tumor cells were derived from diverse tissue origins (human LOVO colorectal adenocarcinoma cells, human SKUT-1B mesodermal tumor, and human HT-29 colorectal adenocarcinoma cells). KP-VR2 significantly inhibited the growth of all three types of tumors (Figure 5). To evaluate the antitumor effects of KP-VR2, we used the LOVO tumor model and treated them with either KP-VR2 or aflibercept at 1 mg/kg. KP-VR2 treatment resulted in 84% and 88% reduction in tumor volume and weight, whereas aflibercept treatment caused a reduction of 66% and 73%, respectively (Figure 5A and 5B). We also assessed the antitumor effect of KP-VR2 on established SKUT-1B tumors. These results showed 80% and 51% reduction in tumor volume after 2 mg/kg KP-VR2 and aflibercept treatment, respectively (Fig. 5C and 5D), implying that KP-VR2 is a promising agent against tumors. In the study using HT-29 colorectal adenocarcinoma cells, a 3-fold lower dose KP-VR2 (1 mg/kg) was tested and found to be equally effective at inhibiting tumor growth and weight (Figure 5E and 5F). Taken together, these results suggest that the improved avidity of KP-VR2 to VEGF-A and PlGF contributes to its higher efficacy in cancer treatment compared to aflibercept.
Figure 5: Inhibition of tumor growth in LOVO, SKUT-1B, and HT-29 tumors by KP-VR2 and aflibercept. Tumor growth inhibition induced by KP-VR2 in LOVO, SKUT-1B, and HT-29 models was compared with that of aflibercept. Tumor growth curves were plotted for KP-VR2, aflibercept, and control groups. Tumor volume (A) and mass (B) in LOVO tumor model. Tumor volume (C) and mass (D) in SKUT-1B tumor model. Tumor volume (E) and mass (F) in HT-29 tumor model. *P < 0.05; values are shown as mean ± SD.
Discussion
Angiogenesis is a viable therapeutic target for pathological conditions, including cancer and age-related macular degeneration. The development and investigation of bevacizumab and aflibercept as therapeutic agents has provided a basis for understanding the clinical potential of biological therapies that target angiogenesis. The validation of VEGF as an important new target against cancer has been suggested by previous clinical studies using bevacizumab, which binds and blocks VEGF. Since anti-VEGF agents block the tumor-associated angiogenesis, essential for several different types of tumors, these approaches are generally efficacious against various cancers [22]. In addition, pathological angiogenesis may contribute to several diseases, such as age-related macular degeneration (AMD) and diabetic retinopathy [23] and psoriasis [24], extending the potential utility of anti-VEGF therapeutics. This highlights the need to optimize anti-VEGF approaches. KP-VR2 (Figure 1A), an Fc fusion protein of two consecutive D2s from VEGFR-1, was designed based on previously published report [25], in which the critical role of VEGFR-1 domain 2 has high affinity binding. VEGFR-1 contains seven Ig-like domains in the extracellular domain (Figure 1A). Among them, VEGFR-1 Domain2 is the main contributor to VEGF-A and PlGF binding. Additionally, residues in VEGFR-1 Domain3 are also involve in the high-affinity binding of VEGF-A and PlGF [26, 27]. Thus, the optimal required domain to bind both VEGF-A and PlGF with good affinity is VEGFR-1 D2-D3.
Although VEGFR-1 binds to VEGF-A and PlGF with higher affinity than VEGFR-2, the development of therapeutic decoy proteins with the VEGFR-1 backbone has proven to be difficult thus far. The major reason behind this is the high pI value of VEGFR-1 owing to the positively charged residues in the VEGFR-1 D3 region, which causes nonspecific extracellular matrix binding and poor pharmacokinetic profiles, leading to a shortened half-life [25]. We assume that aflibercept employed VEGFR-2 D2 instead of VEGFR-1 D2 due to the intrinsic problem of VEGFR-1 D2. However, previous structural analyses have indicated that VEGFR-1 might make greater use of its D2 in contacting VEGF [28]. Therefore, to rule out VEGFR-1 D3, we generated KP-VR2 consisting of two consecutive D2s of VEGFR-1, demonstrating improved decoy efficiency and dramatic increased avidity for both VEGF and PlGF. Creating a new decoy receptor fusion protein with an additional binding site has great advantages. Most importantly, the two consecutive D2s of VEGFR-1 mainly consisted of two putative binding sites for ligands, resulting in greatly improved decoy efficiency, as shown in Figure 2. The combination of high-affinity and improved avidity contributes to making KP-VR2 one of the most potent and efficacious VEGF blockers available. KP-VR2 represented more potent inhibitory activity against VEGF-A and PlGF than aflibercept. In addition, KP-VR2 showed more potent decoy activity against VEGF-A than bevacizumab, but bevacizumab was unable to bind PlGF. This was proved by our in vitro experiments showing the strong suppression of proliferation and migration after KP-VR2 treatment. Consistent results were observed in vivo, where KP-VR2 showed much stronger antitumor effects in implanted tumor models when judged against aflibercept and bevacizumab.
When equimolar doses of KP-VR2 and aflibercept were compared in the LOVO, SKUT-1B, and HT-29 xenograft models, we found that much higher doses of aflibercept were required to inhibit tumor growth (Figure 5). When used at the same dose, KPVR2 showed better efficacy than aflibercept. Furthermore, KP-VR2 and aflibercept have similar circulation times in mice and rats (data not shown). Considering that the HT-29 tumor models are resistant to anti-VEGF therapy [27], our results suggest the possibility of overcoming resistance to anti-VEGF therapy by blockade of PlGF with KP-VR2. An additional advantage is that KP-VR2 is composed entirely of human sequences, minimizing the possibility of immunogenicity in human patients. Additionally, KP-R2 bound all species of VEGF tested, such as human, rabbit, rat, and mouse VEGF (data not shown), rendering it a useful reagent that can be used in any experimental animal model.
Presently, anti-VEGF drugs are approved for clinical use for the treatment of various tumors [22]. FDA approved aflibercept, in combination with leucovorin, 5-fluorouracil, and irinotecan (FOLFIRI), for treating colorectal cancer patients after progression with oxaliplatin regimens [2, 16]. This soluble decoy receptor shows one-to-one high-affinity binding to all isoforms of VEGF and PLGF [18, 29]. Aflibercept is also FDA approved for the treatment of age-related macular degeneration (AMD) and diabetic retinopathy, showing better efficiency than a single monotherapy for the inhibition of VEGF-A [24].
In this study, KP-VR2 was designed to bind and sequester VEGF as well as PlGF with high affinity and avidity. Like aflibercept, KP-VR2 can be used to treat various tumors and angiogenic ocular diseases, including AMD, and will be evaluated in future clinical trials. Our results suggest that KP-VR2 is a potent and effective inhibitor for both VEGF and PlGF. Through the enhanced ligand binding avidity, KP-VR2 inhibited tumor growth and cell migration more effectively than did current VEGF-Traps. The clinical availability of this novel fusion protein should be further explored through preclinical and clinical studies.
Experimental Procedures
Reagents and Materials
Dulbecco’s modified Eagle medium, fetal bovine serum (FBS), RPMI-1640, and trypsin-EDTA were purchased from Gibco (Gaithersburg, MD, USA). F12K and McCoy’s 5A were purchased from ATCC (Rockville, MD, USA). Monoclonal antibodies against VEGFR-2, Erk1/2 phospho-VEGFR-2 (Tyr1175), and phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-human IgG-peroxidase antibodies were purchased from KPL (Gaithersburg, MD, USA). Human VEGF-A165, human VEGF-A121, and human PlGF-1 were purchased from R&D Systems (Minneapolis, MN, USA). Aflibercept (Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA) and bevacizumab (Genentech, Inc., San Francisco, CA, USA) were purchased.
Cell Lines
We used the following cells in the study: HUVECs (C-2519. Lonza, Walkersville, MD, USA), Chinese hamster ovary suspended cells (CHO-S; A1369601, Gibco, Gaithersburg, MD, USA), LOVO (CCL-229, ATCC), SKUT-1B (HIB-115, ATCC), and HT-29 (HIB-38, ATCC).
Cell Culture
HUVECs were cultured in EGM-2 medium supplemented with 2% FBS, hydrocortisone, hFGFB, VEGF, R3-IGF, ascorbic acid, hEGF, GA-1000, and heparin. These cells were used to evaluate the effects of KP-VR2 on VEGFR-2. LOVO, SKUT-1B, and HT-29 cells were grown in F12K, Eagle’s Minimum Essential Medium, and McCoy’s 5A or F12K with 10% FBS, respectively. These cells were allowed to grow to a confluence of approximately 80% and were subcultured 2 or 3 times a week.
Construction and Expression of KP-VR2
KP-VR2 was created by fusing two consecutive second Ig domains of VEGFR-1 to Fc of human IgG1 (D2-D2-Fc). The KPVR2 expression construct was transfected into CHO-S cells using Lipofectamine 2000 (Life Technologies) and cultured as previously reported [19]. KP-VR2 was secreted into the culture media and purified by protein A-Sepharose affinity chromatography, followed by two different cation and anion exchange chromatography runs. The purified KP-VR2 was analyzed using non-reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting. SDS-PAGE analysis of aflibercept and KP-VR2 was resolved on 4-12% gel under non-reducing conditions. In the western blot analysis, the KP-VR2 and aflibercept were resolved on 4-12% gel under non-reducing conditions, transferred to a polyvinylidene difluoride membrane, and the bands were probed with human Fc-specific antibody (KPL, Gaithersburg, MD, USA) and horseradish peroxidase-conjugated Goat anti-Human IgG (Seracare, Milford, MA, USA) and a DAB substrate kit (VECTOR Lab, Burlingame, CA, USA).
Analysis of Binding of KP-VR2, Aflibercept, and Bevacizumab
The binding abilities of KP-VR2, aflibercept, and bevacizumab were measured using ELISA. For this, a 96-well plate was coated with 1-3 nM of KP-VR2, aflibercept, and bevacizumab and blocked with 0.3% bovine serum albumin (BSA) in PBST (Phosphate- Buffered Saline Tween-20). Then, VEGF-A165 and VEGF-A121 were added in gradually increasing amounts from 0.0625 nM to 256 nM, and PLGF-1 from 0.1 to 2000 nM. Next, the plate was washed and reacted with the Goat anti-Human VEGF antibody. Then, the plate was washed again and reacted with peroxidase-conjugated anti-Goat Ig antibody. Next, 3,3’,5,5’-tetramethylbenzidine (TMB) solution (Bethyl Laboratories, Montgomery, TX, USA) was added, and, thereafter, absorbance was measured at 450 nm. The data were analyzed using a four-parameter logistic equation (Origin v7).
Biolayer Interferometry (BLI) Analysis
Binding kinetics were measured on the Octet QK384 System (ForteBio, Pall Life Science, Fremont, CA, USA), which is based on BLI technology. KP-VR2 and aflibercept were immobilized onto anti-Human Fc biosensors (Pall ForteBio) at a concentration of 20 nM for 600 s, followed by biosensor rinsing in a kinetics buffer that served as a background buffer (KB buffer, Pall ForteBio). After they were balanced to the baseline, the biosensors were dipped into serial dilutions of VEGF and PlGF. The association was measured by the incubation of biosensors in various concentrations (0.3125- 20 nM) of VEGF and PlGF for 600 s. Subsequently, dissociation was performed for 3,000 s. The analyses of binding kinetics were evaluated by ForteBio data analysis software using curve fit models of 1:1 binding and 2:1 heterogeneous binding.
Cell Invasion Assay
The HUVEC invasion assay was performed in vitro using a Transwell chamber system with 8.0-μm pore polycarbonate filter inserts (Corning Costar Corporation, Cambridge, MA, USA). Briefly, the 8-μm pore inserts were coated with 5 μg/mL collagen (Collagen Type I, Rat Tail, Upstate). HUVECs (7.5× 104cells/300 μL) were seeded to the upper wells in EBM-2 medium with 0.1% FBS. EBM- 2 medium (750 μL), with 0.1% FBS in the presence of VEGF-A165 (350 ng/mL) and KP-VR2, aflibercept, or bevacizumab (0-200 nM), was placed in the lower wells. The cells were incubated at 37 °C for 48 h. After 48 h, the upper collagen-coated surface was wiped off using a cotton swab. Cells that migrated through the filters were fixed, stained with crystal violet, photographed, and counted. The migrated cells were determined using a multi-detection microplate reader at an excitation of 458 nm and emission of 528 nm (BioTek Instrument, Inc., Winooski, VT, USA). The percentage of total migration (y-axis) was calculated as (FDrug - FBasal)/ (FTotal - FBasal) where FTotal is fluorescence in the presence of VEGF-A165, FBasal is fluorescence in the absence of VEGF- A165, and FDrug is fluorescence in the presence of VEGF- A165 mixed with drug at a specific molar ratio (x-axis) [20]. The values were analyzed using a four-parameter logistic equation (Origin v7).
Tumor Growth Inhibition Experiments
Tumor cells (5.0 × 106 cells/mouse) were suspended in PBS and implanted subcutaneously on the shaved right flank of 8-10-weekold male BALB/c mice (Orient Bio Inc.). Tumor-bearing mice were randomized into control and treatment groups (n=7-8 per group). Then, the mice received an intraperitoneal injection of PBS, KP-VR2, or aflibercept twice weekly during the experiment. The size of the tumor was measured with calipers (tumor volume = length × width × height). For LOVO and HT-29 models, eight mice bearing tumors of 150-300 mm3 were injected intraperitoneally twice a week with 1 mg/kg KP-VR2, 1 and 3 mg/kg aflibercept, and vehicle, 100 μL of PBS. For the SKUT-1B model, the mice were allowed a brief recovery period (one day) and were then injected intraperitoneally twice a week with 2 mg/kg KP-VR2 and aflibercept until the end of the experiment, after which the animals were euthanized and tumors were excised and measured.
Statistical Analyses
Statistical differences between means were determined by an independent samples t test. The statistical significance was set at P < 0.05.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Acknowledgements
Funding: This work was supported by a grant from the Korea Health Technology R&D Project through the KoreaHealth Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI18C1951). The funding organizations had no role in the design or conduct of this research. They provided unrestricted grants. The authors declare that they have no conflict of interest.
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Folkman J, Merler E, Abernathy C, Williams G (1971) Isolation of a tumor factor responsible for angiogenesis. J Exp Med 133(2): 275-288.
- Giordano G, Febbraro A, Venditti M, Campidoglio S, Olivieri, et al. (2014) Targeting angiogenesis and tumor microenvironment in metastatic. colorectal cancer: Role of aflibercept. Gastroenterol Res Pract pp. 526178.
- Saif MW (2013) Anti-VEGF agents in metastatic colorectal cancer (mCRC): are they all alike? Cancer Manag Res 5: 103-115.
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13(1): 9-22.
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, et al. (2000) Vascular-specific growth factors and blood vessel formation. Nature 407(6801): 242-248.
- Shibuya M (2010) Tyrosine kinase receptor Flt/VEGFR family: its characterization related to angiogenesis and cancer. Genes Cancer 1(11): 1119-1123.
- Prager GW, Poettler M, Unsel M, Zielinski CC (2012) Angiogenesis in cancer. anti-VEGF escape mechanisms. Transl Lung Cancer 1(1): 14–25.
- Claesson-Welsh L, Welsh M (2013) VEGFA and tumour angiogenesis. J Inter Med 273(2): 114-127.
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I,et al. (1996) A novel vascular endothelial growth factor, VEGF-C, is aligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. Embo J 15(2): 290-298.
- Jussila, L, Alitalo K (2002) Vascular growth factors and lymphangiogenesis. Rev 82(3): 673-700.
- McColl BK, Baldwin ME, Roufail S, Freeman C, Moritz RL,et al. (2003) Plasmin activates the lymphangiogenicgrowth factors VEGF-C and VEGF-D. J Exp Med 198(6): 863-868.
- Khaliq A, Li XF, Shams M, Sisi P, Acevedo CA, Whittle,et al. (1996) Localisation of placenta growth factor (PIGF) in human term placenta. Factors 13(3-4): 243-250.
- Vuorela P, Hatva E, Lymboussaki A, Kaipainen A, Joukov V,et al. (1997) Expression of vascular endothelial growth factor andplacenta growth factor in human placenta. Biol Reprod 56(2): 489-494.
- Brave SR, Eberlein C, Shibuya M, Wedge SR, Barry ST (2010) Placental growth factor neutralising antibodies give limited anti-angiogenic effects in an in vitro organotypic angiogenesis model. Angiogenesis 13(4): 337-347.
- Salem ME, El Refai SM (2013) Efficacy and safety of aflibercept and its role in the treatment of metastatic colorectal cancer. Rare Cancers Ther 1 p. : 3-19.
- Semeraro F, Morescalchi F, Duse S, Parmeggiani F, Gambicorti E, et al. (2013) Aflibercept in wet AMD: specific role and optimal use. Drug Des Devel Ther 7: 711-722.
- Koh YJ, Ki, HZ, Hwang SI, Lee JE, Oh N, et al. (2010) Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cell. 18, 171-184.
- Wiesmann C, Fuh G, Christinger HW, Eigenbrot C, Wells JA, et al. (1997) Crystal structure at 1.7 A° resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell 91(5): 695-704.
- Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, et al. (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15(2): 171-185.
- Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, et al. (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 99(17): 11393-11398.
- Anisimov A, Leppanen VM, Tvorogov D, Zarkada G, Jeltsch M, et al. (2013) The basis for the distinct biological activities of vascular endothelial growth factor receptor-1 ligands. Sci Signal 6(282) ra52.
- Lee JE, Kim C, Yang H, Park I, Oh N, et al. (2015) Novel glycosylated VEGF decoy receptor fusion protein, VEGF-Grab, efficiently suppresses tumor angiogenesis and progression. Mol Cancer Ther 14(2): 470-479.
- Yoshida A, Anand Apte B, Zetter BR (1996) Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth Factors 13(1-3): 57-63.
- Mésange P, Poindessous V, Sabbah M, Escargueil AE, de Gramont A, et al. (2014) Intrinsic bevacizumab resistance is associated with prolonged activation of autocrine VEGF signaling and hypoxia tolerance in colorectal cancer cells and can be overcome by nintedanib, a small molecule angiokinase inhibitor. Oncotarget 5(13): 4709-4721.
- Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3(5): 391.
- Adamis AP, Miller JW, Bernal MT, D Amico DJ, Folkman J, et al. (1994) Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 118: 445-450.
- Anisimov A, Leppänen VM, Tvorogov D, Zarkada G, Jeltsch M, et al. (2013) The basis for the distinct biological activities of vascular endothelial growth factor receptor-1 ligands. Sci Signal 6(282): ra52-ra52.
- Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, et al. (1994) Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med 180(3): 1141-1146.
- Stewart MW (2012) Aflibercept (VEGF Trap-eye): the newest anti-VEGF. drug Br J Ophthalmol 96(9): 1157-1158.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...