Lupine Publishers Group
Lupine Publishers
Menu
Review ArticleOpen Access 
The Evolution of Sensing Device for the Detection of Histamine and other Biogenic Amines Volume 2- Issue 1
Badri KH*, Munir MA and Lee YH
- Department of Chemical Sciences, Faculty of Science and Technology, University Kebangsaan Malaysia, Malaysia
Received: August 05,2022 Published: August 23,2022
Corresponding author: Badri KH, Department of Chemical Sciences, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Bangi, Malaysia
DOI: 10.32474/JBRS.2022.01.000126
Abstract
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
This paper reviewed the progressive trend in the sensing of biogenic amine such as histamine. A sensor was developed where this sensor is using palm-based polyurethane as an electro-sensor substrate. Palm based polyurethane (PU) was produced via condensation polymerization between palm kernel oil-based monoester polyol (PKOp)and 4,4’-diphenylmethane diisocyanate (MDI). PU is used in this study due to its porosity and its capability to attach onto screen – printed electrode (SPE) sturdily without disintegrate. PU – SPE absorbed histamine inside its pores so histamine can undergo the oxidation process. The oxidation process was detected using cyclic voltammetry (CV) and differential pulse voltammetry (DPV).
Biogenic Amines
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
Biogenic amines (BA) are basic nitrogenous compounds shaped by decarboxylation of amino acids. Biogenic amines can be present in food and can cause several adverse reactions in the consumers [1-3]. Relocation of the alpha-carboxyl group from a proteinogenous amino acid leads to corresponding biogenic amines such as histamine originates from histidine, cadaverine from lysine, tyramine from tyrosine and so on [4]. All kinds of foodstuffs that contain proteins or free amino acids represent important task to be subjected to conditions enabling microbial or biochemical activity of biogenic amines. The total amount of different amines formed strongly depends on the nature of food and the microorganisms present. Food likely to contain high levels of these compounds are dairy products, fish and fish products, meat and meat products, fermented vegetables and soy products and fermented beverages such as milk, wine and beer. The main biogenic amines encountered in food and beverages are histamine, tyramine, putrescine and cadaverine [5,6]. The factors that influence biogenic amines accumulation in food are distribution and storage conditions, food physicochemical parameters (pH, NaCl and ripening temperature), raw material quality, manufacturing processes, presence of decarboxylase- positive microorganisms and free amino acids [7].
Biogenic amines and polyamines have been reported in variety of food, such as fish, meat, cheese, vegetables and wines. They are also described as organic bases with aliphatic, aromatic and heterocyclic structures [8]. Histamine is the most common biogenic amine and can be found in food or beverages. Histamine [(2-1H-imizazol— yl)ethanamine] is a biogenic amine that can be produced in organisms by decarboxylation of the amino-acid histidine through microbial or enzymatic processes. Histamine is involved in several physiological functions in the central nervous system (CNS) as a neurotransmitter, in sleep-wake regulation and body temperature control, and it affects appetite, mood, endocrinal processes, learning, and memory. It is also implicated in inflammatory and immune responses by increasing the permeability of blood capil-laries. In addition, histamine is partly responsible for gastric acid release, stimulatory effects, erection and sexual functions, schizophrenia and multiple sclerosis. Therefore, it is not surprising that elevated levels of histamine may cause immune system disorders and allergies. One of the major sources of health problems caused by histamine is Scombroid food poisoning that is a common type of seafood poisoning caused by eating spoiled fish, mainly mackerel, tuna, sardines and anchovies [9]. At temperatures above 16oC, the enzyme histidine decarboxylase, produced by enteric bacteria, converts histidine to histamine. Unlike food spoiled by bacteria that can be suitable for consumption after cooking, the histamine content in food is not normally destroyed by cooking. It should be noted that histamine is present in a variety of food like wine cheese, fermented sausages and fish, and most members of the public are not affected by moderate consumption of these products. However, people with histamine allergy may develop symptoms that typically include sweating, burning sensation, dizziness, nausea, headache, tachycardia and other symptoms. The National Institutes of Health (NIH) recently published a popular article about food poisoning by seafood products describing these effects [10].
Histamine has an important role in human metabolism, such as the release of stomach acid. In small dosage it has little effect, but in larger dosage is has toxic effects. The intestinal tract of human contains the enzymes diamine oxidase (DAO) and histamine-N-methyl transferase (HMT) which convert histamine to harmless degradation products. Putrescine and cadaverine can inhibit these enzymic reactions and also have potentiate to increase histamine toxicity. The presence of low levels of histamine, in the diet normally has no toxic effect as humans do not absorb histamine efficiently from gastrointestinal tract. If a high level of histamine is present in the diet, then the capacity of DAO and HMT to detoxify histamine will be limited and histamine will enter into the bloodstream resulting in histamine poisoning [11].
Histamine generally comes from the Scombridae family such as mackerel and tuna, sardine, blue fish and mahi-mahi. Histamine has been connected with Scombroid poisoning in several studies. The time of onset of Scombroid poisoning ranges from several minutes to 3 h after ingestion of food containing histamine at concentration higher than 100 mg/100 g fish. The most general symptoms of this toxicity after consumed by human are itching, faintness, a burning sensation in the mouth, dizziness and the inability to swallow, but the victims usually recover within 8 h [12]. Furthermore, histaminolytic (histamine oxidizing) bacteria may allow an equilibrium to develop between histamine production and destruction in food containing high amounts of histamine [13].
Food and Drug Administration (FDA) has allowed to consume 5 mg/100 g fish (FDA, 1998), but in several studies showed histamine at 67 and 180 mg has been given orally to volunteers without any sign of toxicity [14]. Thus, the efficiency of detoxification system in the body influences the histamine toxicity [10]. Histamine poisoning (Scombroid poisoning) is a worldwide problem that occurs after consuming food that containing biogenic amines, particularly histamine at concentrations higher than 500 ppm [15]. Histamine poisoning manifest itself as an allergen-type reaction characterized by difficulty in breathing, itching, rash, vomiting, fever and hypertension. People having deficient natural mechanisms for detoxifying biogenic amines through genetic reasons or through inhibition due to the intake of anti-depression medicines, such as monoamine oxidase inhibitors (MAOIs) are more susceptible to histamine poisoning [13]. Histamine alone may not cause toxicity at a low level, but the presence of other biogenic such as putrescine and cadaverine, at concentrations 5 times higher than histamine, enhance the toxicity of histamine [14].
Histamine fish poisoning is among the most common food borne diseases related to fish consumption. Fifty-six of the 71 food borne disease outbreaks (78.9%) that have been notified in Europe in 2011 were due to histamine fish poisoning (EFSA 2013). The risk is correlated with the number and the histidine decarboxylase activity of the contaminating bacteria that grow in the flesh of fishes that are rich of free histidine, such as tuna, mackerel and bonito [15]. Bacteria of the genus Photobacterium, i.e., P. damselae subsp. Damselae (Pdd) and P. phosphoreum, are strong histamine producers [16-18]. Photobacterium damselae subsp. damselae is considered to be an emerging pathogen of marine fish of importance in aquaculture, with a notable increase in its geographical distribution during the last several years [19].
16] demonstrated that Pdd inoculated on tuna can produce toxic levels of histamine even at 4oC. These authors observed the Pdd displayed the highest performance in accumulating histamine in fish samples stored at refrigeration temperature in comparison with other psychrotolerant marine bacteria, namely P. phosphoreum and Raoultella planticola. The demonstrated that Pdd (strain JCM 8968) can produce more than 500 mg/kg histamine at 4oC in 24h and maintain 60% and 50% of the initial activity in tuna and dried saury for up to 12 weeks at -20oC, respectively. The presence of Pdd in the fish that are stored in melting ice or at chilling temperature and even in the de-frozen and processed seafood can thus pose a significant hazard if the contamination is carried on fish species which are rich in free histidine.
Biogenic amine levels in processed fish products are strongly affected by the quality of raw material and conditions and handling techniques during processing [20]. Therefore, the monitoring of Bas in fish and fish products in considered for two reasons: as a quality index and prevention of potential toxicity to human health [21]. Due to toxicological effects of Bas on human health, 50 ppm of histamine and 100 ppm of tyramine [22] have been suggested by the Food and Drug Administration (FDA) as tolerance levels in fish. Histamine in the causative agent of Scombroid poisoning, a food borne chemical hazard. Scombroid poisoning is usually a mild illness with a variety of symptoms including rash, urticarial, nausea, vomiting, diarrhea, flushing, tingling and itching of the skin. Severity of the symptoms can vary considerably with the amount of histamine ingested and the individual’s sensitivity to histamine. Scombroid fish such as tuna, mackerel, bonito and saury that contain high levels of free histidine in their muscle are often implicated in Scombroid poisoning incidents [9]. However, several species of non-scombroid fish such as mahi-mahi, bluefish, herring and sardine have often been implicated in incidents of Scombroid poisoning [23].
Scombroid syndrome/histamine poisoning occurs worldwide, and it is considered one of the most common forms of toxicity caused by fish consumption [24]. Histamine poisoning is a foodborne disease caused by eating spoiled fish. It recorded in 1828 with many cases reported since that time [25]. Histamine poisoning is the most prevalent form of seafood-borne poisoning and also the most reported cases concern fish, with very limited research on crab histamine poisoning. Fish and crabs are harmless when consumed fresh. Histamine is an inherent substance in species and exist in mast cells and basophils [26]. During decay, however, bacteria inside the body can convert histidine into histamine. Its biological effects include vasodilatation and hypotension, allergic reactions on nasal mucous membranes, sleep-wake regulation and gastric acid release [27]. When the histamine concentration in the body is higher than normal, anaphylactoid reaction can occur [28]. During histamine poisoning, which is relatively common following spoiled fish consumption, the occurrence of anaphylactoid reaction can, albeit rarely, result in death [29].
Analysis of Biogenic Amines using Conventional Methods
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
Analytical approaches for biogenic amines analysis are aimed to
a) Modify the current methods or develop new methods.
b) Determine the concentration of biogenic amines in products from other countries using valid methods.
c) Analyse biogenic amines used to control the effectiveness of methods developed and
d) Understand the relation between the levels of biogenic amines and biogenic amine-producing microorganisms [30].
Several analytical methods have been developed for determination of biogenic amines levels, including histamine, in food products as noted in review articles that surveyed these techniques. In a recent review of the analytical approaches to analysis of biogenic amines in food samples [31], several examples of using liquid chromatography (LC), thin-layer chromatography (TLC), gas chromatography (GC) and capillary zone electrophoresis (CZE) were presented for determination of biogenic amines in a variety of food products including wine, fish and seafood, orange juice, beer, cheese, sausage and fermented meat products, as well as milk products and even in lake water [32].
Analysis of biogenic amines in food is problematic not merely because of their low concentration levels but also due to the complexity of the matrix. Thus, sample clean-up plays an important role for proper isolation and enrichment of biogenic amines prior to their analytical determination. Common clean-up and pre-treatment techniques are liquid-liquid extraction (LLE) [33,34], solid phase extraction (SPE) [35], solid phase microextraction (SPME) (Paleologos & Kontominas 20044), cloud point extraction (CPE) [36] and hollow fibre liquid phase microextraction (HF-LPME) [37- 39]. In recent years, great attention has been focused on analysis of biogenic amines and various methods have been developed for the analysis of biogenic amines [40-42]. These methods are divided into two groups: those (direct) based on the detection of biogenic amines themselves and those (indirect) based upon the detection of the producer microorganisms [43]. The quantitative determination of biogenic amines in food is generally accomplished by HPLC [41,42] and GC [44,45].
Sensor
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
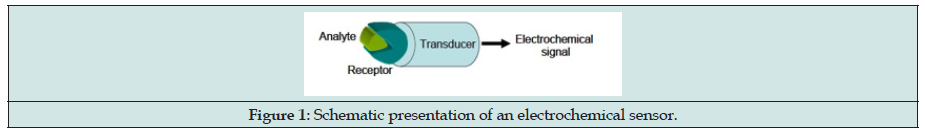
An overview of analytical chemistry development demonstrates that electrochemical sensor represents most rapidly growing class of chemical sensors. A chemical sensor can be defined as a device that provides continuous information about its environment. All chemical sensors consist of a transducer, with transforms the response into a detectable signal on modern instrumentation and a chemically selective layer, which isolates the response of the analyte from its immediate environment. They can be classified according to the property to be determined as electrical, optical, mass or thermal sensors and they are designed to detect and respond to an analyte in the gaseous, liquid or solid state [46].
Compared to optical, mass, and thermal sensors, electrochemical sensors are especially attractive because of their remarkable detectability, experimental simplicity and low cost. They have a leading position among the presently available sensors that have reached the commercial stage, and which have found a vast range of important applications in the fields of clinical, industrial, environmental and agricultural analyses [46]. Amongst many analytical techniques available, the development of chemical sensors has made significant strides in the last three decades. The rapidly growing applicants of chemical sensors reflect the extent to which analytical chemists require these devices for cheap, accurate, convenient and quick analysis of various samples. Chemical sensors are miniaturized analytical devices, which can deliver real-time and online information about the presence of specific compounds or ions in complex samples. Usually, an analyte recognition process takes place followed by the conversion of chemical information into an electrical or optical signal (Figure 1). It converts the activity of a specific ion in a solution into an electrical potential [47].
A number of instrumental techniques such as UV-visible and infrared spectrophotometry, atomic absorption spectrophotometry, flame photometry, fluorometry, mass spectrometry and chromatography etc. are available at the disposal of analytical chemists. Generally, these methods provide reproducible results with high sensitivity and good selectivity. However, all these analytical techniques require sophisticated instruments and chemical manipulation of sample before measurement which may be time consuming and inconvenient and need adequate skill to use it. The chemical sensors have no such requirements. These provide accurate, reproducible, fast and often selective determination of various chemical species. Not only this, but this technique could also be non-destructive, adaptable to small sample volumes and to online monitoring due to these merits. The utility of chemical sensors is being increasingly realized in medicinal, environmental, agricultural and industrial fields [47].
Electrochemical Sensors
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
Classification of Electrochemical Techniques
Generally electrochemical methods are based on the transformation of chemical information into an analytically useful signal. Any sensor used in electroanalytical determination contains two basic functional units; one receptor parts, which transforms the chemical information into a form of energy and one transducer part which transforms the energy, bearing chemical information, into a useful signal. A broad range of electrochemical techniques can be used for this purpose [47].
Typically, in electrochemistry, the reaction under investigation would either generate a measurable current (amperometric), a measurable potential or charge accumulation (potentiometric) or measurably alter the conductive properties of a medium (conductometric) between electrodes [48]. References are also made to other types of electrochemical detection techniques such as impedimetric, which measures impedance (both resistance and reactance) [49] and field-effect, which uses transistor technology to measure current as a result of a potentiometric effect at a gate electrode [50].
Since reactions are generally detected only in close proximity to the electrode surface, the electrodes themselves play a crucial role in the performance of electrochemical sensors. Based on the chosen function of a specific electrode, the electrode material, its surface modification or its dimensions greatly influence its detection ability. Electrochemical sensing usually requires a reference electrode, a counter or auxiliary electrode and a working electrode, also known as the sensing or redox electrode. The reference electrode, commonly made from Ag/AgCl, is kept a distance from the reaction site in order to maintain a known and stable potential. The working electrode serves as the transduction element in the biochemical reaction, while the counter electrode establishes a connection to the electrolytic solution so that a current can be applied to the working electrode. These electrodes should be both conductive and chemically stable. Therefore, platinum, gold, carbon and silicon compounds are commonly used, depending on the analyte [51].
A chemical sensor is a device that transforms chemical information, ranging from the concentration of a specific sample component to total composition analysis, into an analytically useful signal. The chemical information, mentioned above, may originate from a chemical reaction of the analyte or from a physical property of the system investigated. Chemical sensors contain two basic functional units: a receptor part and a transducer part. In the receptor part, part of a sensor the chemical information is transformed into a form of energy which may be measured by the transducer. The transducer part is a device capable of transforming the energy carrying the chemical information about the sample into a useful analytical signal [52]. Chemical sensor research has experienced explosive growth over the last three decades. A chemical sensor is generally defined as an analytical device which converts a chemical response into a quantifiable and processable signal [53]. Chemical sensors can be applied to a large variety of samples including body fluids, environmental samples, food and beverage samples. In order to construct a successful chemical sensor, several conditions must be met:
a) The chemical used as a binder must be highly specific for the purpose of the analysis and be stable under normal storage conditions.
b) The reaction should be independent as manageable of such physical parameters as stirring, pH and temperature. This will allow analysis of samples with minimal pre-treatment.
c) The response should be accurate, precise, reproducible, and linear over the concentration range of interest without dilution and concentration. It should also be free from electrical or other transducer induced noise.
d) If the chemical sensor used for clinical purposes, the probe must be tiny and compatible, having no toxic or antigenic effects.
e) For rapid measurements of analytes from human samples it is desirable that the chemical sensor can provide real-time analysis.
f) The complete chemical sensor should be cheap, small, portable and capable of being used by semi-skilled operators.
Electrochemical Detection Techniques – Biosensor
In biosensing the measurement of electrical properties for extracting information from biological systems is normally electrochemical in nature, whereby a bio electrochemical c o m p o - nent serves as the main transduction element. Although biosensing devices employ a variety of recognition elements, electrochemical detection techniques use predominantly enzymes. This is mostly due to their specific binding capabilities and biocatalytic activity [55,56]. Other biorecognition elements are e.g., antibodies, nucleic acids, cells and microorganisms [48].
Amperometric Devices
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
Are a type of electrochemical sensor, since they continuously measure current resulting from oxidation or reduction of an electroactive species in a biochemical reaction [57]. Clark oxygen electrodes perhaps represent the basis for the simplest forms of amperometric biosensors, where a current is produced in proportion to the oxygen in proportion to the oxygen concentration. This is measured by the reproduction of oxygen at a platinum working electrode in reference to Ag/AgCl reference electrode at a given potential [48]. Typically, the current is measured at a constant potential, and this is referred to as amperometry. If a current is measured during controlled variations of the potential, this is referred to as voltammetry. Furthermore, the peak value of the current measured over a linear potential range is directly proportional to the bulk concentration of the analyte, i.e., the electroactive species [48,50,54]. Since not all protein analytes are intrinsically capable to serve as redox partners in electrochemical reactions, these devices use mostly mediated electrochemistry for the electrochemical reaction of the analyte at the working electrode [48].
Cyclic Voltammetry (CV)
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
Voltammetry belongs to a category of electro-analytical methods, through which information about an analyte is obtained by varying a potential and then measuring the resulting current. It is, therefore, an amperometric technique. Since there are many ways to vary a potential, there are also many forms of voltammetry, such as: polarography (DC voltage) [53], linear sweep, differential staircase, normal pulse, reverse pulse, differential pulse and more [58]. Cyclic voltammetry is one of the most widely used forms and it is useful to obtain information about the redox potential and electrochemical reaction rates (e.g., the chemical rate constant) of analyte solutions. In this case, the voltage is swept between two values at a fixed rate, however, when the voltage reaches V2 the scan is reversed and the voltage is swept back to V1, as is illustrated in Figure 2. The scan rate, (V2 – V1) / (t2 – t1), is a critical factor since the duration of a scan must provide sufficient time to allow for a meaningful chemical reaction to occur. Varying the scan rate, therefore, yields correspondingly varied results [59].
Figure 2: An overview of selected cyclic voltammograms with the reference to the work of Li et al.: i) a single generic linear voltage sweep; ii) a cyclic voltammogram of the Prussian Blue-modified (PB) glassy carbon electrode was prepared in 0.1 M KCl + .1 M HCl at a scan rate 50 mV/s; iii) cyclic voltammograms of the PB sol-gel modified glassy carbon electrode and iv) the cholesterol/PB sol-gel modified glassy carbon electrode in a phosphate buffer (pH 6.8) at varying concentrations of the analytic solution: a) blank solution; b) blank solution + cholesterol 1 x 10-5, 2 x 10-5, 3 x 10-5, 4 x 10-5, 5 x 10-5 mol/L. (Li et al. 2003).
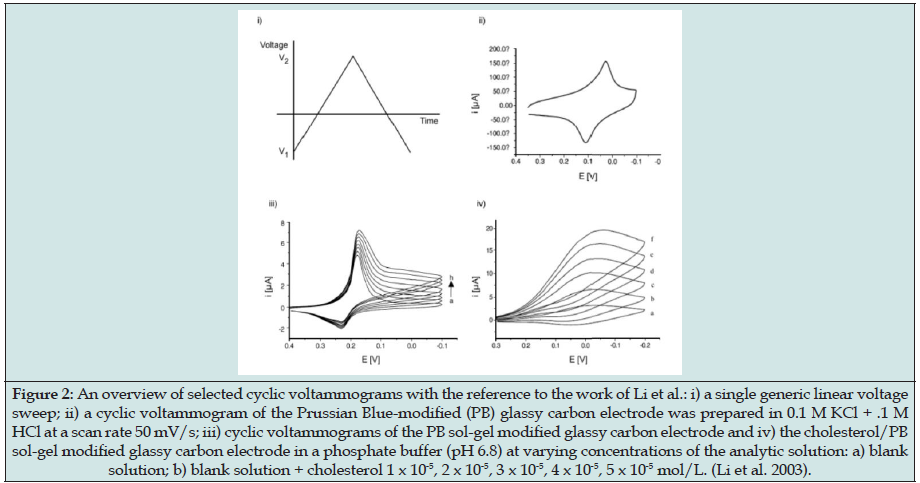
The voltage is measured between the reference electrode and the working electrode, while the current is measured between the working electrode and the counter electrode. The obtained measurements are plotted as current vs. voltage, also known as a voltammogram. As the voltage is increased toward the electrochemical reduction potential of the analyte, the current will also increase. With increasing voltage toward V2 past this reduction potential, the current decreases, having formed a peak as the analyte concentration near the electrode surface diminishes, since the oxidation potential has been exceeded. As the voltage is reversed to complete the scan toward V1, the reaction will begin to re-oxidize the product from the initial reaction. This produces an increase in current of opposite polarity as compared to the forward scan, but again decreases, having formed a second peak as the voltage scan continues toward V1. The reverse scan also provides information about the reversibility of a reaction at a given scan rate [48].
Potentiometric Devices
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
Measure the accumulation of a charge potential at the working electrode compared to the reference electrode in an electrochemical cell when zero or no significant current flows between them [54]. In other words, potentiometry provides information about the ion activity in an electrochemical reaction [60]. For the potentiometric measurements, the relationship between the concentration and the potential is governed by the Nerst equation, where Ecell represents the observed cell potential at zero current. This is sometimes also referred to as the electroactive force or EMF. Eocell is a constant potential contribution to the cell, R the universal gas constant, T the absolute temperature in degrees Kelvin, n is the charge number of the electrode reaction, F is the Faraday constant and Q is the ratio of ion concentration at the anode to ion concentration at the cathode [48].
Potentiometric sensors prove suitable for measuring low concentration in tiny samples volume, since they ideally offer the benefit of not chemically influencing the sample. The variety of ions, for which low detection limits are possible, is currently quite limited and missing such important analytes as: nickel, manganese, mercury and arsenate ions. Detailed information about potentiometry and their limit of detection (LOD) is provided in the review by Bakker et al. Potentiometry is also used as an alternative method to electrically determine the point in a chemical reaction at which equal quantities of opposing solutions reach a state of equilibrium (e.g., 0.1 mol HCl and 0.1 mol NaOH). This is known as measuring a titration endpoint with the technique known as potentiometric titration. By performing a titration at constant or zero current, the end point is identified from the variation in electrode potential, which are caused by changes in solution concentration of the potential- determining ion [48].
Conductometric Devices
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
Measure the ability of an analyte (e.g., electrolyte solutions) or a medium (e.g., nanowires) to conduct an electrical current between electrodes or reference nodes. Although conductometric devices can be considered as a subset of impedimetric devices, techniques for measuring capacitance changes are reviewed later in combination with electrochemical impedance spectroscopy. In most cases conductometric devices have been strongly associated with enzymes, where the ionic strength and thus the conductivity of a solution between two electrodes changes as a result of an enzymatic reaction. Thus, conductometric devices can be used to study enzymatic reaction that produce changes in the concentration of charged species in a solution [55].
Chemical sensors are broadly divided into classes based on transduction principle. The classes of chemical sensors are specifically electrochemical, optical, electrical and mass sensitive. Electrochemical sensors transduce the electrochemical interaction of an analyte at a modified electrode into a voltage or current signal. Major groups within electrochemical sensors are potentiometric sensors – which measure the potential of an indicator electrode (ion-selective electrode) against a reference electrode and voltammetric sensors – which measure current flow at a constant or varying potential. Amperometric sensors, which measure current flow at a constant potential, are very commonly used for electrochemical biosensors [61].
Electrochemical sensors and biosensors have recently found extensive applications in diverse industries. Nowadays, many analytical instruments used in environmental, food, pharmaceutical or clinical laboratories and also most of the commercial point-of-care devices work using chemical sensors and biosensors as a whole or a basic part. Glucose biosensors use widely in glucometers and pH electrodes are the important and known examples of the electrochemical sensors. An electrochemical sensor is a device transforms electrochemical information into an analytically useful signal. Electrochemical sensors usually composed of two basic components, a chemical (molecular) recognition system which is the most important part of a sensor and physicochemical transducer which is a device that converts the chemical response into a signal that can be detected by modern electrical instrumentations. These two parts form a working (or sensing) electrode. A reference electrode and sometimes a counter electrode are also used in electrical measurements. Transduction of a biological or chemical signal into an electrical signal can be done by amperometry, voltammetry, potentiometry or conductometry [62].
Immobilization of enzymes
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
Enzymes are biologic polymers that catalyse the chemical reactions that make biological life possible. They have a wide variety of biochemical, biomedical, pharmaceutical and industrial applications. The major advantage is that the catalysed reaction is not perturbed by a side-reaction, resulting in the production of one required end-product. In addition, the enzymatic reaction took place at mild condition of temperature, pressure and pH with the reaction rates of the order of those achieved by chemical catalysts at more extreme conditions [63]. However, there are some practical advantages of the use of enzymes and one of the majors is that most enzymes operate dissolved in water homogenous catalysis systems, and the main problem is the separation of enzymes, from the reaction media for reuse [64]. A possible solution of this problem can be enzyme immobilization. This is a method of keeping the enzymes molecules confined or localized in a certain defined region of space with a retention of their catalytic activity [65]. Several techniques may be applied to immobilize them on a solid support. They are based on chemical and physical methods. Both physical and chemical immobilization methods offer some advantages and disadvantages. During the chemical methods, a loss of the activity of enzyme is observed; covalent bonds formed as a result of the immobilization can perturb the enzyme’s native structure, but such covalent linkages provide a strong and stable enzyme attachment and, in some cases, can reduce the enzyme deactivation rates. The physical immobilization methods more or less perturb the native structure of the enzyme, but in this case the enzyme does not bind to the carrier. That is why low activity of the immobilized enzyme is observed [66].
Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
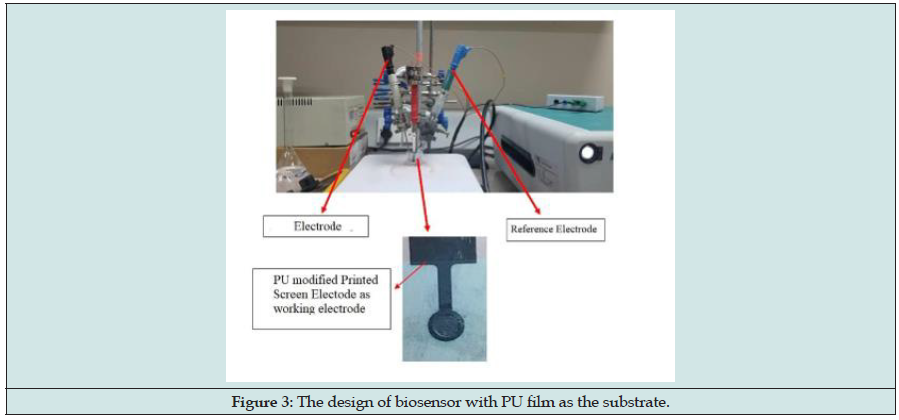
Fish contains high protein. Protein in fish can be converted to biogenic amines. Biogenic amines in fish are histamine, putrescine, cadaverine and others. Histamine needs to be determined because of its toxicity. According to Food and Drug Administration (FDA), histamine can be consumed not more than 50 mg·kg-1 and above it can cause several adverse effects such as headache, nausea and vomit. Histamine can be analysed using chromatography methods where not only that the instrument is expensive, but it also needs specialist, and the process is very tedious. A sensor method was developed where this sensor using palm-based polyurethane as an electro-sensor substrate. Palm based polyurethane (PU) was produced via condensation polymerization between palm kernel oil-based monoester polyol (PKOp) and 4,4’-diphenylmethane diisocyanate (MDI). PU is used in this study due to its porosity and its capability to attach onto screen printed electrode (SPE) sturdily without disintegration. PU has satisfactory tensile and modulus. Verification of the chain structure of PU was identified through spectroscopy analysis using Fourier Transform Infrared and (FTIR) and Nucleus Magnetic Resonance (NMR) spectroscopy. Thermal analysis via TGA showed degradation above 240°C. It can be concluded that PU is very stable at high temperature. The thickness of PU applied onto the SPE was 0.01mm and the electrode modified was activated potentiodynamically. PU – SPE absorbed histamine inside its pores so histamine can undergo the oxidation process. The oxidation process was detected using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). This method showed that histamine was not oxidized until 0.4 V with a 50 mV·s-1 scan rate showed a peak of histamine oxidation exactly at 0.31 V. The histamine was detected in a supporting electrolyte, giving peak oxidation current at potential applied 0.31 V with current 9.63 μA, for 1 mmol·L-1of histamine standard solution. The chemical sensor showed a satisfactory response, a linear response range at 0.015 to 1 mmol·L-1, a good sensitivity of 0.1 mmol·L-1 was attained at 3.07 % during intraday and 9.55 % during interday, respectively. The detection and quantification limits of histamine standard were acquired at 0.17 mmol·L-1and 0.53 mmol·L-1. Various interfering compounds were also examined in order to establish their effect, if any, on the determination of histamine at the PU modified electrode. The interference was from several major interfering compounds such as aniline, cadaverine, hexamine, putrescine, and xanthine.
The sensor showed an excellent anti – interferents property towards other amines (Figure 3). A satisfactory recovery performance towards varying concentration of histamine was obtained at 94 and 103 % for 0.01 mmol·L-1 and 0.1 mmol·L-1 of histamine, respectively. The developed chemical sensor has a good potential to determine histamine level in fish. The determination of histamine was also compared to high performance liquid chromatography (HPLC) where this method also showed a satisfactory response between 0.16 – 5.00 μg·mL-1. The detection and quantification limits of histamine standard obtained at 0.10 ppm (0.89 μmol·L-1) and 0.30 ppm (2.69μmol·L-1), respectively. Although the LOD of sensor method was below chromatography method, the developed chemical sensor has a good potential to determine histamine level in fish [67-108].
Conclusion
- Abstract
- Biogenic Amines
- Analysis of Biogenic Amines using Conventional Methods
- Sensor
- Electrochemical Sensors
- Amperometric Devices
- Cyclic Voltammetry (CV)
- Potentiometric Devices
- Conductometric Devices
- Immobilization of enzymes
- Polyurethane Modified Screen-Printed Electrode for the Electrochemical Detection of Histamine (2-(1h-Imidazol-4-Yl) Ethanamine) in Fish Mackerel (Rastrelliger Brachysoma)
- Conclusion
- References
This article is describing the progress in analytical detection of biogenic amines namely the histamine. A sensor development made use of the palm-based polyurethane as substrate to a biosensor is one of the cases. The evolution is significant in the search of simple yet efficient and cost-effective approach of detection of the histamine.
References
- Abstract
- Introduction
- Water situation in Jordan
- Literature Review
- Methods
- Jawa (Islamic house)
- Traditional houses in Umm El-Jimal
- Great Temple Complex at Petra
- Water harvesting system in Archeological buildings
- Traditional village house
- Water harvesting in Jordan at the present time
- Recent studies about RWH in Jordan- residance
- Results & Recommendations
- Discussion
- Conclusion
- References
- Alizadeh N, Kamalabadi M, Mohammadi A (2017) Determination of histamine and tyramine in canned fish samples by headspace solid phase microextraction based on a nanostructured polypyrene fiber followed by ion mobility spectrometry. Food Analytical Methods p. 1- 8.
- Gardini F, Ozogul Y, Suzzi G, Tabanelli G, Ozugul F (2016) Technological factors affecting biogenic amine content in foods: A review. Frontiers in Microbiology 7: 1218.
- Bilgin B, Genccelep H (2015) Determination of biogenic amines in fish products. Food Science & Biotechnology 24(5): 1907-1913.
- Lazaro CA, Conte-Junior CA (2013) Chromatographic methods for biogenic amines determination in foods of animal origin. Brazil Journal of Veterinary Research Animal Science 50(6): 430-446.
- Mohammed GI, Bashammakh AS, Alsibaai AA, Alwael H, El-Shawai MS (2016) A critical overview on the chemistry, clean-up and recent advances in analysis of biogenic amines in foodstuffs. TrAC Trends in Analytical Chemistry 1-51.
- Onal A, Tekkeli SEK, Onal C (2013) A review of the liquid chromatographic methods for the determination of biogenic amines in foods. Food Chemistry 138(1): 509-515.
- WHO, Publication (2013). Meeting on Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products, Joint FAO/Expert Report.
- Perez E (2013) Poisoning- fish and shellfish, MedlinePlus, National Institutes of Health.
- Taylor SL (1986) Histamine Food Poisoning: Toxicology and Clinical Aspects. CRC Critical Reviews in Toxicology 17(2): 91- 128.
- Bulushi IA, Poole S, Deeth HC, Dykes GA (2009) Biogenic amines in fish: Roles in intoxication, spoilage, and nitrosamine formation--A Critical Reviews in Food Science and Nutrition 49(4): 369-377.
- Naila A, Flint S, Fletcher G, Bremer P, Meerdink G (2010) Control of Biogenic Amines in Food- Existing and emerging approaches. Journal of Food Science 75(7): 139-150.
- Arnold S & Brown W (1978) Histamine toxicity from fish products. Advances in Food Research. Adv Food Res 24: 113-54.
- Yongmei L, Xiaohong C, Mei J, Xin L, Rahman N, Mingsheng D, Yan G (2009) Biogenic amines in Chinese soy sauce. Food Control 20(6): 593- 597.
- Emborg J, Dalgaard P (2006) Formation of histamine and biogenic amines in cold-smoke tuna: An investigation of psychrotolerant bacteria from samples implicated in cases of histamine fish poisoning. Journal of Food Protection 69(4): 897-906.
- Trevisani M, Mancusi R, Cecchini M, Costanza C, Prearo M (2017) Detection and characterization of histamine-producing strains of Photobacterium damselse Damselae isolated from mullets. Veterinary Sciences 4(2): 31- 42.
- Kanki M, Yoda T, Tsukamoto T, Baba E (2007) Histidine decarboxylases and their role in accumulation of histamine in tuna and dried saury. Applied and Environmental Microbiology 73(5): 1467-1473.
- Bjornsdottir K, Bolton GE, McClellan-Green PD, Jaykus LA, Green DP (2009) Detection of gram-negative histamine-producing bacteria in fish: A comparative study. Journal of Food Protection 72(9): 1987-1991.
- Bjornsdottir K, Bolton GE, McClellan-Green PD, Jaykus LA, Green DP (2010) Development of molecular-based methods for determination of highhistamine producing bacteria in fish. International Journal of Food Microbiology 139(3): 161-167.
- Tercetti MS, Ogut H, Osorio R (2016) In the Black Sea: Evidence of a multiclonal origin. Applied and Environmental Microbiology. 82(13): 3736- 3745.
- Zare D, Muhammad K, Bejo MH, Ghazali H (2015) Determination of urocanic acid, a compound implicated in histamine toxicity and assessment of biogenic amines relative to urocanic acid content in selected fish and fish products. Journal of Food Composition and Analytical 37: 95-
- Prester L (2011) Biogenic amines in fish, fish products and shellfish: a review. Food Additive & Contamination 28(11): 1547-
- Hu Y, Huang Z, Li J, Yang H (2012) Concentrations of biogenic amines in fish squid and octopus and their changes during storage. Food Chemistry 135(4): 2604-2611.
- Chen HC, Huang YR, Hsu HH, Lin CS, Chen WC, et al. (2010). Determination of histamine and biogenic amines in fish cubes (Tetrapturus angustirotris) implicated in a food-borne poisoning. Food Control 21: 13-18.
- Dalgaard P, Emborg J, Kjolby A, Sorensen ND, Ballin NZ (2008) Histamine and biogenic amines: formation and importance in In improving seafood products for the consumer. Cambridge Page 292-324.
- Yang Yu, Ping Wang, Ligong Bian, Shijun Hong (2017) Rare death via histamine poisoning following crab consumption: A case report. Journal of Forensic Sciences 63(3): 980-982.
- Smiljkovic D, Blatt K, Stefanzl G, Dorofeeva Y, Skrabs C, et al. (2017). BTK-inhibition is a potent approach to block IgE-mediated histamine release in human basophils. Allergy 72 (11): 1666-
- Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, et al. (2015) International union of basis and clinical pharmacology. XCVIII. Histamine receptors. Pharmacological Reviews 67 (3): 601- 655.
- Dong XX, Yang JY, Luo L, Zhang YF, Mao C, et al. (2017) Portable amperometric immunosensor for histamine detection using Prussian blue-chitosan-gold nanoparticle nanocomposite films. Biosensors and Bioelectronics 98: 305-309.
- Feng C, Teuber S, Gershwin ME (2016) Histamine (Scombroid) fish poisoning: a comprehensive Clinical Reviews in Allergy and Immunology 50(1): 64-69.
- Bedia EF (2013) Recent analytical approaches to analysis of biogenic amines in food samples. TrAC Trends in Analytical Chemistry 52: 239-247.
- Badia-Erim (2013) Recent analytical approaches to analysis of biogenic amines in food sample. TrAC Trends in Analytical Chemistry 52: 239-247.
- Cohen G, Rudnik DD, Laloush M, Yakir D, Karpas Z (2015) A novel method for determination of histamine in tuna fish by Food Analytical Methods 8(9).
- Fernandes JO, MA Ferreira (2000) Combined ion-pair extraction and gas chromatography-mass spectrometry for the simultaneous determination of diamines, polyamines and aromatic amines in Port wine and grape Journal of Chromatography A 886(1-2): 183-195.
- Garcia-Villar N, Saurina J, Hernandez-Cassou S (2006) High performane liquid chromatographic determination of biogenic amines in wines with an experimental design optimization procedure. Analytica Chimica Acta 575(1): 97-105.
- Innocente N, Biasutti M, Padovese M, Moret S (2007) Determination of biogenic amines in cheese using HPLC technique and direct derivatization of acid extract. Food Chemistry 101(3): 1285-1289.
- Carabias-Martinez R, Rodriguez-Gonzalo E, Moreno-Cordero B, Perez-Pavon JL, Garcia-Pinto C, et al. (2001) Surfactant cloud point extraction and preconcentration of organic compounds prior to chromatography and capillary electrophoresis. Journal of Chromatography A 902(1): 251-265.
- Nerin C, Salafranca J, Aznar M, Battle R (2009) Critical review on recent developments in solventless techniques for extraction of analytes. Analytical and Bioanalytical Chemistry 393(3): 809-
- Salafranca J, Pezo D, Nerin C (2009) Assessment of specific migration to aqueous simulants of a new active food packaging containing essential oils by means of an automatic multiple dynamic hollow fibre liquid phase microextraction system. Journal of Chromatography A 1216(18): 3731-
- Pedersen Bjergaard S, Rasmusses KE (2008) Liquid-phase microextraction with porous hollow fibers, a miniaturised and highly flexible format for Liquid-liquid extraction. Journal of Chromatography A 1184(1-2): 132-
- Huang KL, Jin CX, Song SL, Wei CY, Liu YM, Li J, et al. (2011) Development of an ionic liquid-based ultrasonic-assisted Liquid-liquid microextraction method for sensitive determination of biogenic amines: Application to the analysis of octopamine, tyramine and phenethylamine in beer samples. Journal of Chromatography B 879(9-10): 579-584.
- Sagratini G, Fernandez-Frazon M, Berardinis FD, Font G, Vittori S, Manes J (2012) Simultaneous determination of eight underivatized biogenic amines in fish by solid phase extraction and liquid chromatography-tandem mass spectrometry. Food Chemistry 132(1): 537 -543.
- Onal A (2007) A review: Current analytical methods for the determination of biogenic amines in foods. Food Chemistry. 103(4): 1475- 1486.
- Linares DM, Martin MM, Ladero V, Alvarez MA, Ndez MA (2011) Biogenic amines in dairy products. Criticals Review Food Science Nutrition 51(7): 691-703.
- Munir MA, Assim ZB, Ahmad FB (2016) Characterization of biogenic amines in fish collected from Sarawak using gas chromatography. Borneo Journal of Resource Science and Technology 6(2): 21-27.
- Almeida C, Fernandes JO & Cunha SC (2012) A novel dispersive Liquid-liquid microextraction (DLLME) gas chromatography-mass spectrometry (GC-MS) method for the determination of eighteen biogenic amines in beer. Food Control 25(1): 380-388.
- Stradiotto NR, Yamanaka H, Zanoni MVB (2003) Electrochemical sensors: A powerful tool in analytical chemistry. Journal of Brazilian Chemical Society 14 (2): 159-173.
- Rahman Md A, Kumar P, Park D, Shim Y (2008) Electrochemical sensors based on organic conjugated polymers. 8(1): 118-141.
- Chaubey A, Malhotra BD (2002) Mediated Biosensors & Bioelectronics 17(6-7): 441-456.
- Guiseppi-Elie A, Lingerfelt L (2005) Impedimetric detection of DNA hybridization: Towards patient DNA In Immobilization of DNA on Chips I, volume 260 of Topics in Current Chemistry. pages 161- 186. Springer-Verlag Berlin, Berlin.
- Thevenot DR, Toth K, Durst RA, Wilson GS (2001) Electrochemical biosensors: recommended definitions and classification. Biosensors & Bioelectronics. 16(1-2): 121-131.
- Ben AM, Korpan Y, Gonchar M, Elskaya A, Maaref MA, et al. (2007) Formaldehyde assay by capacitance versus voltage and impedance measurements using bi-layer bio-recognition membrane. Biosensors & Bioelectronics 22(5): 575-581.
- Hulanicki A, Glab S, Ingman F (1991) Chemical sensors definitions and International Union of Pure & Applied Chemistry 63(9): 1247-1250.
- Grieshaber D, MacKenzie R, Voros J, Reimhult E (2008) Electrochemical biosensors- Sensor principles and architectures. Sensors 8(3): 1400-1458.
- Eggins B (2002) Chemical sensors and biosensors. Analytical techniques in the Science. Jhon Wiley & Sons, West
- D’ Orazio P (2003) Biosensors in clinical chemistry. Clin Chim Acta 334(1-2):41-69.
- Schoning MJ, Poghossian A (2002) Recent advances in biologically sensitive field-effect transistors (biofest). Analyst 127(9): 1137- 1151.
- Luppa PB, Sokoll LJ, Chan DW (2001) Immunosensors--principles and applications to clinical chemistry. Clinica Chimica Acta 314(1-2): 1-26.
- Katz E, Willner I (2003) Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Route to impedimetric immunosensors, DNA-sensors and enzyme Electroanalysis 15(11): 913-947.
- Pei R, Cheng Z, Wang E, Yang X (2001) Amplification of antigen-antibody interactions based on biotin labelled protein-streptavidin network complex using impedance spectroscopy. Biosensors & Bioelectronic 16 (6): 355-
- Bakker E, Pretsch E (2005) Potentiometric sensors for trace-level analysis. Trac Trends in Analytical Chemistry 24(3): 199-207.
- Kassal P, Steinberg MD, Steinberg IM (2018) Wireless chemical sensors and biosensors: A review. Sensors and Actuators B pp. 1-45.
- Faridbod F, Gupta VK, Zamani HA (2011) Electrochemical sensors and biosensors. International Journal of Electrochemistry p. 1-2.
- Krajewska B (2004) Application of chitin- and chitosan- based materials for enzyme immobilizations: A review. Enzyme & Microbial Technology 35(2-3): 126-139.
- Romaskevic T, Budriene S, Pielichowski K, Pielichowski J (2006) Application of polyurethane-based materials for immobilization of enzymes and cells: a 788 Chemija 17 (4): 74- 89.
- Bakker M, van de Velde F, van Rantwijk F, Sheldon R.A. (2000) Highly efficient immobilization of glycosylated enzymes into polyurethane foams. Biotechnology & Bioengineering 70(3): 342-348.
- Gunzler H, Williams A (2002) Handbook of Analytical WILEY-VCH, Weinheim (Federal Republic of Germany) 1196 pages.
- Munir MA, Badri KH, Lee YH, A Inayatullah, HA Badrul (2020) A Modest Approach of Electrochemical Sensor to Determine Biogenic Amines in Food and Beverages. Journal Sains Indonesia 1 (3):162-173.
- Munir MA, Badri KH, Lee YH (2021) Polyurethane modified screen-printed electrode for the electrochemical detection of histamine in fish. IOP Conference Series Earth and Environmental Science 880(1):
- Munir MA, Badri KH, Lee YH, A Inayatullah, E Nurinda, et al. (2022) The Application of Polyurethane-LiClO4 to Modify Screen-Printed Electrodes Analyzing Histamine in Mackerel Using a Voltametric ACS Omega 7 (7): 5982-5991.
- Munir MA, Badri KH, Lee YH, A Inayatullah, HA Badrul, et al. (2022) Design and Synthesis of Conducting Polymer Bio-Based Polyurethane Produced from Palm Kernel International Journal of Polymer Science pp. 13.
- Muhammad Abdurrahman Munir, Khairiah Haji Badri, Sofian Ibrahim (2020) Synthesis of bio polyurethane from palm kernel oil and characterization using FTIR and NMR. International Journal of Scientific and Research Publications 10(10): 238-246.
- Muhammad Abdurrahman Munir, Lee Yook Heng, Edison Eukun Sage, Muhammad Mukram Mohamed Mackeen and Khairiah Haji Badri (2021) Histamine Detection in Mackerel (Scomberomorus Sp.) and its Products Derivatized with 9- Pak. J. Anal. Environ. Chem 22(2): 243-251.
- Muhammad Abdurrahman Munir, Muhammad Mukram Mohamed Mackeen, Lee Yook Heng, Khairiah Haji Badri (2021) Study of Histamine Detection using Liquid Chromatography and Gas Chromatography. ASM Science Journal 16: 1-9.
- Akdis CA, Akdis M (2015) Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J 8(1): 17.
- An D, Chen Z, Zheng Z, Chen J, Wang S, et al. (2016) Polyoxometalate functionalized tris (2, 2-bipyridyl) dichloroethane (II) as the probe for electrochemiluminescence sensing of histamine. Food Chemistry 194(1): 966-71.
- Aziz AA (2007) Development of a biosensor based on amine oxidase from Cicer arientum for the detection of biogenic amines. University Technology Malaysia, Malaysia p. 89.
- Boyce J (2014) Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel Nutrition Research 31(1): 61-75.
- Byun BY, Mah JH (2012) Occurrence of biogenic amines in miso, Japanese traditional fermented soybean paste. Journal of Food Science 77(12): 216-
- Chytiri S, Paleologos E, Savvaidis I & Kontominas M (2004) Relation of biogenic amines with microbial and sensory changes of whole and filleted freshwater rainbow trout (Onchorynchus mykiss) stored on Journal of Food Protection 67(5): 960-965.
- Ede G (2017) Histamine intolerance: why freshness Journal of Evolution and Health 2(1): 1-8.
- European Food Safety Authority (EFSA), European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in EFSA Journal 11: 3129.
- Food and Drug Administration (1998) Scombrotoxin (histamine) formation in Fish and Fishery Products Hazards and Controls Guide 2. pp. 73-90.
- Hungerford JM (2010) Scombroid poisoning: a review. Toxicon 56(2): 231-243.
- Jarisch R (2015) Histamine Intolerance: histamine and seasickness.
- Jiang S, Peng Y, Ning B, Bai J, Liu Y, Zhang N, Gao Z, et al. (2015) Surface plasmon resonance sensor based on molecularly imprinted polymer film for detection of histamine. Sensors and Actuators B Chemical. 221: 15-21.
- Jones SM, Burks AW, Dupont C (2014) State of the art on food allergen immunotherapy: oral, sublingual and epicutaneous. Journal of Allergy and Clinical Immunology 133(2): 318-323.
- Kofler L, Ulmer H, Kofler H (2011) Histamine 50-skin-prick test: A tool to diagnose histamine intolerance. ISRN Allergy 1-5.
- Kovacova-Hanuskova E, Buday T, Gavliakova S, Plevkov J (2015) Histamine, histamine intoxication and intolerance. Allergol Immunopathol (Madr) 43(5): 498-506.
- Li JP, Peng TZ, Peng YQ (2003) A cholesterol biosensor based on entrapment of cholesterol oxidase in a silicic sol-gel matrix at a Prussian blue modified Electroanalysis 15(12): 1031-1037.
- Lorenzo JM, Martinez S, Franco I, Carballo J (2007) Biogenic amine content during the manufacture of dry-cured lacon, a Spanish traditional meat product: Effect of some additives. Meat Science 77(2): 287-293.
- Maintz L, Novak N (2007) Histamine and histamine The American Journal of Clinical Nutrition 85(5): 1185-1196.
- Martuscelli M, Arfelli G, Manetta AC, Suzzi G (2013) Biogenic amines content as a measure of the quality of wines of Abruzzo (Italy). Food Chemistry 140(3): 590-597.
- Paleologos EK, Kontominas MG (2004) On-line solid-phase extraction with surfactant accelerated on-column Derivatization and micellar liquid chromatographic separation as a tool for the determination of biogenic amines in various food substrates. Analytical Chemistry 76(5): 1289-
- Perez S, Bartroli J, Fabregas E (2013) Amperometric biosensor for the determination of histamine in fish samples. Food Chemistry 141(4): 4066- 4072.
- Phuvasate S, Su YC (2010) Effects of electrolyzed oxidizing water and ice treatments on reducing histamine-producing bacteria on fish skin and food contact surface. Food Control 21(3): 286-
- Pospiskova K, Safarik I, Sebela M, Kuncova G (2013) Magnetic particles-based biosensor for biogenic amines using an optical oxygen sensor as a transducer. Microchemical Acta 180: 311-
- Ramani D, De Bandt JP, Cynober L (2014) Aliphatic polyamines in physiology and diseases. Clinical Nutrition. 33 (1): 14-
- Ramon-Marquez T, Medina Castillo AL, Fernandez Gutierrez A, Fernandes Sanchez JF (2016) Novel optical sensing film based on a functional nonwoven nanofiber mat for an easy, fast and highly selective and sensitive detection of tryptamine in beer. Biosensors & Bioelectronic 79: 600-
- Restuccia D, Spizzirri UG, Parisi OI, Cirillo G, Picci N (2014) Brewing effect on levels of biogenic amines in different coffee samples as determined by LC-UV. Food Chemistry 175: 143-
- Restuccia D, Spizzirri UG, Puoci F, Picci N (2015) Determination of biogenic amine profiles in conventional and organic cocoa-based products. Food Additives 782& Contaminants: Part A 32 (7): 1156-
- Rivas B, Gonzalez R, Landete JM, Munoz R (2008) Characterization of a second ornithine decarboxylase isolated from Morganella morganii. Journal of 785Food Protection 71(3): 657-
- Ruiz Capillas C, Jimenez Colmenero F (2004) Biogenic amines in meat and meat products. Critical Reviews in Food Science & Nutrition 44(7-8): 488-
- Seifert R, Strasser A, Schneider EH, Neumann D, Dove, S, et al. (2013) Molecular and cellular analysis of human histamine receptor subtypes. Trends in Pharmacological Sciences 34 (1): 33-
- Shakila R, Vijayalakshmi K, Jeyasekaran G (2003) Changes in histamine and volatile amines in six commercially important species of fish of the Thoothukkudi coast of Tamil Nadu, India stored at ambient Food Chemistry 82(3): 347- 352.
- Tapingkae W, Tanasupawat S, Parkin KL, Benjakul S, Visessanguan W (2010) Degradation of histamine by extremely halophilic archaea isolated from high salt- fermented fishery products. Enzyme and Microbial Technology 46(2): 92-
- Wang QH, Fang GZ, Liu YY, Zhang DD, Liu JM, et al. (2017) Fluorescent sensing probe for the sensitive detection of histamine based on molecular imprinting ionic liquid-modified quantum dots. Food Analytical Methods p. 1-
- Wood RA (2016) Food allergen immunotherapy: Current status and prospects for the future. Journal of Allergy and Clinical Immunology 137 (4): 973-
- Yu W, Freeland DMH, Nadeau KC (2016) Food allergy: immune mechanisms, diagnosis and immunotherapy. Nature Reviews Immunology 16 (12): 751- 765.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...