Lupine Publishers Group
Lupine Publishers
Menu
Research ArticleOpen Access 
Management of Rhizoctonia solani, Fusarium solani and Meloidogyne incognita by Silicon dioxide nanoparticles and Rhizobium ciceri alone and in combination on chickpea Volume 1 - Issue 2
Zaki A. Siddiqui* and Sumaiya Aziz
- Department of Botany, Aligarh Muslim University, India
Received: September 08, 2020 Published: September 23,2020
Corresponding author: Zaki A. Siddiqui, Department of Botany, Aligarh Muslim University, India
DOI: 10.32474/JBRS.2020.01.000109
Abstract
Effects of silicon dioxide nanoparticles (SiO2 NPs) and Rhizobium ciceri was observed alone and in combination on Rhizoctonia
solani/ Fusarium solani / Meloidogyne incognita on the growth, photosynthetic pigments (chlorophyll and carotenoid) and proline
contents of Cicer arietinum.
Inoculation of M. incognita resulted in a greater reduction in plant growth, photosynthetic pigments and higher increase in
proline contents than other test pathogens. Inoculation of R. ciceri or foliar spray of SiO2 NPs (0.10 mg ml-1) with pathogens under
study resulted in increased plant growth, photosynthetic pigments and proline contents than without R. ciceri / NPs. Combined
application of NPs with R. ciceri resulted in a greater increase in plant growth, photosynthetic pigments and proline contents in
plants with pathogens than with NPs or R. ciceri. Plants without R. ciceri had a very poor root nodulation but nodulation was high in
plants with R. ciceri. Pathogens under study and NPs had adverse effect on nodulation caused by R. ciceri. Wet rot and black root rot
indices were 4 when R. ciceri and R. solani were inoculated respectively. R. ciceri / NPs reduced wet root rot, black root rot indices,
galling and population of M. incognita. Disease indices, galling and population of M. incognita were reduced greatly by use of R. ciceri
plus SiO2 NPs.
Keywords:Black Root Rot; Cicer Arietinum; Disease Index; Root Knot Nematode; Root Nodule Bacterium; Wet Root Rot
Introduction
Chickpea (Cicer arietinum L.) is widely grown as major food
legume because of its rich nutrient values. It has high protein and
starch suitable for textile sizing Duke [6]. Rhizobium Frank is root
nodule bacterium bacterium associated with roots of legumes for
symbiotic nitrogen fixation. Rhizobium inoculation has positive
effect on growth attributes, yield components and quality of
chickpea. Application of rhizobial isolates significantly suppress
root rotting fungi and root knot nematode Parveen et al. [20] and
reduced damaged caused Siddiqui and Mahmood [23].
Important diseases of chickpea include; Rhizoctonia wet rot
(Rhizoctonia solani Kuhn) and Fusarium black root rot (Fusarium
solani Mart. Amend Sacc.) and root-knot disease by Meloidogyne
incognita (Kofoid and White) Chitwood. Parasitism by M. incognita
in chickpea involves giant cells formation and root galling Vovlas et
al. [29]. Rhizoctonia wet root causes severe losses from seedling to
maturity stages Jayalakshmi et al. [12]. Its characteristic symptoms
include root rotting and gradual yellowing and wilting of foliage,
the rotted, discolored tissues are soft and wet. Fusarium black root
rot also results in severe losses to of chickpea crop Jayalakshmi et
al. [12]. The root system is generally rotted with most of the finer
roots destroyed, while the taproot remains intact, but dark and
necrotic.
Silicon (Si) application is useful for increasing plant growth.
Use of Si overcome biotic stresses and also improves plant’s physiological and mechanical properties Epstein [7]; Ma and
Yamaji [10]. Si also enhances disease resistance against pest and
pathogens Marschner [18].
Frequent occurrence of F. solani, R. solani and M. incognita was
observed in the chickpea fields of Aligarh, U.P. India. Infected plants
were found with symptoms of diseases having poor plant growth.
Silicon dioxide nanoparticles (SiO2 NPs) and Rhizobium ciceri were
used alone and in combination management of F. solani, R. solani
and M. incognita on chickpea. Effects of SiO2 NPs and R. ciceri were
also observed on chickpea growth, photosynthetic pigments and
also on proline contents.
Materials And Methods
Chickpea fields were surveyed for the collection disease materials of Aligarh U.P. Samples (root and soil) were collected and placed at 4oC until processing in refrigerator. The presence of plant parasitic nematodes and fungi in the collected samples were examined.
Isolation of fungi from infected chickpea
Roots showing disease symptoms were washed with sterilized water three times to remove soil particles. Isolation of fungi was made from infected plant parts Siddiqui et al. [24] on potato dextrose agar (PDA) medium. Identity of Rhizoctonia solani and Fusarium solani was confirmed. Later, pure cultures were separately prepared of both R. solani and F. solani
Root-knot nematode M. incognita
Root-knot nematodes females were dissected out from roots of chickpea having symptoms of root-knot. Later, perineal patterns were prepared for the identification of Meloidogyne sp. Taylor and sasser [27].
Preparation of SiO2NPs suspension
SiO2 NPs (Sigma-Aldrich, product No. 637246-50G) was used to prepare 0.10 mg ml-1 suspension by dissolving 100 mg nanapowder in 1-liter sterilized water. Foliar spray of 10 ml suspension was made on seedlings (15-day old) in a greenhouse with small spray pumps. Distilled water (10 ml) was sprayed on control plants and each treatment had five replications.
Effect of SiO2 NPs on M. incognita
The effect of SiO2 NPs was studied on the M. incognita. Egg masses (20) of average size from eggplant roots were picked with sterilized forceps and effect of 0.10 mg ml-1 NPs on the hatching and mortality was observed as described by Siddiqui et al. [24].
Effect of SiO2 NPs on the fungi
Activity of SiO2 NPs against both fungi was observed separately. Ten ml NPs (0.10 mg ml-1 conc.) was added in 100 ml autoclaved PDA to observe effect as described by Siddiqui et al. [24]
Preparation and sterilization of soil mixture
Collected loam soil was sieved through 10 meshes. Clay pots were filled with 1 kg of soil mixed with loam soil and river sand (3:1). Before sterilization, soil surface of pots was wet with water, sterilized at 137.9 kPa for 20 minutes and allowed for cooling 24 hours.
Raising and maintenance of test plants
Chickpea (Cicer arietinum L.) seeds Pusa-3022 were sterilized and sown in 15 cm pots Siddiqui et al. [24]. Seedlings were inoculated and un-uninoculated control were placed in a glass house at 20 ± 2oC (Table 2). Pots were arranged on a bench and each treatment had five replications. Watering of plants was done as required. Ninety days after inoculation plants were harvested Inocula of fungi
Richard’s liquid medium was used for culturing of both fungi separately Riker and Riker [22] for obtaining inocula. Eighty ml liquid medium after filtration through muslin cloth was placed 250 ml flasks, autoclaved at 103.4 kPa for 15 minutes. Each fungus was separately inoculated, incubated, mycelia mats were collected, blotting sheets were used to remove excess water and inoculum of each fungus was prepared in Waring blender as described Siddiqui et al. [24]. Ten ml of this suspension was used as inculum (1 g fungus).
Nematode inoculum
Infected chickpea roots were used to collect Meloidogyne incognita, eggplants for its multiplication. Later, inoculum was prepared, juveniles were counted and 2000 juveniles were inoculated as described by Siddiqui et al. [24]
Rhizobium inoculum
Rhizobium ciceri (100 g) charcoal culture was dissolved in 1 liter sterilized water. Ten ml (1 g inoculum) around the seeds per pot was inoculated where required.
Inoculation technique
Rhizobium ciceri was used with seeds at sowing in about half seeds. Inoculation of R. solani, F. solani and M. incognita, chickpea seedlings (two weeks old) were used and inoculations were done Siddiqui et al. [24]. There were 2 treatments i.e. (1) Control; (2) Rhizobium ciceri. Each of these was tested with (I) Control; (II) R. solani; (III) F. solani; (IV) M. incognita (4×2=8). These 8 treatments were tested in two combinations (i) without SiO2 NPS spray (ii) With SiO2 NPs spray (8×2= 16). Each treatment had 5 replications (16×5=80 pots).
Evaluation of the experiment
Ninety days after inoculation the plants were harvested. Plant growth attributes, photosynthetic pigments, proline and disease index, galling and nematode population were recorded Siddiqui et al., [24].
Disease index
Disease index were determined on disease symptoms. Disease rating was done on 0 (no disease) to 5 (severe rot / blight) Nesha and Siddiqui [19] Estimation of photosynthetic pigments and proline content Photosynthetic pigments (chlorophyll and carotenoid) in the fresh leaf samples were estimated by Mackinney [17] and proline content by Bates et al. [3].
Statistical analysis
The data were analysed through three ways (Rhizobium cicer × SiO2 NPs × Pathogens) analysis of variance (ANOVA) in R (version 2.14.0) statistical software (package- library agricolae). Least significant difference (L.S.D) were calculated at p=0.05. Duncan’s new multiple range test (DNMRT) were employed to denote the significant differences between treatments. Graphs of nematode population and galls per root system were prepared using Sigma Plot™ and error bars showing standard error.
Results
Effects on SiO2 NPS on pathogenic fungi and on M. incognita
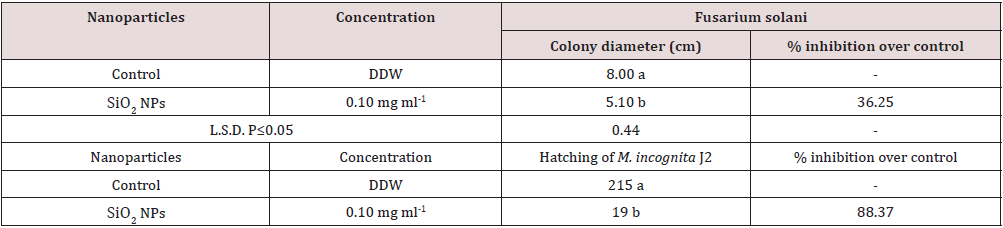
Effects of SiO2 NPs were studied on R. solani and F. solani (Table 1). It had a higher adverse effect on the growth of F. solani followed by R. solani. SiO2 NPs caused 36.25 and 34.60% inhibitions in the growth of F. solani and R. solani respectively (Table 1). SiO2 NPs also had adverse effect on the hatching of M. incognita. It caused 88.37% inhibition in hatching of M. incognita while 43.75% mortality of M. incognita J2 was observed after 48 hours of incubation over control (Table 1).
Table 1:Effect of SiO2 NPs on the growth of R. solani and F. solani in potato dextrose agar medium and on the hatching and mortality of M. incognita.
Pot experiment
Three ways ANOVA revealed that individual effect of Rhizobium ciceri, SiO2 NPs and pathogen, and interaction of R. ciceri × SiO2 NPs, Pathogen × R. ciceri, SiO2 NPs × pathogen and R. ciceri × SiO2 NPs × pathogen was significant in proline content and root nodulation at p=0.05 (ANOVA not shown). However, effect of interaction of R. ciceri × SiO2 NPs × pathogen on shoot length, root fresh weight, root dry weight and photosynthetic pigments was non-significant at p=0.05. Similarly, effect of interaction of Pathogen × R. ciceri on root length, shoot fresh weight, shoot dry weight, root dry weight, chlorophyll was also non-significant at p=0.05. Effect of interaction on SiO2 NPs × pathogen on shoot fresh weight, root dry weight and photosynthetic pigments were also non-significant at p=0.05. However, Individual effect of R. ciceri, SiO2 NPs and pathogen and their interactions were significant on other parameters studied (ANOVA not shown).
Effect on plant growth and nodulation
Inoculation of plants with pathogens under study i. e. M. incognita, F. solani and R. solani resulted in a significant reduction
in plant growth parameters over uninoculated control (Table 2).
Inoculation of M. incognita resulted in a higher reduction (26.07%)
in shoot dry weight followed by R. solani (23.92%) and F. solani
(20.10%). Similarly, inoculation of M. incognita resulted in a
higher reduction (25.42%) in root dry weight followed by R. solani
(23.73%) and F. solani (20.33%). Plants without R. ciceri had poor
nodulation while plants with R. ciceri had higher nodulation (Table
2).
Foliar spray of SiO2 NPs resulted in a significant increase in
plant growth parameters over plants with and without pathogen
(Table 2). Spray of SiO2 NPs to plants without pathogen caused
25.36% increase in shoot dry weight over uninoculated control.
Foliar spray of SiO2 NPs to plants with M. incognita caused 33.01%
increase in shoot dry weight while plants with F. solani and R. solani
resulted in 39.22% and 39.31% increase in shoot dry weight over
plants with respective pathogen alone (Table 3). Foliar spray of SiO2
NPs caused 38.98% increase in root dry weight over uninoculated
control. Spray of SiO2 NPs to plants with M. incognita, F. solani and R. solani resulted in 45.45%, 53.19% and 53.33% increase in root dry
weight respectively. Nodulation in plants without R. ciceri remain
unaffected by inoculation of pathogens and SiO2 NPs spray because
of very poor nodulation (Table 2).
Table 2:Effects of SiO2 NPs and Rhizobium ciceri alone and both together on F. solani, R. solani and M. incognita and on the growth attributes and nodulation of chickpea.Values within a column followed by the same letter are not significantly different at p=0.05 by DNMRT.
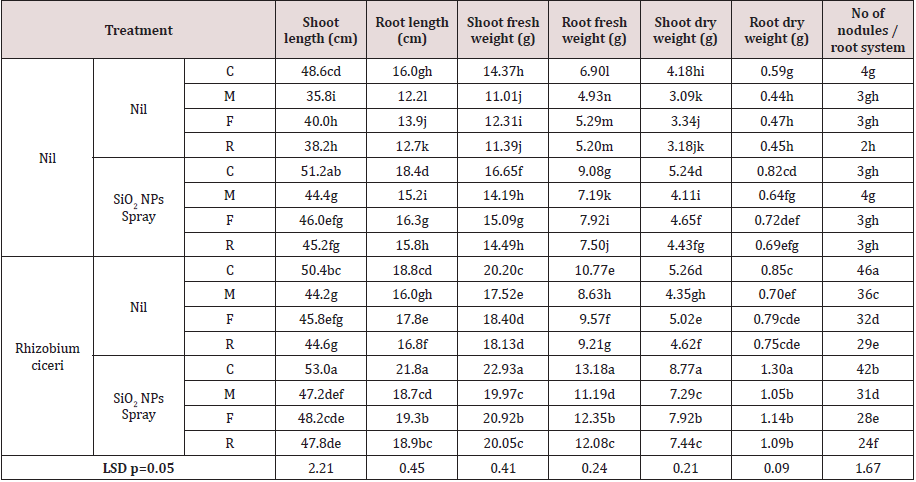
Table 3: Effects of SiO2 NPs and Rhizobium ciceri alone and both together on F. solani, R. solani and M. incognita and on the photosynthetic pigments (chlorophyll and carotenoid) proline content and disease index of chickpea.
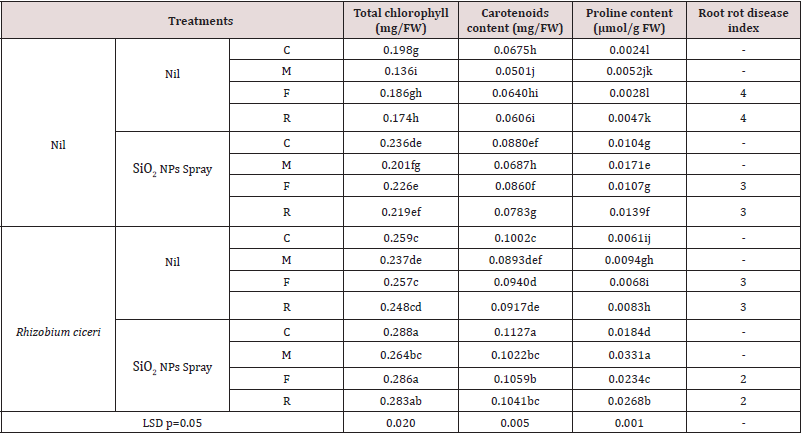
Values within a column followed by the same letter are not significantly different at p=0.05 by DNMRT.
Inoculation of R. solani resulted in a significant increase in plant
growth parameters over pathogen inoculated and un-inoculated
control (except shoot length in uninoculated plants) (Table2).
Inoculation of R. solani resulted in 25.84% increase in shoot dry
weight over uninoculated control. Use of R. solani to plants with M.
incognita, F. solani and R. solani resulted in 40.78, 50.30 and 45.28%
increase in shoot dry weight respectively over their respective
inoculated control (Table 2). Inoculation of R. solani caused 44.06%
increase in root dry weight over uninoculated control. Similarly,
chickpea plants grown with R. solani and with M. incognita,F. solani
and R. solaniresulted in 59.09, 68.09 and 66.67% increases in root
dry weight over respective pathogen inoculated plants. High root
nodulation was observed in plants with R. solani while pathogens
under study had adverse effect on root nodulation caused by R. solani (Table 2).
Foliar spray of SiO2 NPs together with R. solani caused a highest
increase in plant growth parameters over both un-inoculated and
pathogen inoculated control (Table 2). Spray of SiO2 NPs with R. solani resulted in 109.80% increase in shoot dry weight over control.
Similarly, spray of SiO2 NPs plus inoculation of R. solani to plants
with M. incognita,F. solani and R. solani caused 139.92, 137.13
and 133.96% increase in shoot dry weight over their respective
inoculated control (Table 2). Use of R. solani with SiO2NPs resulted
in 120.33% increase in root dry weight over control. However,
spray of SiO2NPs to plants with R. solani and test pathogens i.e.
M. incognita,F. solani and R. solani resulted in 138.64, 142.55
and 142.22% increase in root dry weight over respective control.
Pathogen under study had adverse effect on root nodulation caused
by with R. solani and spray of SiO2 NPs also adversely affect root
nodulation (Table 2).
Effect on photosynthetic pigments
Inoculation of plants with M. incognita, and R. solani caused a significant reduction in photosynthetic pigments i. e. chlorophyll and carotenoid contents over uninoculated plants (Table 3). Foliar spray of SiO2 to plants with pathogens under study caused a significant increase in photosynthetic pigments over plants with pathogen alone. Foliar application of SiO2 caused 19.19 and 30.37% increases in chlorophyll and carotenoid respectively over control. Similarly, foliar spray of SiO2 to plants with M. incognita, R. solani and F. solani resulted in 21.50 to 47.79% increase in chlorophyll and 29.21 to 37.13% increase in carotenoid over respective inoculated control. Inoculation of R. ciceri caused 30.80 and 48.44% increase in chlorophyll and carotenoid respectively over control. Similarly, R. ciceri inoculation to plants with M. incognita, R. solani and F. solani resulted in 42.53 to 74.26% increase in chlorophyll and 46.88 to 78.24% increase in carotenoid contents. Combined use of R. ciceri together with SiO2 NPs caused 45.45 and 66.96% increase in chlorophyll and carotenoid respectively over control. Similarly, use of R. ciceri with SiO2 NPs to plants with M. incognita R. solani and F. solani caused 53.76 to 94.11% increase in chlorophyll and 65.47 to 103.99% increase in carotenoid contents (Table 3).
Effect on proline contents
A significant increase in proline contents was observed in plants with M. incognita, R. solani and R. ciceri over control (Table 3). Plants with M. incognita, caused a higher increase in proline contents than by R. solani. Foliar spray of SiO2 or inoculation of R. ciceri also increased proline contents. Use of SiO2 NPs together with R. ciceri resulted in highest increase in proline contents over other treatments (Table 3).
Disease indices
Wet root rot and black root rot disease indices were found 4 in plants with R. ciceri and R. solani respectively (Table 3). Disease indices were 3 in plants with R. solani / R. solani and sprayed with SiO2 NPs or inoculated with R. ciceri. Combined use of SiO2 NPs with R. ciceri reduced disease indices to 2 (Table 3).
Root galling and nematode population
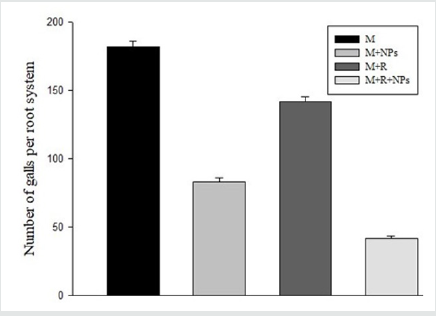
Inoculation of M. incognita inoculated alone resulted in high root galling and nematode population (Figure 1, 2). Foliar spray of SiO2NPs or inoculation of R. ciceri reduced galling and nematode population. SiO2NPs spray caused a higher reduction in galling and nematode population as compared to R. ciceri inoculation. Spray of SiO2NPs together with R. ciceri caused highest reduction in nematode population and galling (Figure 1, 2).
Figure 1: Effect of SiO2 NPs (NPs) and Rhizobium ciceri (R) on the population of M. incognita (M) on chickpea.
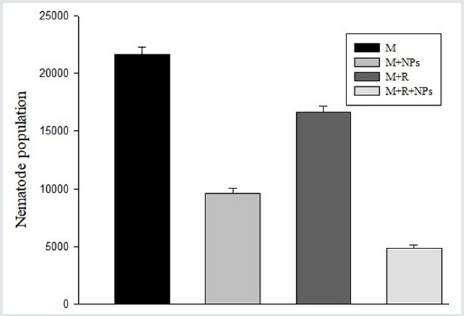
Figure 2: Effect of SiO2 NPs (NPs) and Rhizobium ciceri (R) on the galling of M. incognita (M) on chickpea.
Discussion
SiO2 NPs showed antifungal effect in PDA medium because
growth of R. ciceri, and F. solani was inhibited. Similarly, Akpinar
et al. [1] found that SiO2 NPs resulted in maximum inhibition of F. oxysporum f. sp. radicis lycopersici. SiO2 NPs also showed adverse
effect on M. incognita J2 hatching. Observation of hatched J2 showed
straight body shape and intake of NPs in J2 of M. incognita within
48 h of incubation. Silica nanoparticles caused degeneration of
reproductive organs of nematodes Pluskota et al. [21]. The silicon
carbide nanoparticles had lethality as dead nematodes exhibited
black internal organs Al Banna et al. [2]. It is possible that SiO2
NPs taken by the nematodes were translocated to primary and
secondary organs Wu et al. [32]; Al Banna et al. [2] resulting in
deformation of J2 of M. incognita.
In pot experiment, foliar spray of SiO2 NPs increased growth,
chlorophyll and carotenoids contents over uninoculated control.
Application of Si in plants results in its accumulation and exerts
various beneficial effects on plants Liang et al. [14]. Absorbed Si
alleviates various abiotic stresses Wang et al. [31]. Si contributes
to overcome stresses also may improves plant’s mechanical and
physiological properties Epstein [7]; Ma and Yamaji [16]. Increased
growth of plants without pathogen (uninoculated control) by SiO2
application can be due to above reasons.
Foliar spray of SiO2 NPs increased growth attributes of plants
with pathogen. Applications of Si provide resistance against
diseases Fauteux et al. [8]; Marschner [18]. Various mechanisms are
involved plant disease resistance i.e. defense barriers after pathogen
infection, activation enzymes (defense-related), production of
antimicrobial compounds and regulation of genes Wang et al. [31].
Si mediated resistance against pathogens was associated with the
changes in activity of soil enzyme and soil microorganisms Wang
et al. [31]. Silicon amendment also reduced disease incidence of
tomato Diogo and Wydra [5].
Foliar spray of SiO2 can prevent pathogen penetration by
formation of a cuticle-Si double layer and decrease disease
incidence Ma and Yamaji [16]. Most Si improves mechanical
properties and regeneration by cross-linking with hemicellulose in
cell walls Guerriero et al. [11].
Proline contents were found to be increased by the spray
of SiO2 NPs in this study. Proline is known to confer tolerance to
plants Lehmann et al. [13]. It is a multi-functional amino acid and
accumulation of proline acts as a major stress response against
pathogens Grote et al. [10]. A correlation between increase in
proline contents and increase defense response against pathogens
Cecchini et al. [4] has been observed. Increased proline content was
also observed in Arabidopsis thaliana Verslues and Sharma [28] in
plant defense against pathogen. Therefore, in this study increase in
proline contents in plants with pathogen was also observed.
Disease indices were found 4 in plants with fungal pathogens viz. R. solani and F. solani. Foliar spray of SiO2 reduced disease indices to 2. Reduction in disease indices by application of SiO2 also confirms antifungal nature of SiO2. SiO2 NPs demonstrated its nematicidal activity by reduction in galling and nematode population. R. ciceri also showed antifungal and nematicidal activity by reducing disease indices, galling and M. incognita population therefore, increase in plant growth. Rhizobial strains have biocontrol properties and can lead to potential control Gopalkrishnan et al. [9]. Rhizobium enhanced resistance level against pathogen by inducing changes in seed proteome and metabolome Sistani et al. [26]. Foliar application of SiO2 NPs had mild adverse effect on rhizobial root nodulation while combined application of NPs and R. ciceri reduce disease severity. This may be possible because of least concentration NPs used as foliar spray. The influence of broad-spectrum of even pesticides and their tolerance to the rhizobia are reported Singh and Wright [25]. It is possible that the R. ciceri used in the present study may have tolerance to SiO2 NPs thereby resultant increase in growth attributes, chlorophyll, carotenoid and proline contents.
Conclusion
SiO2 NPs had displayed antifungal and nematicidal activities towards tested fungi and root knot nematode. Similarly, R. ciceri also provided biocontrol of M. incognita and root rot fungi. Use of SiO2 NPs with R. ciceri was better for the management of pathogens under study than their use alone. Therefore, researches on characterizing of resistant rhizobia are needed so they can be used for the management of chickpea diseases along with SiO2 NPs.
Acknowledgements
Necessary facilities provided by Aligarh Muslim University Aligarh, India are thankfully acknowledged.
References
- Akpinar I, Sar T, Unal M (2017) Antifungal effects of Silicon dioxide nanoparticles (SiO2 NPs) against various plant pathogenic fungi. Conference: International Workshop Plant Health: Challenges and Solutions 23-28.
- Al-Banna L, Salem N, Ghrair A. M, Habash SS (2018) Impact of silicon carbide nanoparticles on hatching and survival of soil nematodes Caenorhabditis elegans and Meloidogyne incognita. Appl Ecol Env Res 16(3): 2651-2662.
- Bates LS, Waldren RT, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39: 205-207.
- Cecchini NM, Monteoliva MI, Alvarez ME (2011) Proline dehydrogenase contributes to pathogen defense in Arabidopsis. Plant Physiol 155(4): 1947-1959.
- Diogo RVC, Wydra K (2007) Silicon-induced basal resistance in tomato against Ralstonia solanacearum is related to modification of pectic cell wall polysaccharide structure. Physiol Mol Plant Pathol 70 (4-6): 120-129.
- Duke JA (1981) Handbook of legumes of world economic importance. Plenum Press New York 52-57.
- Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50: 641-664.
- Fauteux F, Remus-Borel W, Menzies JG, Belanger RR (2005) Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol Lett 249(1): 1-6.
- Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK, Gowda CLL, et al. (2018) Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech 5(4): 355-377.
- Grote D, Schmit R, Claussen W (2006) Water uptake and proline index as indicators of predisposition in tomato plants to Phytophthora nicotianae infection as influenced by abiotic stresses. Physiol Mol Plant Pathol 69(4-6): 121-130.
- Guerriero G, Hausman JF, Legay S (2016) Silicon and the plant extracellular matrix. Front Plant Sci 7: 463.
- Jayalakshmi SK, Usharani S, Benagi VI, Mannur DM (2008) Sources of resistance to dry root rot of chickpea caused by Rhizoctonia bataticola. Agric Sci Digest 28 (2): 147 -148.
- Lehmann S, Funck D, Szabados L, Rentsch D (2010) Proline metabolism and transport in plant development. Amino Acids 39: 949-962.
- Liang YC, Wong JWC, Wei L (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil Chemosphere 58(4): 475-483.
- Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci., 11(8): 392-397.
- Ma JF, Yamaji N (2008) Functions and transport of silicon in plants. Cell. Mol. Life Sci., 65(19): 3049-3057.
- Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140: 315-322.
- Marschner P(2012) Marschner’s Mineral Nutrition of Higher Plants. London: Academic Press pp 672.
- Nesha R, Siddiqui ZA (2013) Interactions of Pectobacterium carotovorum pv. carotovorum, Xanthomonas campestris pv. carotae, and Meloidogyne javanica on the Disease Complex of Carrot. Int J Veg Sci 19(4): 403-411.
- Parveen G, Noreen R, Shafique HA, Sultana V, Haque SE, et al. (2019) Role of rhizobia in suppressing the root diseases of soybean under soil amendment. Planta daninha 37.
- Pluskota A, Horzowski E, Bossinger O, von Mikecz A (2009) In Caenorhabditis elegans nanoparticle-bio-interactions become transparent: silica-nanoparticles induce reproductive senescence. PLoS One 4(8): e6622.
- Riker AJ, Riker RS (1936) Introduction to Research on Plant Diseases: A Guide to the Principles and Practice for Studying Various Plant-disease Problems. John’s Swift Co. Inc., St. Louis, Chicago, New York, India 3: pp. 119.
- Siddiqui ZA, Mahmood I (2001) Effects of rhizobacteria and root symbionts on the reproduction of Meloidogyne javanica and growth of chickpea. Bioresour Technol 79(1): 41-45.
- Siddiqui ZA, Parveen A, Ahmad L, Hashem A (2019) Effects of graphene oxide and zinc oxide nanoparticles on growth, chlorophyll , carotenoids, proline contents and diseases of carrot. Sci Hort 249: 374-382.
- Singh, G, Wright D (2002) In vitro studies on the effects of herbicides on the growth of rhizobia. Lett Appl Microbiol 35(1):12-16.
- Sistani NR, Kaul HP, Desalegn G, Wienkoop S (2017) Rhizobium Impacts on Seed Productivity, Quality, and Protection of Pisum sativum upon Disease Stress Caused by Didymella pinodes: Phenotypic, Proteomic, and Metabolomic Traits. Front Plant Sci 8: 1961.
- Taylor AL, Sasser JN (1978) Biology, Identification and Control of Root Knot Nematodes (Meloidogyne species). A cooperative publication of North Carolina State University, Dept of Plant Pathology and USAID, Raleigh NC, USA 7: pp. 111.
- Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book 8: e0140.
- Vovlas N, Rapoport HF, Jiménez Díaz RM, Castillo P (2005) Differences in feeding sites induced by root-knot nematodes, Meloidogyne spp., in chickpea. Phytopathology 95(4): 368-375.
- Wang L, Cai K, Chen Y, Wang G (2013) Silicon-mediated tomato resistance against Ralstonia solanacearum is associated with modification of soil microbial community structure and activity. Biol Trace Elem Res 152(2): 275-283.
- Wang M, Gao L, Dong S, Sun Y, Guo SQ (2017) Role of Silicon on Plant–Pathogen Interactions. Front Plant Sci 8: pp. 701.
- Wu S, Lu J, Rui Q, Yu S, Cai T, et al. (2011) Aluminum nanoparticle exposure in L1 larvae results in more severe lethality toxicity than in L4 larvae or young adults by strengthening the formation of stress response and intestinal lipofuscin accumulation in nematodes. Environ Toxicol Phar 31(1): 179-188.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...



