Lupine Publishers Group
Lupine Publishers
Menu
Mini reviewOpen Access 
Ethanol Synthesis from Renewable Biogas and Ladfill Gas Sources Volume 2 - Issue 3
Savvas Vasileiadis1, Artemis Vasileiadou2, Zoe Ziaka1*, Elias Fatmelis1 and Christina Spourtoudi1
- 1Department of Catalysis and Environmental Protection, School of Technology and Physical Sciences, Hellenic Open University, Greece
- 2Department of Physics, Aristotle University of Thessaloniki, Greece
Received: March 15, 2023; Published: March 21, 2023
Corresponding author: Zoe Ziaka, Department of Catalysis and Environmental Protection, School of Technology and Physical Sciences, Hellenic Open University, Greece
DOI: 10.32474/JBRS.2023.02.000140
Abstract
An efficient catalytic method of ethanol synthesis is presented from synthesis gas based on waste biogas and landfill gas resources. A significant interest for wise utilization of waste biogas and landfill gases can be essential in the renewable area of energy and chemicals production. Improved practices for ethanol production that contribute to enhanced utilization of renewable resources are investigated in this article. Ethanol can be produced via a direct catalytic one-step process from synthesis gas coming out from a conventional reactor or from a membrane type reformer. The flowsheet of the corresponding process is presented to show the pathway of the reactors system.
Keywords: Ethanol production; biogas and landfill-gas; waste utilization; renewable energy; catalytic reformer; membrane reformer; hydrogen and syn-gas production
Introduction
The production of biogas and landfill gas resources have been growing steadily for the last 25 years. There is a number of different sources today that produce these gases at global scale, especially as the earth population increases. Today, most of the developed places in the world are looking for a relief from the increasing demand of energy and the large cost of oil and natural gas. This fact has heightened the focus in utilizing alternative and renewable energy sources. It is predicted that after 2050’s, more than 50% of world energy demand will be produced from renewable energy resources. Energy security, economic development and protection of the today’s world resources are the priorities of the national energy policy for many countries in the modern world. Utilization of wastegases can be a partial solution to the requirements and expectations of the proposed renewable energy sources development. In this short communication we report a new design and process for the capability of ethanol synthesis /production from renewable feedstocks such as biogas and landfill gas type sources. Ethanol is a very important chemical component in the chemical industry. It can be used as antiseptic, antidote, anesthetic, medical solvent, for making several drugs in the pharmacology area, as an engine and rocket fuel, in a DEFC (direct ethanol fuel cell), in household heating and cooking, as a feedstock and chemical solvent, and in other uses as well. The chemical compositions of typical waste gases, coming from various sources, are shown in detail in the Table 1 [1].
Results and Discussion
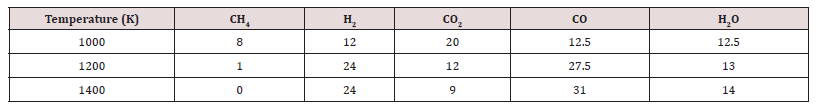
Syngas coming from the reforming of renewable sources such as biogas and landfill gases can be converted directly into ethanol in a catalytic synthesis reactor with significant yield and selectivity [2,3]. The syngas production outcome at various temperatures is presented in the Table 2 below [1]. Reforming of the above gases can take place catalytically usually in a fixed bed catalytic reactor or in a catalytic membrane reactor [4-6]. Mostly Ni, Cr, Rh, and Ru catalysts and their mixtures, are used in the reforming of biogas and landfill gases after their initial purification. Pd, Pt, Co and Fe catalysts have been also used but with lower yields in synthesis gas. The direct ethanol process from the CO and H2 gas constituents is projected more economic and efficient from other competent methods of ethanol synthesis [2,7,8].
Table 2: Syngas production (kmol/ton of biomass) at various Temperatures (K) and Pressure of 24 atmospheres.
The direct exothermic synthesis reaction is given below:
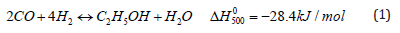
CO and hydrogen are the direct products from the reforming reactions of such renewable gases as described in previous communications [8-11]. The optional use of the membrane in the reforming reactor (membrane reformer or permreactor) provides more hydrogen at the exit streams so that the stoichiometry of the reactants in equation (1) is more easily satisfied. Various catalysts and their mixtures can be used for ethanol synthesis such as Cu, Co, and Rh, Ru, Pd, Pt and Fe metals as described in previous pertinent communications [2,3,7,8,10,11]. Depending on the catalyst used and the other details of the reaction, the conversion, yield, and selectivity to ethanol varies at different levels. The corresponding analysis is a subject of future study as well. A detailed flowsheet of the processes is shown in Figure 1.
In Figure 2 there are experimental and simulation/modeling results for the methane conversion from the catalytic plug flow reactor (PFR) of the reforming reaction. The conversion is increased as the temperature increases as it is expected since it is an overall endothermic reaction. It also increases as the space time of the reactor increases. The significance of this work is the fact that there is a satisfactory agreement between the modeling and experimental data (Figure 2). Beyond ethanol synthesis the valuable synthesis gas coming out of the reformer can be used in other usages such as a feed in high temperature solid oxide and molten carbonate fuel cells for electricity generation. Work in this area is continuing within our research group [12].
Figure 2: Methane Conversions vs Space times at various Temperatures from both experimental and simulation/modeling data of a Plug Flow Catalytic Reactor.

Conclusion
In this paper, the direct production of ethanol from synthesis gas has been discussed using biogas and/or landfill gas sources. A catalytic reformer or membrane reformer for syngas production is presented including experimental and modeling results that show satisfactory fitting at various temperatures and space times. The various catalysts needed are proposed for the examined reaction systems. The entire process is shown in a detailed flowsheet. Energy security, economic improvement, and environmental protection of the various resources have to be the priorities for every country in the modern society. Concluding, turning waste gases into chemicals (such as ethanol) is not only a viable solution with important potential to reduce or even eliminate dependence on fossil fuels, but also a wise and efficient way to produce decentralized energy with a smaller carbon footprint.
References
- Spourtoudi C (2012) Production of Methanol from Biomass: Problems & Prospects. MSc thesis, Hellenic Open University, Greece.
- Ziaka Z, Vasileiadis S (2009) Membrane Reactors for Fuel Cells and Environmental Energy Systems. Xlibris Publishing.
- Vasileiadis S, Ziaka Z (2005) Permreactor and separator type fuel processors for production of hydrogen and hydrogen, carbon oxides mixtures. US Patent No 6,919,062 B1.
- Vasileiadis S, Ziaka-Vasileiadou Z (2004) Biomass reforming process for integrated solid oxide-fuel cell power generation. Chem Eng Sci 59(22): 4853-4859.
- Panteloglou A A, Ziaka Z D, Vasileiadis S P (2021) An Alternative to Flare Gas Processing: A Feasibility Study of Natural Gas to Liquid Processes. J Mater Sci Eng A 11(1-3): 11-25
- Xu J and Froment GF (1989) Methane Steam Reforming, Methanation and Water-Gas Shift: Intrinsic Kinetics. AIChE Journal 35(1): 88-96.
- Zuo ZJ, Peng F, Huang W (2016) Efficient Synthesis of Ethanol from CH4 and Syngas on a Cu-Co/TiO2 Catalyst Using a Stepwise Reactor. Sci Rep 3(6): 34670.
- Kang I, He S, Zhou W, Shen Z, Li Y, et al. (2020) Single-pass transformation of syngas into ethanol with high selectivity by triple tandem catalysis. Nat Commun 11(1): 827.
- Vasileiadis S, Ziaka-Vasileiadou Z (2004) Efficient Catalytic Reactors-Processors for Fuel Cells and Synthesis Applications. Sep Purif Technol 34(1): 213-225.
- Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, et al. (2015) Synthesis of Ethanol from Syngas over Rh/MCM-41 Catalyst: Effect of Water on Product Selectivity. Catalysts 5(4): 1737-1755.
- Choi YM, Liu P (2009) Mechanism of Ethanol Synthesis from Syngas on Rh (111). J Am Chem Soc 131(36): 13054–13061.
- Vasileiadis S, Ziaka Z, Vasileiadou A, Dova M (2022) Utilization of Renewable Biogas and Landfill gases as chemical Production and Power Sources. J Biosens & Renew Sci 1(5): 130-131.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...