
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5752
Case Report(ISSN: 2690-5752) 
Efficiency and Safety of Prophylaxis for Pregnancy Carrier of Mutation in Gene F12 Volume 8 - Issue 2
Victoria López MD PhD1*, Gabriel Antonio Colamarco MD1, Maria Dolores de las Marinas MD PhD2, Raquel Rodríguez López MD PhD3, Daniela Duica1 and Kiri Duke1
- 1Allergology Department, Denia Hospital, Alicante, Spain
- 2Allergology Department, Consorcio Hospital General Universitario, Valencia, Spain
- 3Genetics Laboratory, Consorcio Hospital General Universitario, Valencia, Spain
Received:April 20, 2023; Published: April 27, 2023
Corresponding author:Victoria López, Specialist in Allergology (MD: Medical Doctor, PhD: Doctor), Denia Hospital, Alicante, Spain
DOI: 10.32474/JAAS.2023.08.000282
Abstract
Hereditary angioedema type 3 (HAE III) is an entity with very precise clinical and molecular characteristics, whose confirmatory diagnosis is made by genetic study. In large series of patients, the recurrent mutation c.983C>A, p. (Thr328Lys) in the F12 gene coding for coagulation Factor XII (FXII) has been demonstrated. It is widely accepted that the gene expression and plasma levels of FXII are regulated by oestrogens, both endogenous and exogenous, making pregnancy the main risk factor for women carrying this and/or other alterations on this major susceptibility gene. C1 inhibitor concentrate remains the only treatment available for pregnant women with this disease. We present the case of a 34-year-old woman heterozygous carrier of the recurrent mutation of the F12 gene, who, from her pregnancy showed a worsening of the angioedema, predominantly affecting the limbs and abdomen. The establishment of long-term personalized prophylaxis with intravenous C1 inhibitor concentrate (Cinryze) controlled the angioedema crises. She also required short-term prophylaxis and prior to fetal eversion, which she passed without incident. Following the recommendations of the national and international guides, this case reaffirms the safety and efficacy of treatment with C1 inhibitor for pregnant women with hereditary angioedema and their fetuses.
Keywords:Hereditary angioedema type 3; F12 gene mutation; congenital FXII deficiency; pregnancy; prophylaxis
Introduction
Bradykinin-mediated, non-histaminergic, angioedema is established after a temporary increase in vascular permeability secondary to a local and transient increase in bradykinin levels due to activation of the contact system. Familial or hereditary cases (HAE) have an estimated average prevalence of one case per 50,000 inhabitants [1,2], for which more than 300 genetic alterations involving the C1INH gene, abnormalities in the coagulation factor XII gene, alterations in the plasminogen gene, the angiopoietin-1 gene, the kininogen-1 gene or the myoferlin gene are known [2- 4]. Hereditary angioedema type 3 (HAE III) (OMIM#610618) is a specific entity, and with significant frequency among cases of angioedema, whose episodes are much more evident in women; very frequently it is diagnosed, precipitated or worsened by oestrogen elevation (pregnancy or taking oral contraceptives) [1].
Biochemically, it is pathognomonic to obtain normal C1 inhibitor (C1NH; 606860) concentrations and function. The molecular basis of the pathology is Coagulation Factor XII deficiency, due to alterations in the F12 gene (OMIM#610619), with an autosomal dominant inheritance pattern, with high penetrance but variable expressivity. The population distribution of the recurrent NM_000505.4 (F12): c.983C>A, p. (Thr328Lys) mutation in this type of patient has been extensively described [5,6], with a comprehensively detailed phenotype in both female and male carriers [7].
The symptoms of angioedema are variable, with differences in their frequency, intensity and severity among individuals, among members of the same family, and even in the same subject at different times of their life [8]. It is impossible to predict why and when an episode of angioedema will occur, although some triggers such as trauma, stress and hormonal changes have been identified [9]. In reference to this last factor, Deroux et al. [10] described one of the largest series of patients with hereditary angioedema due to mutation of factor XII and identified that 89% of the angioedema episodes in their patients were related to hormonal changes (pregnancy, menstruation, menopause or contraceptive treatment), suggesting that this type of hereditary angioedema is the most influenced by hormonal changes. The administration of C1 inhibitor concentrate is indicated as short- and long-term treatment and prophylaxis in patients with HAE crisis in whom other drugs are ineffective or are even contraindicated. For the time being, it is the only one available for pregnant women [9], and to date no adverse health events have been reported in pregnant women or their fetuses as a consequence of its administration [11].
Description of the Case
We present the case of a 34-year-old female, since she was 18 years old, presented between 4 and 6 episodes a year of facial oedema (HP:010066/000028), occasionally associated with abdominal pain (HP:000201) or sensation of laryngeal oppression (HP:001227), without improvement with treatment with corticosteroids and oral antihistamines that she has occasionally received. She related her crises preferentially to situations of emotional stress and/or hormonal changes, presenting a history of similar episodes of unknown origin in her mother and two other second to fourth degree relatives in the maternal line. After the suspected diagnosis of HAE, the complement factor determinations (C4, C1 inhibitor and activity) were within normal ranges, therefore genetic was asked to study the hereditary mutation responsible for HAE III or HAE with normal C1 inhibitor, confirming the presence of a c.983C>A p. (Thr328Lys) variant in heterozygosis in the coagulation factor XII gene. Genetic counseling and diagnosis in eight more family members determined the status of heterozygous carrier of the causal recurrent variant of HAE in the F12 gene in the patient’s mother, her only brother, a maternal uncle, a maternal aunt and a daughter of that maternal aunt.
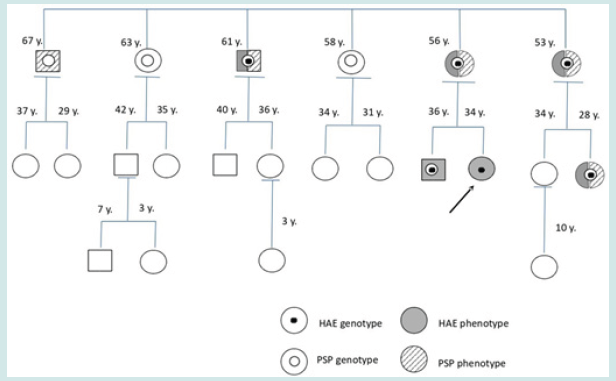
Simultaneously with the study of hereditary angioedema, counseling and family genetic study of PMP22 gene deletion (OMIM*601097) was conducted. Both members, maternal uncles, were carriers and affected by hereditary polyneuropathy due to increased sensitivity to pressure (PSP; OMIM:162500). The existence of the causative deletion in the PMP22 gene was also confirmed in the patient`s mother and brother, all her maternal aunts and uncles, and her HAE- carrying cousin; the deletion was ruled out in our patient (Figure 1) [12]. During the years of follow-up, the patient required self-administration of icatibant acetate (Firazyr) in isolation and with good response, during some of the angioedema episodes, which were predominantly localized on the face (HP:00028) or extremities. She also received short-term prophylaxis with 1000 IU of intravenous C1 inhibitor concentrate (Cinryze) prior to several dental procedures, which were conducted without incident. However, from the initial weeks of her first pregnancy, the episodes of angioedema appeared with more frequency and intensity, mainly affecting the abdomen and lower limbs. Long-term prophylaxis with intravenous C1 inhibitor concentrate (Cinryze) was indicated, requiring for disease control 500 IU weekly during the first trimester, 1000 IU every 7 days during the second trimester and 1500 IU every 4 days in the last few weeks of pregnancy (being instructed for home self-administration at home).
Figure 1: Family pedigree of angioedema and/or hereditary polyneuropathy. Circles indicate female gender, squares indicate male sex. The described case is marked with an arrow.
At 38 weeks of gestation, obstetrics proposed performing a fetal avoidance maneuver from breech presentation to the head due to intrauterine malposition. The procedure was successfully conducted after the administration of an additional dose of 1000 IU of intravenous C1 inhibitor concentrate (Cinryze) as a short-term pre-procedure prophylaxis one hour before the procedure. The delivery occurred vaginally and without incidents. Currently, the patient is breastfeeding, and she has discontinued Cinryze, having presented no new episodes of angioedema since delivery.
Discussion
Bradykinin-mediated angioedema is a rare disease, to which a wide list of functional genetic variants in the F12 gene have been associated [1,2], so the genetic study of each index case and its direct relatives is essential to obtain a precision diagnosis, as we demonstrated in the case presented. The association between angioedema crises and the oestrogen hormone is one of the main characteristics of angioedema with normal C1 inhibitor, especially in cases due to a mutation of coagulation factor XII, in which the regulatory function of this hormone on the synthesis of factor XII protein and on other proteins of the coagulation cascade and of the kinin-kallikrein system is lost [13,14]. However, the influence of pregnancy on the disease is highly variable [9], which is why Czaller et al. [15] described a number of predictors of the course of hereditary angioedema during pregnancy. Thus, they identified that episodes of angioedema were more frequent and intense in pregnant women who had manifested the disease from an early age and found that women who presented angioedema in relation to menstruation presented episodes more frequently in the last trimester of pregnancy, while those patients who manifested symptoms throughout pregnancy related angioedema crises in other periods of their lives to trauma.
The abdomen was the most frequently affected location during our patient’s pregnancy, which could be explained by mechanical trauma secondary to uterine growth and fetal movements. These are episodes that should be taken into consideration in the evolution of the patients in order to rule out other possible obstetric complications during pregnancy, since an increase in the percentage of spontaneous abortions has been identified in pregnant women with this disease [9,16]. There is no doubt that patients with hereditary angioedema in general and in this case in particular, need a personalized treatment and prophylaxis regimen to achieve disease control and improve their quality of life [9]. In this sense, we recognize that we have encountered the limitation of the COVID-19 epidemic in the registry using the AEQoL questionnaire for the objective determination of changes in quality of life. However, we consider the proximity and availability of our Allergology Unit to be strengths of our workplace, as well as the great capacity for multidisciplinary collaboration, following the recommendations of the experts [16], favoring the integration of the different diagnoses of the patient, so that Gynecologists and Allergists know the described case and the implications of her pathologies, favoring the prevention of exacerbations and remaining alert to her crises.
Ethical considerations do not allow prospective studies in pregnant women, and since hereditary angioedema is a disease with a very low distribution in the general population, data on the treatment of angioedema episodes in pregnant women are limited. For this reason, Baker et al. [11] conducted a retrospective analysis of short and long-term angioedema prophylaxis in 16 women with hereditary angioedema in the gestational period, and their results were consistent with previous studies, demonstrating the safety and efficacy of intravenous C1 inhibitor therapy for this investigated population compared to attenuated androgens, tranexamic acid, and other newer therapeutic options. Subsequently, Brooks et al [14] after a literature review of 40 publications on the use of C1 inhibitor in women with angioedema during pregnancy as short and long-term treatment or prophylaxis, concluded that it is a welltolerated drug by women and their fetuses. Thus, international guidelines advise that C1 inhibitor concentrate should be the drug of choice in acute crises for the treatment of angioedema, for short-term prophylaxis prior to caesarean section, and for longterm prophylaxis in pregnant patients with recurrent or severe episodes of angioedema, or with high-risk pregnancies or a history of spontaneous abortions [9,17,18]. This clinical case provides new information on the administration of C1 inhibitor in a pregnant woman with hereditary angioedema due to F12 gene mutation as prophylaxis prior to the practice of fetal eversion, which was successfully performed with no clinical repercussion for the patient or risk to her fetus.
Conclusions
a. Hereditary bradykinin-mediated angioedema encompasses a group of diseases with the same pathogenesis and identical clinical manifestation but with different genetic causes, so patients with suggestive symptoms and a family history of angioedema require a genetic study to reach an accurate diagnosis.
b. It has been shown that in this case of hereditary angioedema due to mutation of coagulation factor XII with normal levels of C1 inhibitor, short- and long-term personalized prophylaxis during pregnancy via the administration of intravenous C1 inhibitor (Cinryze) controlled the episodes of angioedema with no adverse effects for the patient or the fetus [11,14].
Conflict of Interest
None of the authors have any conflict of interest with respect to this scientific work.
Acknowledgements
All the authors contributed to the writing of the manuscript and reviewed the intellectual content for the publication of this scientific work.
References
- Caballero T, Farkas H, Bouillet L, Bowen T, Gompel A, et al. (2012) International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibidor deficiency. JACI 129(2): 308-316.
- Betschel S, Badiou J, Binkley K, Borici Mazi R, Hérbert J, et al. (2019) The International/Canadian Hereditary Angioedema Guideline. Allergy, asthma and clinical immunology 15(1): 72-75.
- Gibbons KR, Abraham T, Sandhu M, Peppers BP, Girzhel JF, et al. (2017) Successful perinatal management of hereditary angioedema with normal C1 esterase inhibitor and factor XII mutation using C1 esterasa inhibitor therapy. Ann Allergy Asthma Inmunol 119(6):558-559.
- Bork K, Machnig T, Wulff K, Witzke G, Prusty S, Hardt J. (2020) Clinical features of genetically characterized types of hereditary angioedema with normal C1 inhibitor: a systematic review of qualitative evidence. Orphanet Journal of Rare Diseases 15(1): 289-295.
- Moreno AS, Valle SOR, Levy S, Franca AT, Serpa FS, et al. (2015) Coagulation Factor XII Gene Mutation in Brazilian Families with Hereditary Angioedema with Normal C1 Inhibitor. Int Arch Allergy Immunol 166(2): 114-120.
- Grumach AS, Stieber C, Veronez CL, Cagini N, Constantino Silva RN, et al. (2016) Homozygosity for a factor XII mutation in one female and one male patient with hereditary angio-oedema. Allergy 71(1): 119-123.
- (2023) Human Phenotype Ontology. https://hpo.jax.org/app/browse/disease/OMIM:610618. (Accessed February 8, 2023).
- Lopes C, Moreno AS, Constantino Silva RN, Maia LSM, Ferriani MPL, et al. (2018) Hereditary angioedema with normal C1 inhibitor and F12 mutations in 42 Brazilian families. JACI PRACT 6(4): 1209-1216.
- Sánchez Jareño M, Cabañas R, Caballero T (2018) Management of pregnancy in hereditary angioedema. Alergia Astma Immunologia 23(4): 186-192.
- Deroux A, Boccon Gibod I, Fain O, Pralong P, Ollivier Y, et al. (2016) Hereditary angioedema with normal C1 inhibitor and factor XII mutation: a series of 57 patients from the French National Center of Reference for Angioedema. Clin Exp Imm 185(3): 332-337.
- Baker JW, Craig TJ, Riedl MA, Banerjy A, Fitts D, et al. (2013) Nanofiltered C1 esterasa inhibitor (human) for hereditary angioedema attacks in pregnant women. Allergy Asthma Proc 34(2): 162-169.
- (2023) Human Phenotype Ontology. Última revisión 8 de febrero.
- Guth de Freitas Batista de Moraes C, Mikami LR, Pereira Ferrari L, Pesquero IB, Chong Neto HJ, Filho NAR (2020) Short-term Prophylaxis for Delivery in Pregnant Women with Hereditary Angioedema with Normal C1-Inhibitor. Rev Bras Ginecol Obstet 42(12): 845-848.
- Brooks JP, Radojicic C, Riedl MA, Newcomer SD, Banerji A, et al. (2020) Experience with Intravenous Plasma-Derived C1-Inhibitor in Pregnant Women with Hereditary Angioedema: A Systematic Literature Review. JACI In practice 8(6): 1875-1880.
- Czaller I, Visy B, Csuka D, Füst G, Tóth F, et al. (2010) The natural history of hereditary angioedema and the impact of treatment with human C1-ihibitor concentrate during pregnancy: a long-term survey. Eur J Obstet Gynecol Reprod Biol 152(1): 44-49.
- Gabriel N, Marcelino F, Ferriani MPL, Arruda LK, Campos RA, et al. (2022) Pregnancy in patients with hereditary angioedema and normal C1 inhibidor. Frontiers Allergy 3(1): 846968-846970.
- Caballero T, Canabal J, Rivero Paparoni D, Cabañas R (2014) Management of hereditary angioedema in pregnant women: a review. International Journal of Women’s Health 6(1): 839-848.
- Maurer M, Magerl M, Betschel S, Aberer W, Ansotegui IJ, et al. (2022) The international WAO/EAACI guideline for the management of hereditary angioedema - The 2021 revision and update. Allergy 77(7): 1961-1990.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




