Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5752
Research Article(ISSN: 2690-5752) 
Biodynamics of Bronze Age Populations in the Northern Mediterranean Volume 6 - Issue 5
Daniel Turbón*
- Zoology and Anthropology Sub Dept of Evolutionary Biology, Ecology and Environmental Sciences. Faculty of Biology, University of Barcelona, Avda. Diagonal 643, 08028 Barcelona, Spain
Received:March 18, 2022; Published: March 30, 2022
Corresponding author: Daniel Turbón, Zoology and Anthropology Sub Dept of Evolutionary Biology, Ecology and Environmental Sciences. Faculty of Biology, University of Barcelona, Avda. Diagonal 643, 08028 Barcelona, Spain
DOI: 10.32474/JAAS.2022.06.000247
Summary
Ancient DNA from archaeological bones has brought to light relevant information on the molecular characterization of European Bronze Age populations. In this article we reconsider, with multivariate statistical techniques, the craniological studies of several samples from four thousand years ago, from the northern shore of the Mediterranean, carried out during the first half of the twentieth century, to respond to the typological vision that prevailed then. The results indicate that during the Bronze Age, there was a diffusion, from east to west, of minority groups with short and broad heads, probably metal prospectors, which were integrated into the local populations and had different cranial characteristics. This analysis complements the recent study of ancient DNA and provides new insights into the biodynamics of those populations.
Keywords: Migration Craniometrics; Genetic Data; Bronze Age; Mediterranean Basin
Introduction
Major technical advances in Paleogenomics have led to a better understanding of the geographical and demographic expansion of modern humans since the Neolithic period their adaptation to a wide range of climates and ecosystems [1] the relationship between the dispersion of languages and genomics [2]. They have also increased our knowledge of recent human prehistory particularly in Europe [3-5]. Genetic studies in modern populations could only offer limited responses to such questions. Paleogenomics on the other hand can provide a reasonable reconstruction of certain human migrations the dissemination of domestication processes farming and diseases and even the kinship of prehistoric family clans. We now have a better idea of the genomes of Upper Paleolithic genomes [6-8] the Eurasian expansion from the Neolithic period onwards [9-14] and the Y-Chromosomal Genetic Variation in Native South Americans [15].
It has recently been proposed [16] that the mostly male individuals associated with the burials of the Yamnaya nomadic shepherds and who migrated towards Eastern Europe went on to mix with local females. From there, the males migrated principally to Central Europe and mixed with local women as part of a process called the Corded Ware Culture. The theory of a predominantly male migration in the third millennium BC has been debated by Haak et al. [2,17] and also by Lazaridis & Reich [18]. Goldberg et al. [17] estimated a major male bias with approximately 5 to 14 migrating males for every migrating female in the migrations from the Pontic Steppe during the late Neolithic/ Bronze Age. They found evidence of ongoing primarily male migration from the steppe to central Europe over a period of multiple generations with a level of sex bias that excludes a pulse migration during a single generation.
The archaeological and osteological evidence shows that males formed a majority in the Yamnaya burials in Eastern Central Europa and the first Corded Ware burials [19-21]. However, Furholt [19] has pointed out that genetic steppe ancestry is mainly connected to a new kind of burial, rather than to Corded Ware or Bell Beaker materials. Closer integration of anthropological models of mobility and social group and the molecular biological compositions should be explored. In this article we shall use powerful statistical tools to analyses the cranial diversity of the Bronze Age burials of some regions in the Northern Mediterranean coast and we compare our results to the data from the ancient DNA of the regions studied here [9, 22-25].
In some modern human populations, there is a tendency towards cranial brachycephalisation or globalization. Short broad and high crania characterize some human groups. In the database of W.W. Howells [26-28] the series of Asian Buriats and the Austrian Alps present the highest known population averages of the Cephalic Index (CI) in men and women. Cranial globalization at lower values can also be found in the series of Peru Philippines Japan and the Pygmies of the Andaman Islands. The causes of the cranial globalization or brachycephaly are as yet unknown although it is known that it did not take place before the Mesolithic [29].
A set of twin studies recently reaffirmed the strong heritability of cephalometric variables although influenced to a variable degree by changes in DNA methylation a key epigenetic mechanism involved in the developmental regulation of gene expression (see Discussion).
This article explores whether the frequency of brachycephalic skulls in European tombs of the Bronze Age could be related to some expansion from East to West similar to the one mentioned above since in this case the wide and short skulls from Western Europe which are overwhelmingly dolichocephalic stand out in the populations. If so this expansion could be associated with metal prospecting which was already intense at that time (Figure 1).
Figure 1: Metallurgy Diffusion in the Bronze Age. Credit Wikimedia Commons, the free media repository. Public domain.
Material And Method
Samples studied
A number of prehistoric series of the Bronze Age were analyzed (Table 1, Figure 2). An outgroup was also added to our analysis: a sample of 20th century brachycephalic skulls (22 male 18 female) of young adults from the Dinaric Alps (Southern Tyrol and Carinthia) studied by Toldt [30]. The Bronze Age series (2nd millennium BC) are represented by samples of major historical value of the northern and western Mediterranean coasts: from the East
a. Mikénai (Helladic Bronze Age) whose crania come from the burials at Mikénai-Kalkoni Dendra Heraion and Asine [31] and date from 1600 BC to 700 BC including three later crania from the Hellenistic period.
b. Cyprus [32] the sites at Melia Enkomi and Lapithos all with a chronology similar to that of Mikénai.
c. Catalonia [33] whose bone remains come from dolmens (simple chamber tombs) and sepulchral caves from the Bronze Age from the 2nd millennium BC with associated brachicephalies in copper mines at Solsona (Figures 2 and 3) and
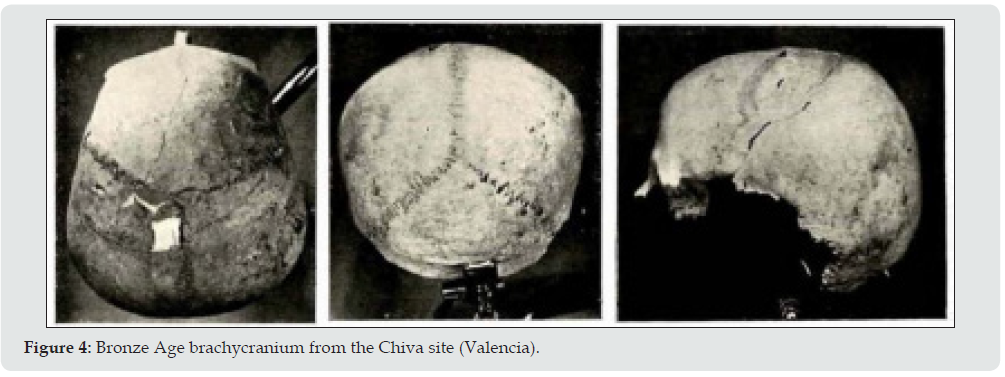
d. Valencia the area with fewest brachycephalics such as Chiva (Figure 4) who were undoubtedly foreigners due to their extreme values [34].
Figure 3: Trepanned and surgically operated Bronze Age brachycranium from the Torre d’en Cornet site (Catalonia).

The face and the base of the cranium are not usually conserved in many human crania found in excavations, which introduces an inevitable bias. However, prehistoric crania are irreplaceable as a form of bone documentation of ancient populations. This lack combined with the fact that the deformed or fragmented crania have been discounted means that the number of crania studied here (Table 1) is not as balanced as we would like.
Variables selected
Brachycephaly affects the entire cranium, not just the length and wide but also the height and perimeters. We decided to study few variables to ensure a low number of missing values and for the minimum number of studied cases to be no less than fifteen to prevent deviations from statistical normality. We therefore did not include some variables of the face or cranial heights although they are in fact very closely correlated with the selected variables. The missing values were replaced with the interpolation method with each sex taken separately since the archaeological origins of the cases analyzed appear to indicate that they are family clans, although some groups may be distant from each other in time. The seven cranial measurements studied here were taken by the authors in accordance with the technique of R. Martin [35] and Martin-Saller [36]. The seven measurements with their identification according to Martin [35] and their equivalence in the technique of W.W. Howells [26] are: Glabello-occipital-length [1, GOL] Maximum cranial breadth [8, XCB] Minimum frontal breadth [9, WFB] Nasion-bregma arch [26, FAR] Nasion-bregma chord (Frontal chord) [29, FCR] Bregma-lambda arch [27, PAR] Bregma-lambda chord (Parietal chord) [30, PAC].
Statistical methodology
The archaeological information and the geographical proximity of the samples studied here imply that the craniological variation is similar and that there will be considerable overlap. Since it would be interesting to establish the presence of brachycephalics the most adequate statistical technique in this case is discriminant analysis as it allows groups and the variables that characterize them to be identified to highlight the main differences of the groups and their causes instead of their similarities. We started by purging the quantitative variables and analyzing their normality and homoscedasticity with the SPSS v. 26 statistical package. Given that the samples analyzed here do not have more than 50 cases for each one we applied the Shapiro-Wilks test and the tests of Levene and Friedman respectively.
The variables were initially standardized to prevent the magnitude effect. Interferences between magnitudes of long dimensions with those of less range of variation were therefore avoided. However, the standardization did not eliminate the correlation with the original measurements in mm since the same r of Pearson is obtained and the cranial size is shown. Thus, the C-Scores were calculated as follows: from the standardized variables (Z-Scores) the cranial size (PENSIZE) of each sample studied was calculated finding the individual value of each case subtracting it from the general average of each sample divided by the number of measurements. Then the Z-scores were centered once again by subtracting the individual’s PENSIZE from each one so that the sum of these deviated scores is zero [27]. Friedman’s non-parametric normality test was applied to the C-Scores variables.
Once the effect of the cranial size is removed the inter- and intra- population variation were interpreted by discriminant analysis and the variability of the cranial shape represented by ellipses at 95% of confidence. The objective of this study is not to classify since group membership has already been established but rather to detect the cranial shape differences. The fact that there is a reduced capacity for discrimination once cranial size is removed should be taken into consideration. The sexes were kept separate and both the normality and homoscedasticity tests as well as the discriminant analysis were computed with the statistical package IBM SPSS v. 26. The 95% variation ellipses were computed with PAST (Paleontological statistics software package v. 3.25) [37]. Finally, the incidence of cranial size (PENSIZE) in the previous analyses of the cranial shape was represented in 3D graphs.
Results
According to the Shapiro-Wilk test few cases were not very significant in the measurements taken in millimeters. None of them deviated from normality in the variables transformed into C-Scores by Friedman’s non-parametric normality test. According to Wilks’ test, the averages of the five groups are significantly distinguished by CGOL (lambda .520) and by CXB (lambda .535) which are the maximum dimensions in length and width of the human cranium. They are followed by the chord of the parietal bone in men: CPAC (lambda .0709) and the minimum width of the forehead: CWBC (lambda.734) in women. CPAC is the causative variable that leads to the M of Box or intra-group variance tolerance test being significant indicating that that fact some groups are more variable than others is mainly due to the length of the parietal bone in most of the samples. The correlations of the canonical discriminant functions are similar in both sexes: .754 for men and .797 for women which are medium-high values which is not surprising given that when the variables in C-Scores are transformed the cranial size is eliminated and therefore the efficacy of the classification is reduced.
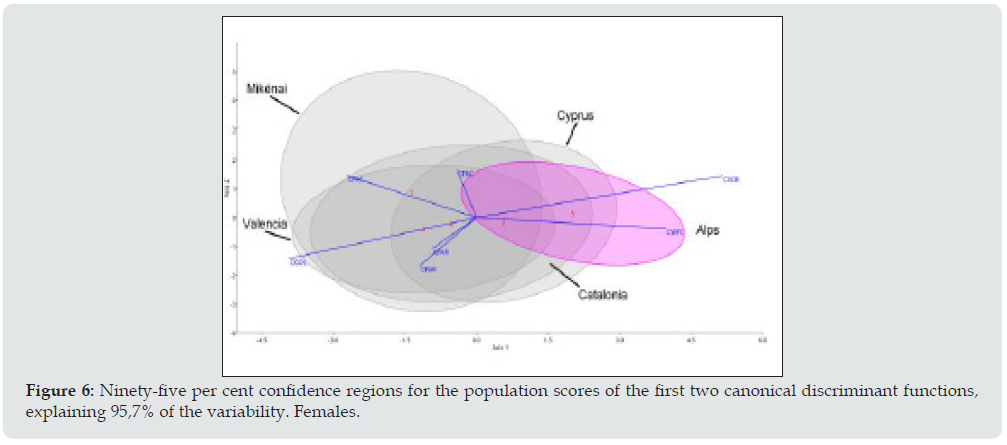
The first canonical discriminant functions explain the 93.5% variation in men (Figure 5) and the 95.7% variation in women (Figure 6). As regards the contribution of the standardized coefficients of the discriminant functions there is a clear dimorphism, although the effect of the cranial size has been removed. In men, the first function is notable for the statistical weight of CGOL (negative) and CXCB (positive) maximum horizontal dimensions of the cranium and the frontal and parietal chords in the second function (CFRC CPAR). Women are distinguished mainly by a considerable weight in the maximum cranial breadth (CXCB) and minimum frontal breadth (CWFC) in the first discriminant function and coincide with men in CGOL and CXCB in the second function.
Figure 5: Ninety-five per cent confidence regions for the population scores of the of the first two canonical discriminant functions, explaining 93,5% of the variability. Males.
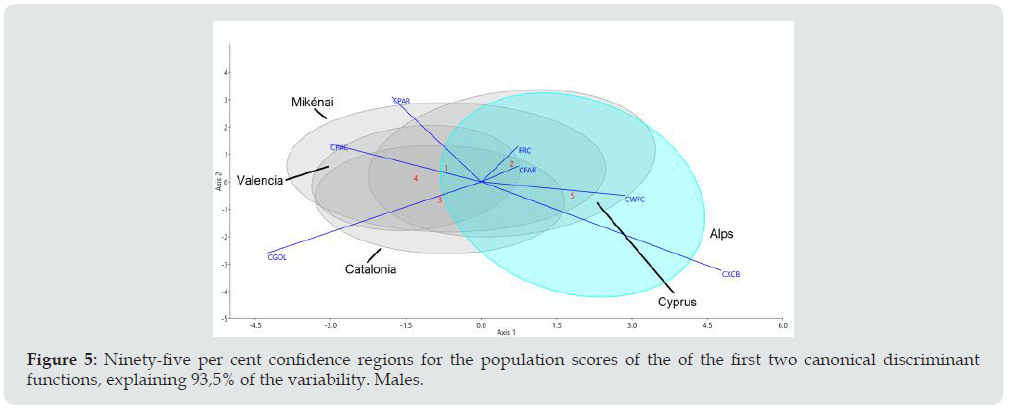
Figure 6: Ninety-five per cent confidence regions for the population scores of the first two canonical discriminant functions, explaining 95,7% of the variability. Females.
The results of the masculine series can be seen in Figure 5 where the outstanding feature is the outgroup series of the Tyrolean Alps and the Slovenian minority of Carinthia (Austria) studied by Toldt [30]. Positive CXCB (cranial breadth and negative CGOL (Glabello-occipital-length)) define the longest distances in the horizontal axis, which is the principal function (75.5% of the variation). The second canonical function provides the other 18% to make 93.5% of the explanation of the variation. Of the four series of the European Bronze Age Cyprus contains the most brachycephalics due to its greater overlap with the hyper-brachycephalics of the Alps. Mikénai and Catalonia also contain brachycephalics although most of the crania there are long and narrow in men which indicates a major intra-group variation, while the centroid of Valencia is the most distant one from the Alpine series.
The variation ellipses at 95% of the female series (Figure 6) are somewhat different from the male ones. Firstly, although the positive values coincide with the men in the first axis (86.1%) the second axis (9.6%) is influenced by two dimensions of the frontal bone (CWFC and CFAR) and CPAR the parietal arch which highlights the fact that the female cranium is rounder and has a prominent forehead. It is worth noting that the female ellipse of Mykénai is very different from the male one due to the second axis, which is the same as the Alpine ellipse. The overlap of the female ellipses is nonetheless similar to the one in men as can be seen in the position of the centroids indicating that in Cyprus there are more brachycephalic women than in Mikénai. This last series is distant from the others, which signifies a considerable intra-group morphological heterogeneity.
The influence of the cranial size (PENSIZE) can be seen in the vertical axis of Figure 7 in the C-Scores values for the cranial length and width dimensions and in values solely standardized for PENSIZE which are correlated with the dimensions in millimeters that were initially taken. In the three axes, the average values are zero with the extreme values at both ends of zero. The Alps series presents a moderate sexual dimorphism and are located in the righthand side of the three-dimensional representation since the crania are very short and broad. The male crania of Cyprus Mikénai and Catalonia series have larger dimensions than the female ones respectively as was expected. However, the women of Valencia are big when compared to the other women in our series and indicate that they are of relatively larger size in comparison to the women. They are the women with the largest cranial size of the entire set. Thus it is clear that the cranial size of a skull can be misleading for gender diagnosis since there are women who have a large cranial size.
Discussion
Cephalometry and twins
The usefulness of the head shape has been widely questioned and discussed in the 20th century. Studies on the children of immigrants to the United States in 1910 to 1912 noted that the children’s cephalic index differed significantly from their parents’ implying that local environmental conditions had a significant impact on the development of head shape [38]. The result of Sparks and Jantz’s reanalysis [39] showed that the genetic component was in fact strong and the heritability of the index was high although not as high as that reported by Osborne and De George [40] which was based on heritability calculated from monozygotic and dizygotic twin data and was therefore stronger [29].
More recently, a number of studies in twins have underlined the strong genetic control of cephalometric variables although DNA methylation differences are apparent already in early childhood even between genetically identical individuals and that individual differences in methylation are not stable over time. A longitudinal- developmental study suggests that environmental influences are important factors in accounting for interindividual DNA methylation differences and that these influences differ across the genome [41-47].
Morphological diversity in the North Mediterranean Basin
Dolichocephalic (long headed) was a term invented by Andreas Retzius (1742-1821) to denote skulls where the diameter of which the transverse diameter is small in comparison with the longitudinal diameter as opposed to brachycephalic (where the head shape becomes globular). The French anthropologist Paul Broca (1824- 1880) labelled skulls with a cephalic index of 75.0 and under as dolichocephalic. Values above 80.0 (80.0% of the longitudinal diameter) are considered brachycephalic. Although there may be brachycephalics in many populations only three of the 28 studied by W.W. Howells [27] show populations averages of over 80.0: modern Asian Buriats Alpine Austrians of the Tyrol and Carinthia and the Pygmies of the Andaman Islands.
In the context of the European Bronze Age brachycephaly turns out to be a useful population marker since the short and wide heads differ from the long and narrow heads of the previous populations among which the former end up being diluted. Their arrival may well be linked to the Bronze Age spread of Yamnaya Steppe pastoralists [14]. In our ellipses of variation, it can be seen that the Cyprus Bronze Age sample is more brachycephalic since it overlaps considerably with the Alpine population of Toldt [30]. There is also a smaller number of brachycephalics amongst the Bronze Age crania of Mikénai which are almost contemporary with the Homeric Achaeans as is the case with the ones from Catalonia where the Valencia series is the one least influenced by the presence of brachycephalics. This may be the outcome of a migratory spread related to the search for metals from east to west on what may have been land routes from Central Europe on the one hand and through pioneer seafaring colonization [9, 48].
The spread of brachycephalics from Central Europe has been recorded as a demographic expansion from the Neolithic period [49] in the French Mediterranean region [50-51] amongst the historical Achaeans of the Argolid [31] Cyprus [32] and Southeast Iberia (El Argar) [52]. But it does not appear in the Epipaleolithic peoples of the Maghreb of Taforalt [53, Figure 2]. There are certain morphological similarities to be found in the crania studied here, which probably correspond to family groups, although the periods of the settlements cover several centuries. In Iberia (Figure 2) there are brachycephalics in the prehistoric copper mines of Asturias (Spain) [34] Catalonia and in Urbiola (Navarra Spain) which are definitely associated with copper mines and in Valencia and the El Argar culture (Figure 2). The latter example is chronologically later and there the excavated material brought to light by the Siret brothers [52] included 13.5% brachycephalics of the total frequency possibly from contacts with the Near East [34]. But such brachycephalics are a minority in Iberia and were probably subsequently diluted amongst the Iberian populations with a tendency towards long and narrow crania as can be seen in other settlements of the El Argar culture.
It is worth noting that there are no brachycephalics amongst the Epipaleolithic peoples of the Maghreb of Taforalt [53, Figure 2) in Dynastic Egypt [27] or in the ancient Egyptian skulls from Thebes [54]. Neither are there brachycephalics in the historical settlements of the Near East mentioned by Angel [55]: Troy (3000-2300 BC Anatolia) Copper Age (3000-2300 BC) Greece Neolithic and Old Helladic Periods (3000-2300 BC) Egypt Badari (4000 BC) Egypt Sedment 9th dynasty (2400 BC) Mesopotamia Al Ubaid (3200- 2800 BC) Mesopotamia Kish (3200-2400 BC). Neither are there Epipaleolithic brachycephalics in Israel until the final period of the Natufian culture [56-57] which has been attributed to processes of local gracilization [58].
Biodynamics and molecular diversity in the Northern Mediterranean Basin
Agricultural and husbandry practices originated 10 000 years ago in a region of the Near East known as the Fertile Crescent. According to the archaeological record this phenomenon known as the Neolithic rapidly expanded from these territories into Europe. However there is heated debate as to whether this diffusion was accompanied or not by human migrations. Fernández et al. [9] analized sixty-three skeletons from the Pre-Pottery Neolithic B (PPNB) sites of Tell Halula Tell Ramad and Dja’de El Mughara dating between 8700–6600 cal. BC. They identified K and N-derived mitochondrial DNA haplogroups as potential markers of the Neolithic expansion whose genetic signature would have reached both the Iberian coasts and the Central European plain. Moreover, the observed genetic affinities between the PPNB samples and the modern populations of Cyprus and Crete seem to suggest that the Neolithic phenomenon was first introduced into Europe through pioneer seafaring colonization.
Zilhao [48] claimed that Early Neolithic Sea voyaging and organized colonization of large islands involving crossings in the range of 100 km are well documented in the Eastern and Central Mediterranean. In the west the distribution of obsidian from Tyrrhenian sources and the lack of human settlement in the Balearic archipelago until later prehistoric times suggest a pattern of contact exchange and dispersal where navigation would have been restricted to small-scale in-sight-of-land crossings and voyages with cargo [59, 60]. This concurs with our results since the major overlap of the variation ellipses (Figures 5 and 6) indicate similarities between the samples that were compared. The geographical and demographic expansions of the Neolithic with the demographic increase tend to maintain the morphological characteristics of the populations. On the other hand, Zilhao [48] considers that the material culture similarities perceived in the Early Neolithic of southern Iberia and the Maghreb may indicate a north-south diffusion of farming across the Straits of Gibraltar but not the reverse.
The permanent settlement of Cyprus began with pioneering agriculturalists in ca. 11000 BP. The analysis of haplotypes from 574 samples of the Y-Chromosome showed potential genetic associations of Greek Cypriots with neighboring populations consistent with two stages of prehistoric settlement. E-V13 and E-M34 are widespread and PCA suggests that their source may be the Balkans and Levant/Anatolia respectively [22].
As regards the origins of the Bronze Age Minoan and Mycenaean cultures Lazaridis et al [23] studied the entire genome of 19 ancient individuals including the Cretan Minoans the Mycenaeans of continental Greece and their eastern neighbors of southwest Anatolia. The result showed that the Minoans and Mycenaeans were genetically similar and owed at least three quarters of their descent to the first Neolithic farmers from western Anatolia and the Aegean, while the other ancestors were related to ones from the Caucasus and Iran. However, the Mycenaeans differed from the Minoans inasmuch as they had other additional ancestors who were hunter-gatherers from Eastern Europe and Siberia. In our study, the second axis of the female variation (9.6%) of the female ellipse of Mykénai is very different from the male one, which may be the outcome of a sampling bias or the result of male migrants who reached Mikénai.
As regards the Western Mediterranean Fernandes et al. [24] after generating genome-wide ancient-DNA data from 61 individuals concluded that the oldest sample of the Balearic Islands (~2400 BC) came from steppe pastoralists that probably derived from migration from Western to Eastern Iberia. Steppe pastoralists arrived in Sicily in about 2200 BC, partly from Iberia. The ancestry related to Iran arrived in the middle of the second millennium BC at the same time as the previously mentioned expansion into the Aegean. There was a major replacement of the population after the Bronze Age. In Sardinia, almost all the ancestry came from the first agriculturalists on the island until the first millennium BC.
Conclusions
The ellipses studied in this paper are long throughout the axis of the first canonical function, which indicates that there is a certain number of brachycephalics on the right-hand edge of the respective ellipses (Figures 5 and 6). The brachycephalics of Iberia are closely associated with copper mining, at least in Navarra and Catalonia. No brachycephalics have been recorded anywhere in Iberia before the Bronze Age. The ones that do appear were probably proto-Celtic prospectors from continental Europe.
Our results confirm that there were small migrations most likely from minorities of metal prospectors in the Bronze Age from the north of the Mediterranean Basin via Roussillon and Provence (Figure 2) although we do not exclude the possibility that they came from the north along the basin of the river Rhone. They were in any case a minority in Iberia. The variations in the shape of the head have proved useful in confirming this phenomenon.
Disclosure Statement
The author reports no conflict of interest.
References
- Allentoft ME, Sikora M, Sjogren KG, Rasmussen S, Rasmussen M, et al. (2015) Population genomics of Bronze Age Eurasia. Nature 522(7555): 167-172.
- Haak W, Lazaridis I, Patterson N, Rohland N, Swapan Mallick, et al. (2015) Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522(7555): 207-221.
- Serrano JG, Ordóñez AC, Fregel R (2021) Paleogenomics of the prehistory of Europe: human migrations, domestication and disease. Annals of Human Biology 48(3): 179-190.
- Gamba C, Jones ER, Teasdale MD, Mc Laughlin RL, Gloria Gonzalez Fortes, et al. (2014) Genome ?ux and stasis in a ?ve millennium transect of European prehistory. Nature Communications 5(1): 5257-5266.
- Malmström H, Linderholm A, Skoglund P, Storä J, Per Sjödin et al. (2015) Ancient mitochondrial DNA from the northern fringe of the Neolithic farming expansion in Europe sheds light on the dispersion process. Philosophical Transactions of the Royal Society B 370(1660): 20130373-20130379.
- Jones ER, Gonzalez Fortes G, Conne S, Siska V, Anders Eriksson, et al. (2015) Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nature Communications 6(1): 8912-8919.
- García O, Fregel R, Larruga JM, V Álvarez, I Yurrebaso, et al. (2011) Using mitochondrial DNA to test the hypothesis of a European post-glacial human recolonization from the Franco-Cantabrian refuge. Heredity 106(1): 37-45.
- Villalba Mouco V, Van de Loosdrecht MS, Posth C, Rafael Mora, Jorge Martínez Moreno, et al. (2019) Survival of Late Pleistocene Hunter-Gatherer Ancestry in the Iberian Peninsula. Current Biology 29(7): 1169-1179.
- Fernández E, Pérez Pérez A, Gamba C, Prats E, Pedro Cuesta, et al. (2014) Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands. PLoS Genetics 10(6): e1004401-e1004411.
- Lazaridis I, Nadel D, Rollefson G, Merrett DC, Nadin Rohland, et al. (2016) Genomic insights into the origin of farming in the ancient Near East. Nature 536(1): 419-426.
- Olalde I, Brace S, Allentoft ME, Armit I, Kristiansen K, et al. (2018) The Beaker phenomenon and the genomic transformation of northwest Europe. Nature 555(1): 190-196.
- Schaefer NK, Shapiro B (2019) New middle chapter in the story of human evolution. Science 365(6457): 981-982.
- Narasimhan VM (2019) The formation of human populations in South and Central Asia. Research Article Summary. Science 365(1): 999-1020.
- Narasimhan VM, Patterson N, Moorjani P, Rohland N, Rebecca Bernardos, et al. (2019) The formation of human populations in South and Central Asia. Science 365(6457): eaat7487-eaat7495.
- Roewer L, Nothnage M, Gusmao L, Gomes V, Miguel González, et al. (2013) Continent-Wide Decoupling of Y-Chromosomal Genetic Variation from Language and Geography in Native South Americans. PLoS Genetics 9(4): e1003460-e1003465.
- Kristiansen K, Allentoft MC, Frei KM, Iversen R, Niels N Johannsen, et al. (2017) Re-theorising mobility and the formation of culture and language among the Corded Ware Culture in Europe. Antiquity 356(1): 334-347.
- Goldberg A, Günther T, Rosenberg NA, Jakobsson M (2017) Ancient X chromosomes reveal contrasting sex bias in Neolithic and Bronze Age Eurasian migrations. Proceedings of the National Academy of Sciences 114(10):2657-2662.
- Lazaridis I, Reich D (2017) Failure to replicate a genetic signal for sex bias in the steppe migration into Central Europe. Proceedings of the National Academy of Sciences 114(20): E3873-3874.
- Furholt M (2014) Upending a ‘Totality’: re-evaluating Corded Ware variability in Late Neolithic Europe. Proceedings of the Prehistoric Society 80(1): 67-86.
- Frînculeasa A, Preda B, Heyd V (2015) Pit-Graves, Yamnaya and Kurgans along the lower Danube: disentangling IVth and IIIrd millennium BC burial customs, equipment and chronology. Praehistorische Zeitschrift 90(1-2): 45-113.
- Furholt M (2019) Re-integrating Archaeology: A Contribution to aDNA Studies and the Migration Discourse on the 3rd Millennium BC in Europe. Proceedings of the Prehistoric Society 85(1): 115-129.
- Voskarides K, Mazières S, Hadjipanagi D, Jacques C, Anastasia Ignatiou, et al. (2016) Y-chromosome phylogeographic analysis of the Greek-Cypriot population reveals elements consistent with Neolithic and Bronze Age settlements. Investigative Genetics 7(1): 1-14.
- Lazaridis I, Mittnik A, Patterson N, Swapan M, Nadin R, et al. (2017) Genetic origins of the Minoans and Mycenaeans. Nature 548(7666): 214-218.
- Fernandes DM, Mittnik A, Olalde I, Lazaridis I, Olivia Cheronet, et al. (2017) The spread of steppe and Iranian-related ancestry in the islands of the western Mediterranean. Nature Ecology & Evolution 4(3): 334-345.
- Clemente F, Unterlander M, Dolgova O, Carlos Eduardo GA, Francisco Coroado Santos, et al. (2017) The genomic history of the Aegean palatial civilizations. Cell 184(10): 2565-2586.
- Howells WW (1973) Cranial Variation in Man. Papers of the Peabody Museum. A Study by Multivariate Analysis of Patterns of Differences Among Recent Human Populations. Papers of the Peabody Museum of Archaeology and Ethnology, India.
- Howells WW (1989) Skull Shapes and the Map. Craniometric Analyses in the Dispersion of Modern Homo. Papers of the Peabody Museum of Archaeology and Ethnology, India.
- Howells WW (1996) Notes and Comments. Howells’ Craniometric Data on the Internet. American Journal of Physical Anthropology 101(3): 441-442.
- Holloway RL (2002) Head-to-head with Boas: Did he err on the plasticity of head form? Proceedings of the National Academy of Sciences 99(23): 14622-14623.
- Toldt C (1910) Untersuchungen über die brachycephalie der alpeländischen Bevö (Investigations into the brachycephaly of the Alpine population). Mitteilungen der Anthropologischen Gessellschaft. Wien 30(1): 68-257.
- Fürst CM (1930) Zur anthropologie der prähistorischen griechen in Argolis. (On the anthropology of the prehistoric Greeks in Argolis). Lund.
- Fürst CM (1933) Zur kenntnis der anthropologie der prähistorischen bevölkerung der Inseln Cypern. (On the knowledge of the anthropology of the prehistoric population of the islands of Cyprus). Lund.
- Turbon D (1981) Antropología de Cataluña en el II Milenio A. C. (Anthropology of Catalonia in the II Millennium BC) University of Barcelona Ed. Barcelona.
- Fusté M (1957) Estudio antropológico de los pobladores neo-eneolíticos de la región valenciana (Anthropological study of the neo-neolithic settlers of the Valencian region). Servicio de Investigación Prehistórica, Institución Alfonso el Magnánimo, Valencia.
- Martin R (1928) Lehrbuch der Antropologie [Textbook of anthropology]. 2nd Edition Jena.
- Martin R, Saller K (1962) Lehrbuch der Anthropologie in Systematischer Darstellung [Systematic presentation of anthropology textbook]. 3rd Edition Vol III: 1575-2416.
- Hammer O, Harper DA, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1): 1-9.
- Boas F, Boas HM (1913) American Anthropologist 15(1): 163-188.
- Sparks CS, Jantz RL (2002) A reassessment of human cranial plasticity: Boas revisited. Proceedings of the National Academy of Sciences USA 99(23): 14636-14639.
- Osborne RH, De George FV (1959) Genetic Basis of Morphological Variation. Harvard University Press, Cambridge, England.
- Carels C, Cauwenberghe N Van, Savoye I Willems G, R Loos, et al. (2001) A quantitative genetic study of cephalometric variables in twins. Clinical Orthodontics and Research 4(3): 130-140.
- Sherwood RJ, Duren DL, Havill LM, Rogers J, Laura AC, et al. (2008) A Genomewide Linkage Scan for Quantitative Trait Loci In?uencing the Craniofacial Complex in Baboons (Papio hamadryas spp.). Genetics 180(1): 619-628.
- Wong CCY, Caspi A, Williams B, Craig IW, Renate H, et al. (2010) A longitudinal study of epigenetic variation in twins. Epigenetics 5(6): 516-526.
- Sreedevi G, Srinivas B, Reddy TP, Krishna Prasad CMS, B Sunil Kumar, et al. (2013) Heritability of Thirty Cephalometric Parameters on Monozygotic and Dizygotic Twins: Twin Study Method. The Journal of Contemporary Dental Practice 14(2): 304-311.
- Weinberg SM, Parsons TE, Marazita ML, Maher BS (2013) Heritability of face shape in twins: A preliminary study using 3D stereophotogrammetry and geometric morphometrics. Dentistry 3000 1(1): 14-19.
- Šidlauskas M, Šalomskiene L, Andriuškeviciute I, Monika S, Žygimantas Labanauskas, et al. (2016) Heritability of mandibular cephalometric variables in twins with completed craniofacial growth. European Journal of Orthodontics 38(5): 493-502.
- Tiro A, Dzemidzic VV, Salaga Ne?c S, Redzic I, Nakas E (2019) Heritability of Craniofacial Characteristics in Twins - Cephalometric Study. Medical Archives 73(3): 205-208.
- Zilhao J (2015) Early prehistoric navigation in the Western Mediterranean: implications for the Neolithic transition in Iberia and the Maghreb. Eurasian Prehistory 11(1-2): 185-200.
- Coon CS (1939) The Races of Europe. Greenwood Press. Connecticut.
- Riquet R (1956) Les populations néo-énéolithiques du bas Languedoc (The neo-eneolithic populations of lower Languedoc). Bulletins et Mémoires de la Société d'Anthropologie de Paris 7(5-6): 316-358.
- Riquet R (1962) Les crânes préhistoriques de la collection Héléna (Narbonne) (Prehistoric skulls from the Héléna collection (Narbonne). Bulletins et Mémoires de la Société d'Anthropologie de Paris 3(4): 480-522.
- Siret L, Siret E (1890, 2006) Las Primeras Edades del Metal en el Sudeste de España (The First Ages of Metal in the Southeast of Spain). (Facsimile edition 2006). Archaeological Museum of Murcia (Spain).
- Ferembach D (1965) Diagrammes craniens sagittaux et mensurations individuelles des squelettes Iberomaurusiens de Taforalt (Maroc Oriental) (Sagittal cranial graphs and individual measurements of iberomaurusian skeletons from Taforalt (Eastern Morocco). Centre de Recherches Anthropologiques, Préhistoriques et Ethnographiques. Alger. Printed in Paris.
- Komáry ZE, Fóthi E (2013) Ancient Egyptian skulls from Thebes in the Anthropological Collection of the Natural History Museum of Paris. Annales historico-naturales Musei nationalis hungarici (Budapest) 105(1): 259-290.
- Angel JL (1951) Troy, the human remains. Supplementary Monograph. I. Princeton University Press, USA.
- Bar-Yosef O, Arensbourg B, Smith P (1971-72) Algunas notas acerca de la cultura y la antropología de los natufienses. (Some Notes on the Culture and Anthropology of the Natufians). Ampurias (Barcelona) 33-34: 111-152.
- Crognier E, Dupouy Madre M (1974) Les Natoufiens du Nahal Oren (Ouadi Fallah). Etude anthropologique. Paleorient 2(1): 103-121.
- Lahr M, Arensburg B (1995) Skeletal robusticity in the Epipaleolithic of North Africa and the Levant. Paleorient 21(2): 87-96.
- Ramis D, Alcover JA, Coll J, Trias M (2002) The Chronology of the First Settlement of the Balearic Islands. Journal of Mediterranean Archaeology 15 (1): 3-24.
- Cherry JF, Leppard TP (2018) The Balearic Paradox: Why Were the Islands Colonized So Late? Pyrenae 49(1): 49-70.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...