Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Research Article(ISSN: 2641-1687) 
What is Abnormal? The Utility of C-Reactive Protein as a Marker of Sepsis Post Major Urological Surgery Volume 2 - Issue 5
Ruairidh Crawford1*, Charlie Khoo1, Tina Rashid1 and William Cook2
- 1Imperial College Healthcare NHS Trust, Department of Urology, Charing Cross Hospital, London, UK
- 2King’s College Hospital, Denmark Hill, London, UK
Received: March 13, 2020; Published: July 17, 2020
Corresponding author: Ruairidh Crawford,Imperial College Healthcare NHS Trust, Department of Urology, Charing Cross Hospital, Fulham Palace Road, London, W6 8RF, UK
DOI: 10.32474/JUNS.2020.02.000150
Abstract
Background: C-reactive protein (CRP) is an acute phase reactant released in response to cell injury of any cause. A rise in CRP in the immediate postoperative period may be misattributed to surgical tissue damage and not to infection, posing a diagnostic challenge for the clinician. We have evaluated its performance as a marker of infective complications following major urological surgery.
Materials and Methods: We reviewed all patients undergoing major urological surgery between March-December 2014. Data including operation, route, Charlson index, post-operative infection, and CRP measurements were recorded. We plotted receiver operating characteristic curves to evaluate the utility of CRP as a marker of infection and explored procedure specific and patient specific risks for CRP elevation.
Results: 117 patients were included. Differences in post-operative CRP measurement between procedures are statistically significant on days 1 to 3 (p <0.05). Using receiver operator characteristics, CRP performs well as a marker of infection from postoperative days (POD) 2 to 8. Discriminatory power is best for patients with septic shock, peaking at POD 5 (<0.0001). In binary logistic regression, adjusting for operation, route, and Charlson Index, CRP remained a statistically significant independent marker of infection from POD 2 to 6.
Conclusion: CRP has high discriminatory power on PODs 2 to 6, particularly for septic shock. The individual major procedures and the route of access have a large influence on postoperative CRP.A larger cohort is required to accurately define normal ranges for CRP adjusted to both procedure specific and patient specific factors.
Keywords: C-reactive protein;Urology; Postoperative;Infection;Complication
Introduction
Post-operative infection following major urological surgery is
associated with significantly increased morbidity and mortality.
C-reactive protein (CRP) is an acute phase reactant synthesised in
the liver with a half-life of 19 hours [1].
The normal value of CRP in a healthy adult is <3.0mg/L.
Measurement of this biomarker is recommended by the National
Institute for Health and Care Excellence (NICE) to help guide
management of suspected sepsis [2]. However, a rise in CRP in
the immediate postoperative period can be misattributed to
surgical tissue damage and not to infection. This poses a diagnostic
challenge for the clinician.
In the field of Colorectal surgery, several studies have reported
the benefits of CRP both as a diagnostic marker of postoperative
sepsis and as a predictor of anastomotic leakage [3-5]. Within
Urology, CRP has been extensively examined as a prognostic
indicator in urological cancers [6-8]. However, there is a paucity of
evidence evaluating post-operative response of CRP to infection
In many institutions, CRP is routinely measured from postoperative day 1 whether an infective complication is suspected or not. With a better understanding of the behaviour of CRP following major urological surgery, there is potential to both improve the patient recovery pathway through early detection of infective complications (enabling prompt treatment) and reduce the number of blood tests patients are exposed to, providing a cost saving. This study aims to evaluate the performance of CRP as a marker of infective complications following major urological surgery.
Patients and Methods
We retrospectively reviewed all patients undergoing partial or radical nephrectomy, nephroureterectomy, cystectomy, radical prostatectomy and retroperitoneal lymph node dissection at a single institution over a 10-month period. Patient characteristics, operation and route, Charlson index to grade morbidity [9], postoperative infection and CRP measurements for post-operative days 1-10 were recorded. All patient data were unidentifiable. Patients must have had at least 2 CRP measurements on different postoperative days to be included.
Definitions
We used the 3rd international consensus definitions for sepsis and septic shock, with sepsis defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, and septic shock defined as sepsis with persisting hypotension requiring vasopressors to maintain mean arterial pressure ≥65 mm Hg, and raised a serum lactate level >2 mmol/L despite adequate volume resuscitation [10]. Using those definitions, infectious complications were classified into the three categories of simple infection, sepsis and septic shock.
Statisticalanalysis
We report descriptive statistics including median, mean and standard deviation. We plotted receiver operating characteristic (ROC) curves evaluating the utility of CRP as a marker of infection and used binary logistic regression to adjust for potential confounders. We tested for differences between groups using the Kruskal-Wallace test. Finally, we compared outcomes for operative route using Fisher’s exact test. All statistical analyses were conducted using Stata 12.
Results
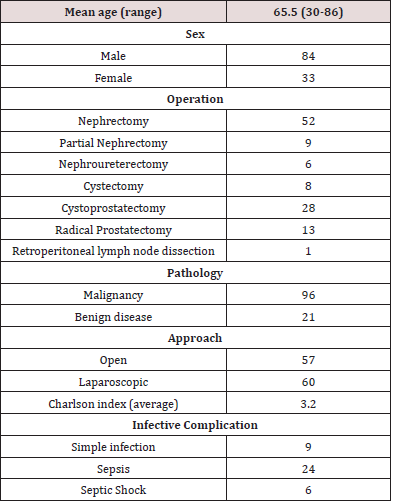
Patient demographics and characteristics are outlined in Table 1. 117 patients were included in the study, with an average age of 65.5 years, and range 30-86. There were 84 males and 33 females. 60 patients underwent laparoscopic surgery and 57 had surgery via an open approach. The average Charlson index was 3.2, meaning the average grade of comorbidity was moderate. 96 patients (82.1%) had malignant disease, and 21 (17.9%) had benign disease. Of the 21 patients with benign disease histologically, 7 were suspected of having malignancy pre-operatively.
39 patients (33.3%) developed an infectious complication
post operatively. 9 patients (7.69%) developed a simple infection,
24 patients (20.5%) developed sepsis, and 6 patients (5.13%)
developed septic shock. Sources of infection included wound
infection, pneumonia, urosepsis and infected abdominal collections,
with diagnoses made on the basis of clinical signs, blood, urine or
sputum culture results and radiological findings.
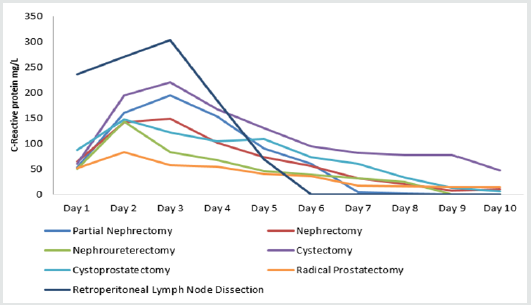
In all patients, CRP rose from day 1 after major surgery and
peaked on post-operative day 3 before falling (Table 2) (Figure 1).
We noted a CRP elevation on day 9, which was attributable to the
few cases who developed late abdominal sepsis. With only a small
number of cases with available CRP data from post-operative day
(POD) 8 onwards, the data on these days did not reach significance.
Table 2: Median, mean and standard deviation for C-reactive protein (CRP) on days 1 to 10 after major urological procedures.
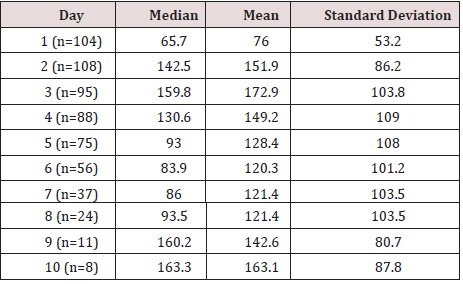
Figure 1: Median, mean and standard deviation for CRP on days 1 to 10 after major urological procedures.
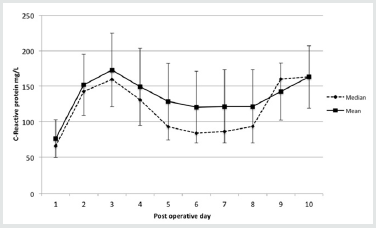
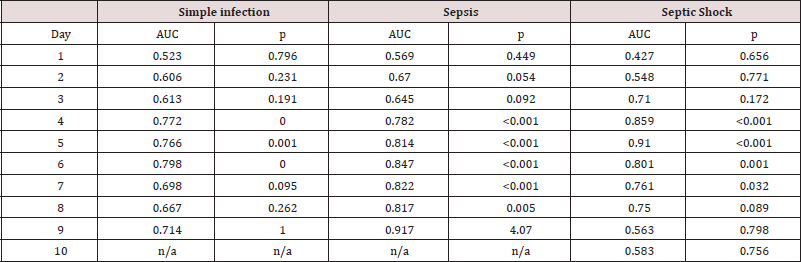
Overall ROC analysis shows that CRP performs well as a marker
of infection from day 2 to day 6. Discriminatory power is best for
more serious infections, with best performance of CRP testing on
day 5 as a marker of septic shock (p = <0.001) (Table 3). In binary
logistic regression, adjusting for operation, route, and Charlson
Index, CRP remained a statistically significant independent
association of simple infection from day 2 to 6, of sepsis from day 2
to 6, and of septic shock from day 3 to 6.
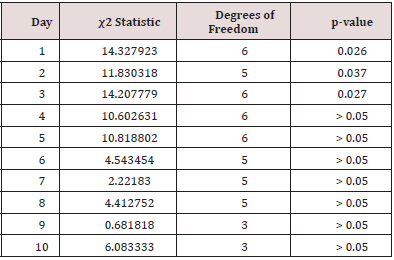
Differences between procedures were statistically significant
(Kruskal-Wallace test) on days 1 to 3 (p all < 0.05) (Table 4).
Among seven major procedures, prostatectomy results in the least
perturbation of CRP (Figure 2). Cystectomy resulted in the highest
rates of septic shock (12.5%) and sepsis (37.5%). Simple infections
occurred most commonly after nephroureterectomy (16.7%). The
1 RPLND case included in this study had no infective complications.
Table 3: Receiver operator characteristics for C-reactive protein (CRP) levels on days 1 to 10 post-operative. AUC = area under curve.
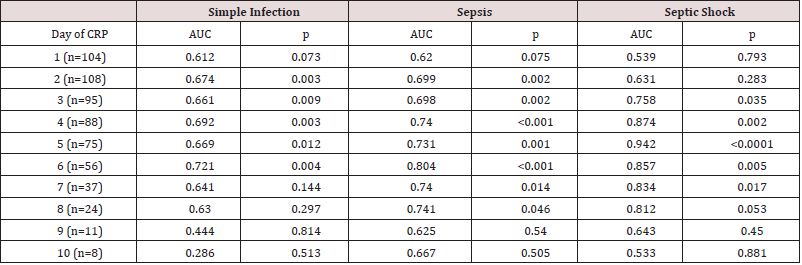
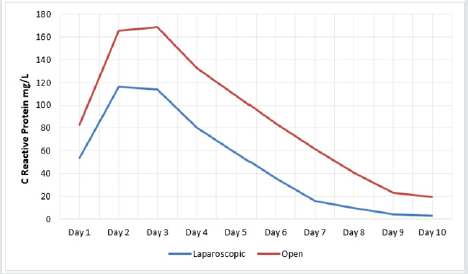
as opposed to open surgery measurably conferred advantages, with lower CRP levels on all days (statistically significant differences were observed on post-operative days 1, 2, 3, 5, 6, 7 and 8) (Figure 3). ROC analysis shows that, following a laparoscopic procedure, CRP has the highest AUC on post-operative days 2 and 3 in the diagnosis of simple infections and sepsis. No patients whose procedure was conducted laparoscopically developed septic shock (hence valid AUC could not be calculated) (Table 5). Following open procedures, CRP had greater value as a marker of infection, with high AUC noted for simple infection on days 4-6 and from day 4 onwards for sepsis and septic shock (Table 6).
Table 5: Receiver operator characteristics for C-reactive protein (CRP) levels on post-operative days 1 to 10 in laparoscopic procedures. AUC = area under curve.
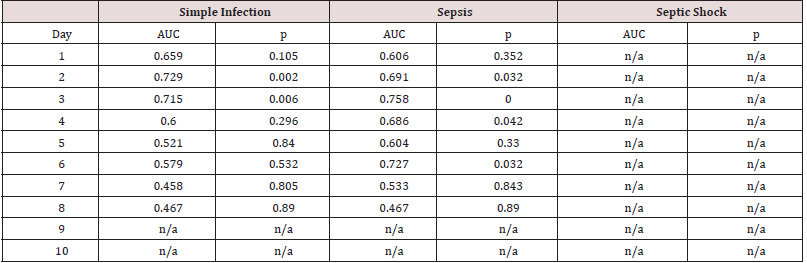
Table 6:Receiver operator characteristics for C-reactive protein (CRP) levels on post-operative days 1 to 10 in open procedures. AUC = area under curve.
Discussion
CRP was first discovered by Tillet and Francis in 1930 when
looking at serological reactions in pneumococcus infection [11]and
named for its reaction with the C polysaccharide of pneumococcus.
In the presence of calcium, CRP binds to the phosphocholine
(PCh) residues in polysaccharides, as well as PCh expressed on
damaged and apoptotic cells [12]. This initiates activation of the
classical complement pathway via assembly of C3 convertase with
opsonisation of the pathogen or damaged host cells [13].
Increased levels of inflammatory cytokines, particularly
interleukin 6 (IL-6), stimulate expression of the CRP gene in
hepatocytes [14]. IL-6 is mainly a pro inflammatory cytokine
involved with the early host response to infection.It is secreted
by many different cell lines, including adipocytes, myocytes,
osteoblasts and immune cells such as macrophages. Changes in the
immune response after tissue damage from surgery is a normal
physiological reactionand correlates with the degree of injury [13].
Other pro-inflammatory cytokines include tumour necrosis factor
alpha (TNF-a) and interleukin-8 (IL-8), and both have been shown
to increase circulating levels of CRP [14,17]. Following a stimulus,
serum concentrations of CRP will rise and peak at approximately 48
hours, before falling rapidly once the stimulus ceases [18].
It is clear from the evidence that both tissue damage and
infection will stimulate the immune system, increase levels of
circulating pro-inflammatory cytokines and CRP. The immune
pathways leading to the rise in CRP in both infection and tissue
injury are almost identical; an elevation in CRP following surgery
may be entirely normal, even if the rise is significant.
The results from this study show that CRP performs well
as a marker of infection in post-operative days 2 to 6, with best
performance on post-operative day 5 (AUC 0.942, p=<0.0001).
Measuring CRP on post-operative day 1 does not differentiate
between surgical tissue injury or an infective process. Trabelssi et
al recently looked at CRP as a marker of complications after radical
cystectomy, and to date the only other urological study looking at
post-operative CRP.They concluded that CRP >150mg/L on postoperative
day 4 was strongly associated with risk of complication
after cystectomy (p<0.001). Measurement of CRP on this day was
also reliable in excluding infective complications, with high negative
predictive value observed [19]. Our data on post-operative day 4 is
concordant with these findings; however, we also found that CRP
has high discriminatory power as a marker of infection on postoperative
days 2-6. CRP has a half-life of 19 hours and peaks after
approximately 48 hours [19], therefore a sustained rise over a few
days with an infective process is expected.
Our data demonstrates lower CRP levels following laparoscopic
surgery as opposed to open surgery, reaching statistical significance
on the majority of post-operative days. The laparoscopic approach
allows less tissue damage and stimulation of the immune response,
so a greater rise in CRP following open surgery is expected.
This has been looked at extensively in colorectal surgery, but
not in urology. Straatman et al evaluated post-operative CRP
following colonic surgery as a substudy of the LAFA randomised
controlled trial (laparoscopy in combination with fast track
multimodal management is the best perioperative strategy in
patients undergoing colonic surgery) [20, 21].They found that
in uncomplicated cases, the rise in CRP levels was significantly
lower at 24 and 72 hours following laparoscopic resections in
comparison to open, in keeping with our results. In patients with
major complications, they found no difference in CRP levels when
comparing laparoscopic to open surgery. Similar results following
colonic surgery were found by Ramanathan et al, who noted a
greater rise in CRP following open surgery; however, in those with
an infective complication, there was no significant difference in CRP
levels on post-operative days 1-4 between open and laparoscopic
resections [22]. In contrast to these studies, we found statistically
significant differences CRP levels between open and laparoscopic
surgery in the septic shock group (p=0.012).
Limitations of this study include the retrospective design
and heterogeneity of the study population. Although interesting
to compare the behaviour of CRP following different urological
procedures and approaches, a larger cohort would be required to
accurately define procedure specific normal ranges for CRP.
Conclusion
CRP has little predictive value as a marker of infection or sepsis on the first post-operative day, or after 6 days, but has surprisingly high discriminatory power on days 2 to 6, particularly for more severe definitions of sepsis. Procedure and route of access have a large influence on CRP from day 1 and should be taken into account when considering what represents an abnormal CRP. Further prospective research examining the behaviour of CRP after major urological surgery is required and could enhance post-operative recovery whilst allowing a cost saving to be made.
References
- Pepys MB, Hirschfield GM (2003) C-reactive protein: A critical update. J Clin Invest 111(12): 1805-1812.
- NICE guideline [NG51] (2016). Sepsis: Recognition, diagnosis and early management.
- Ortega-Deballon P, Radais F, Facy O, d'Athis P, Masson D, et al. (2010) C reactive protein is an early predictor of septic complications after elective colorectal surgery. World J Surg 34(4): 808-814.
- Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, etal. (2012) C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol 19(13): 4168-4177.
- Scepanovic MS1, Kovacevic B, Cijan V, Antic A, Petrovic Z, et al. (2013) C-reactive protein as an early predictor for anastomotic leakage in elective abdominal surgery. Tech Coloproctol 17(5): 541-547.
- Tanaka N1, Kikuchi E, Shirotake S, Kanao K, Matsumoto K, etal. (2014) The Predictive Value of C-reactive Protein for Prognosis in Patients with Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy: A Multi-institutional Study. Eur Urol 65(1): 227-234.
- Zhou L, Cai X, Liu Q, Jian ZY, Li H, et al. (2015) Prognostic Role of C-Reactive Protein in Urological Cancers: A Meta-Analysis. Sci Rep 5: 12733.
- Teishima J, Ohara S, Shinmei S, Inoue S, Hayashi T, et al. (2018) Normalization of C-reactive protein levels following cytoreductive nephrectomy in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors is associated with improved overall survival. Urol Oncol 36(7): 339.e9-339.e15.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases 40(5): 373-383.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, etal. (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8): 801-810.
- Tillet WS, Francis T (1930) Serological reactions in pneumonia with a non- protein somatic fraction of Pneumococcus. J Exp Med 52(4): 561-571.
- Agrawal A1, Simpson MJ, Black S, Carey MP, Samols D (2002) A C-reactive protein mutant that does not bind to phosphocholine and pneumococcal C-polysaccharide. J Immunol 169(6): 3217-3222.
- Volanakis JE (2001) Human C-reactive protein: Expression, structure, and function. Mol Immunol 8(2-3): 189-197.
- Boras E, Slevin M, Alexander MY, Aljohi A, Gilmore W, et al. (2014) Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine 69(2): 165-179.
- Hildebrand F, Pape HC, Krettek C (2005) The importance of cytokines in the posttraumatic inflammatory reaction. Unfallchirurg 108(10): 793-794, 796-803.
- Wigmore SJ, Fearon KC, Maingay JP, Lai PB, Ross JA (1997) Interleukin-8 can mediate acute-phase protein production by isolated human hepatocytes. Am J Physiol 273(4): E720-6.
- Calabró P, Willerson JT, Yeh ET (2003) In ammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation 108(16): 1930-1932.
- Trabelssi M, Thuret R, Droupy S, Costa P, Rebillard X, et al. (2018) Postoperative C-reactive protein is a reliable marker to detect complications after radical cystectomy. Prog Urol 28(5): 282-290.
- Pepys MB1, Hirschfield GM (2003) C-reactive protein: A critical update. J Clin Invest 111(12): 1805-1812.
- Straatman J, Cuesta MA, Tuynman JB, Veenhof AAFA, Bemelman WA, et al. (2018) C-reactive protein in predicting major postoperative complications are there differences in openand minimally invasive colorectal surgery? Substudy from a randomized clinical trial. Surg Endosc 32(6): 2877-2885.
- Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, et al. (2011) Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 254(6): 868-875.
- Ramanathan ML, MacKay G, Platt J, Horgan PG, McMillan DC (2015) The impact of open versus laparoscopic resection for colon cancer on C-reactive protein concentrations as a predictor of postoperative infective complications. Ann Surg Oncol 22(3): 938-943.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...