
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Review Article(ISSN: 2641-1687) 
Radiology; USG and Colour Doppler of Post Renal Transplant Complications Volume 3 - Issue 3
Kajal Patel*, Nitin Patel, Shruti Mehta, Vaidehi Patel and Harsh Sutariya
- Associate Professor in Radiology, Institute of Kidney disease and research center, Gujrat University of transplantation sciences, India
Received: February 18, 2022; Published: February 25, 2022
Corresponding author: Kajal Patel, Associate Professor in Radiology, Institute of Kidney disease and research center, Gujrat University of transplantation sciences, India
DOI: 10.32474/JUNS.2022.03.000164
Abstract
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
Kidney transplant is the treatment of choice for patients with end-stage renal disease. Kidney transplant offers better quality of life. It is more cost effective than hemodialysis. Advances in surgical technique, along with improvement in organ preservation and immunosuppression have improved patient outcomes. Post-operative complications, however, can limit this success. Ultrasound and Doppler study is the primary imaging modality for evaluation of renal transplant, providing real –time information about complication in graft. A multimodality approach including CT scan, MRI or conventional angiography may be necessary in cases when sonography and Doppler are inconclusive to diagnose the etiologies of these complications. Radiologists play an integral role within the multidisciplinary team in care of transplant patient at every stage of the transplant process. Therefore, the radiologist should always be aware when evaluating the failing renal graft, whether the cause is renal or extrinsic. In this pictorial essay we tried to gather the most common complication of transplant kidney in different cases that occurred in our hospital, with an emphasis on Ultrasound and Doppler study.
Keywords: USG; Colour Doppler; Post renal transplantation; Complications
Introduction
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
The preferred modality for renal replacement is renal transplantation, and its superiority in prolonging the longevity of patients with end-stage renal disease is well established [1]. Kidney transplantation is typically classified as deceased-donor (formerly known as cadaveric) or living-donor transplantation depending on the source of the donor organ. Due to improvement in transplantation technology, advancement in immunosuppressant and graft preservation techniques the 1-year survival rates for grafts, are reported to be 80% for mismatched cadaveric renal grafts; 90% for nonidentical living related grafts; 95% for human lymphocyte antigen-identical grafts [2]. Radiologists play a major role at every stage of the transplant process with transplantation team. Ultrasonography with colour Doppler is the principal modality used for evaluation of renal allograft. USG is a relatively cheap, noninvasive, and non-nephrotoxic modality. It is applied for diagnostic and monitoring purposes in the post-transplant period. This pictorial essay describes USG and Doppler imaging appearances of the major complications that may occur in renal transplantation. All our images have been obtained from a single our center which is major transplantation center in India. All post renal transplant patients undergo a USG and comprehensive Doppler evaluation on post-operative day one. The sonographic examination algorithm includes gray-scale evaluation of the graft and spectral Doppler. Further imaging is based on clinical follow-up including daily monitoring of laboratory values. If clinical parameters are abnormal, repeat sonography is performed and depending on the results, CT, MRI, or angiography may be requested.
Surgical Technique
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
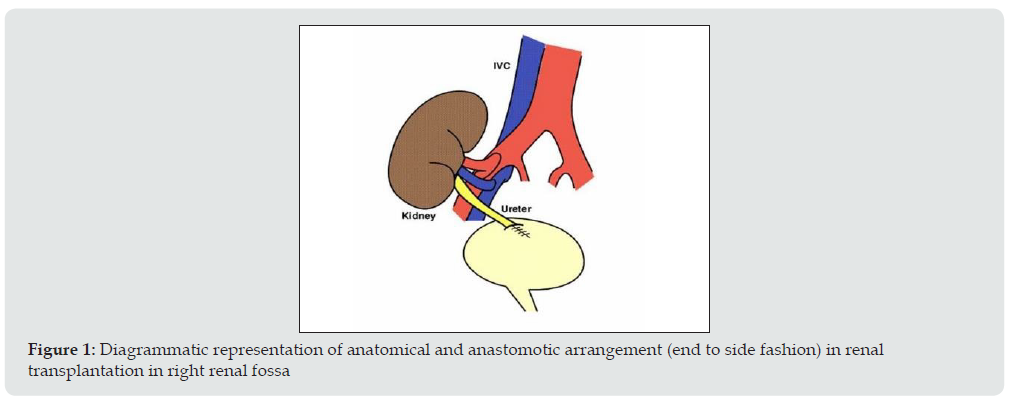
Surgical technique and location of placement of renal allograft depends on the variation in arterial and venous anatomy of donor. The transplanted kidney is usually placed extraperitoneally in the patient’s right iliac fossa (less commonly in left iliac fossa), with end-to-side or end to end anastomosis to the external iliac vasculature. The currently preferred method for restoring urinary drainage is ureteroneocystostomy, a procedure by which the ureter is implanted directly into the dome of the bladder (Figure 1).
Figure 1: Diagrammatic representation of anatomical and anastomotic arrangement (end to side fashion) in renal transplantation in right renal fossa
Post renal transplantation complications
Urologic Complications
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
The prevalence of urologic complications varied from 10% to 25% with a mortality rate ranging from 20% to 30%. Incidence rate is decreased range between 3% and 9% in the current era because of advancement in surgical techniques and frequent use of ureteral stents [3,4].
Urinary Obstruction
a) Incidence: 2%-5% of kidney transplant recipients.
b) Site of obstruction: Approx. 90% of stenoses occur in the distal third of the ureter due to its poor vascular supply.
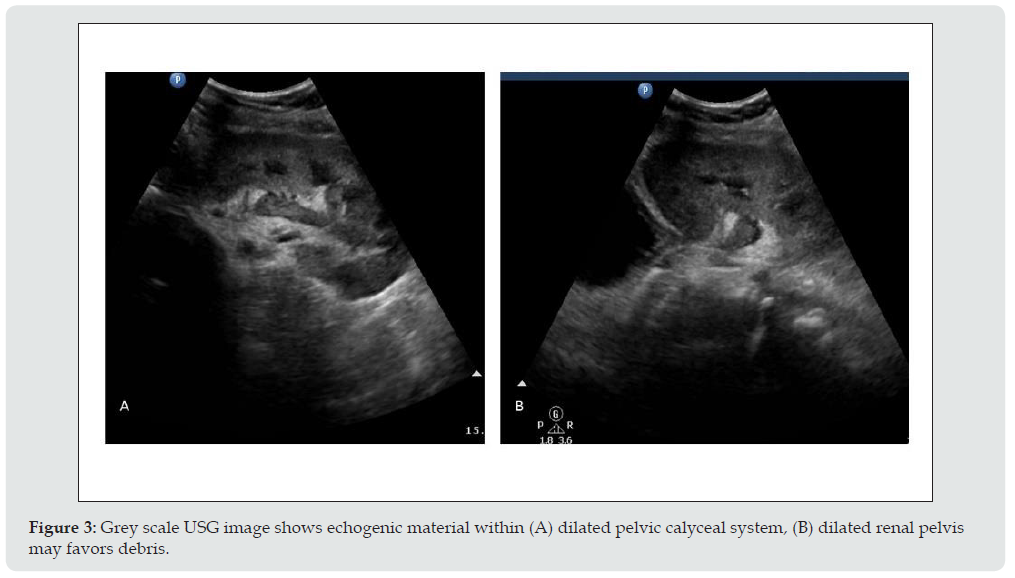
c) Imaging appearance: US can easily confirm the diagnosis of hydronephrosis and dilated renal pelvis and thus determine the level of ureteral obstruction (Figure 2). When contents of pelvic calyceal system are echogenic and weakly shadowing, fungus balls should be considered, whereas low-level echoes suggest pyonephrosis (Figure 3).
Figure 2: Grey scale USG image shows (A) dilated pelvic calyceal system, (B) dilated ureter may favors urinary obstruction.
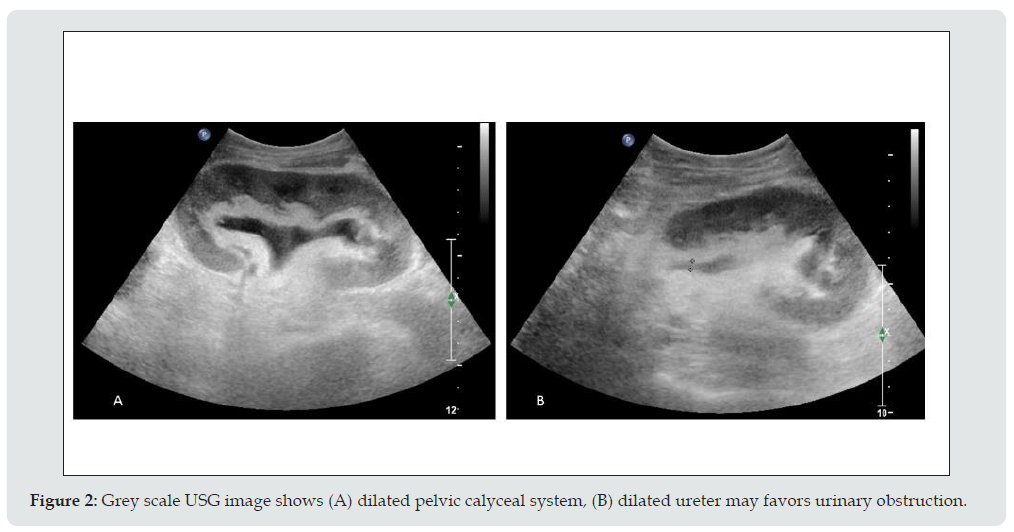
Figure 3: Grey scale USG image shows echogenic material within (A) dilated pelvic calyceal system, (B) dilated renal pelvis may favors debris.
Urine Leaks and Urinomas
a) Incidence: up to 6 % of renal transplant recipients [5]
b) Location: extraperitoneal or intraperitoneal, if intraperitoneal may present with ascites.
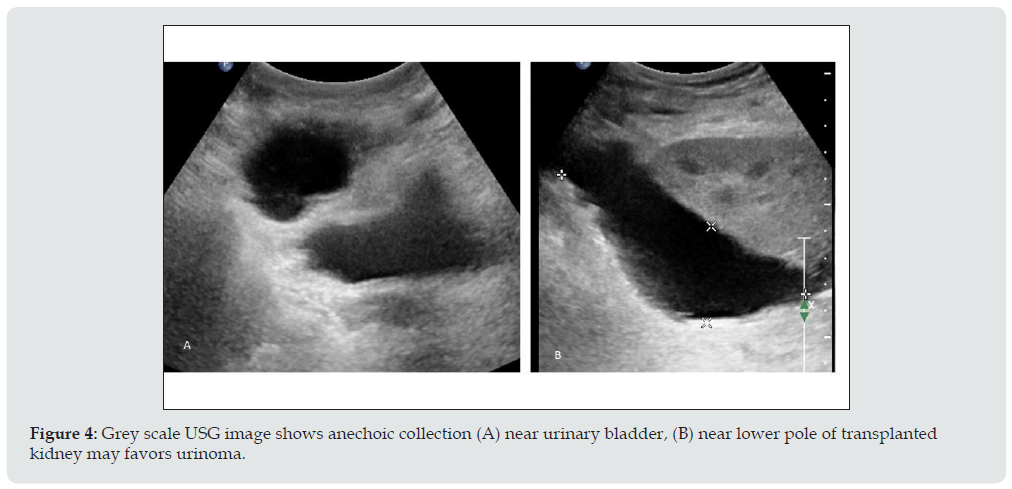
c) Imaging appearance: USG findings are nonspecific, well defined anechoic fluid collection with septa or without septation, adjacent to the lower pole of the transplant in most of the cases (Figure 4).
Figure 4: Grey scale USG image shows anechoic collection (A) near urinary bladder, (B) near lower pole of transplanted kidney may favors urinoma.
Drainage of fluid under ultrasound guidance and testing the fluid for creatinine helps to differentiate it from seromas or lymphoceles. High concentration of creatinine will be found in case of urinoma if we compare with serous fluid.
Calculous Disease
a) Incidence: 1% to 2 % of post-transplant cases develops clinically relevant stones as compared to general population [6]. As the kidney is denervated patient will not suffer typical renal colic.
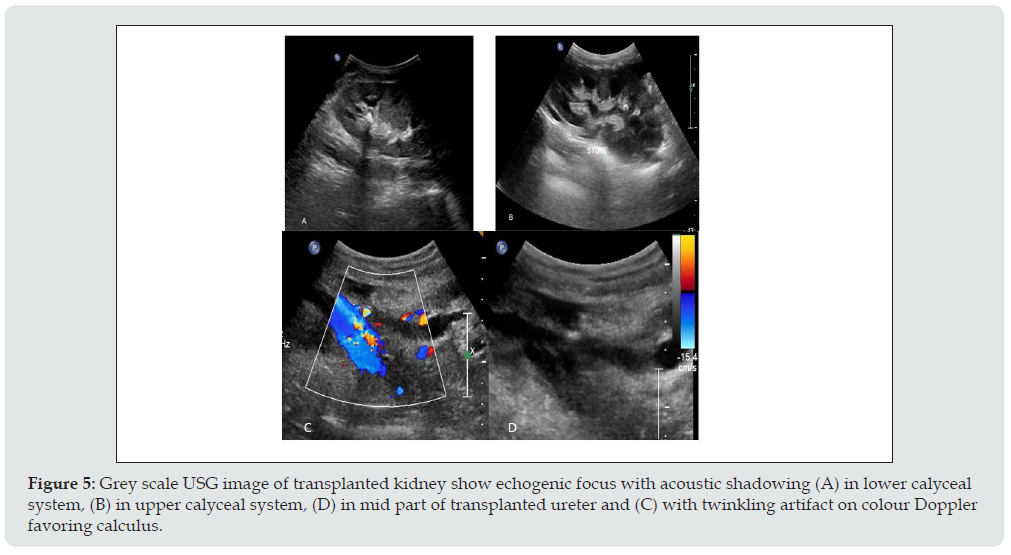
b) Imaging appearance: Calculus appears as strongly reactive focus of variable size producing acoustic shadowing on USG and twinkling artifact on colour Doppler (Figure 5). Other rare urologic complications are ureteric necrosis and vesico-ureteric reflux.
Figure 5: Grey scale USG image of transplanted kidney show echogenic focus with acoustic shadowing (A) in lower calyceal system, (B) in upper calyceal system, (D) in mid part of transplanted ureter and (C) with twinkling artifact on colour Doppler favoring calculus.
Peritransplant Fluid Collections
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
Fluid collection in peritransplant region has been reported in up to 50 % of renal transplantation. They are urinomas, hematomas, lymphocele and abscess, the clinical relevance of these collection is largely determined by their size, location and possible growth. In immediate postoperative period, small hematomas or seroma are almost expected. Their size should be documented at baseline examination. Rarely do they lead to graft dysfunction or obstruction of collecting system. Urinomas and hematomas are most likely to develop immediately after transplantation, whereas lymphoceles generally develop after 4 to 8 weeks. The ultrasonography characteristics of peritransplant fluid collections, however, are entirely nonspecific, correlation with clinical findings helps to restrict differential diagnosis. Ultimately, diagnosis may be made only with percutaneous aspiration and then biochemical analysis. Differentiation between Peritransplant and subcapsular collection is important. A subcapsular collection likely to cause mass effect on parenchyma, usually cresenteric and show extension along the contour of kidney deep to the renal capsule
Hematoma
a. Incidence: Varies from 4 to 8 % [7]
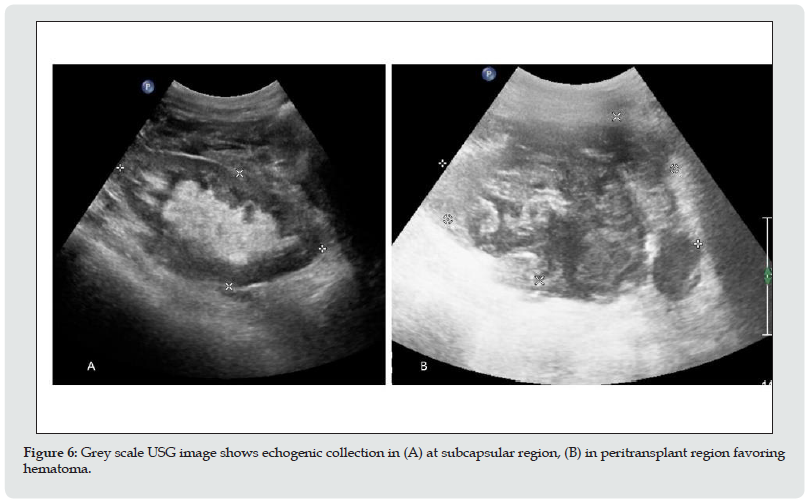
b. Imaging appearance: Hematomas have a complex appearance. Hematomas appears echogenic in acute case and progressively become less echogenic with time (Figure 6). Chronic hematomas even appear anechoic, more closely resembling fluid and septation may develop later on.
Figure 6: Grey scale USG image shows echogenic collection in (A) at subcapsular region, (B) in peritransplant region favoring hematoma.
Lymphocele
a. Incidence: Affecting up to 20% of the patients [8] It occurs after surgery owing to the surgical disruption of the normal lymphatic channels along the iliac vessels or around the hilum of the graft.
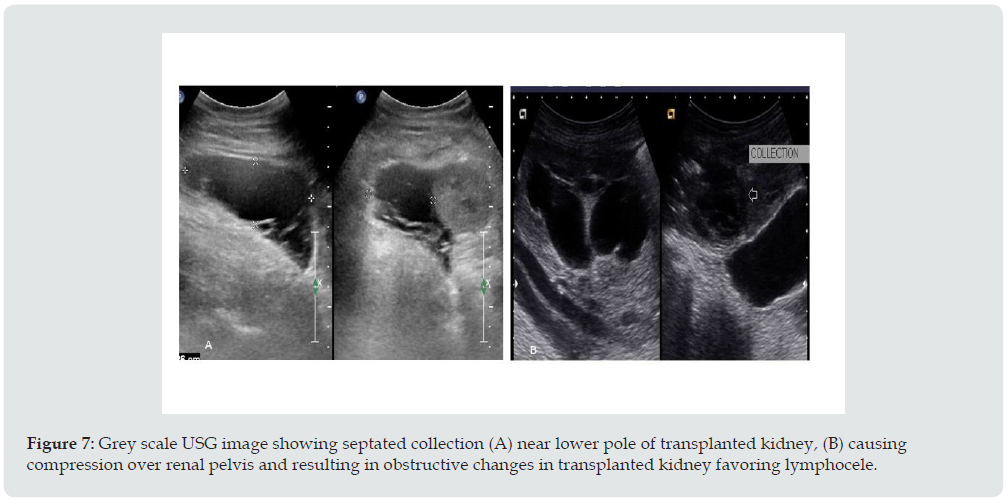
b. Imaging appearance: on Ultrasound it appears as anechoic bur may contain septation. They may become infected and gave more complex appearance (Figure 7).
Peritransplant abscesses
a) Imaging appearance: USG cannot always differentiate an abscess from other collection. Collection may show low level echoes and thick irregular wall. If gas is seen, an abscess is probable. In any pyrexia patient, any perinephric collection should be considered infected until proven otherwise through the appropriate imaging and guided diagnostic aspiration.
Figure 7: Grey scale USG image showing septated collection (A) near lower pole of transplanted kidney, (B) causing compression over renal pelvis and resulting in obstructive changes in transplanted kidney favoring lymphocele.
Parenchymal Complication / Graft Dysfunction
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
Diseases of the renal parenchyma are usually diffuse and often leads graft dysfunction. Differential diagnosis is difficult by imaging alone. USG is not sensitive or specific for evaluation; differential will be relying on biopsy. USG still has a central role in qualitative assessment of graft perfusion and to guide the biopsy.
Acute tubular necrosis (ATN)
Acute tubular necrosis is due to reversible ischemic damage to the renal tubular cells prior to engrafting and reperfusion injury.
i. Incidence: affects 20–60 % of cadaveric renal grafts.
ii. Time of onset: in the first 48 hours after transplantation.
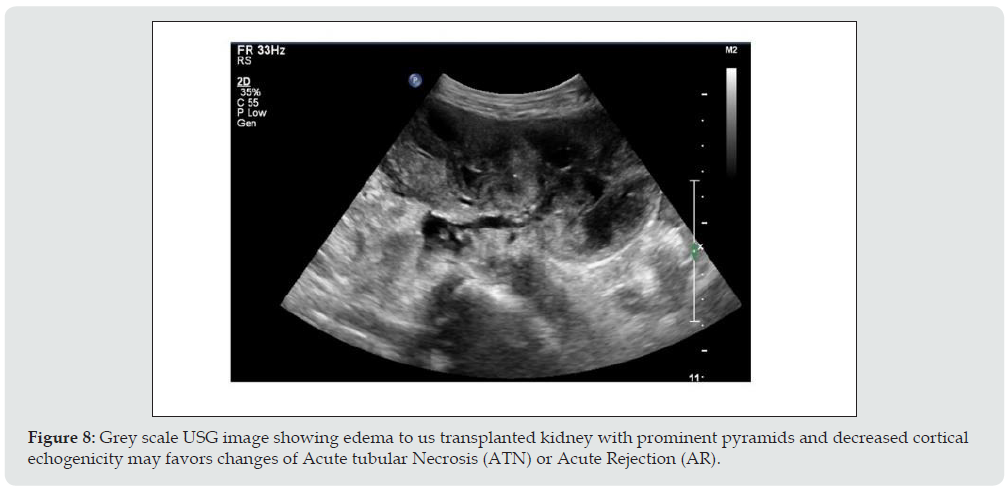
iii. Imaging appearance: No specific imaging pattern for the diagnosis of ATN. The kidney may appear normal, in severe cases it looks enlarged, edematous and echo poor with loss of corticomedullary differentiation (Figure 8) and shows elevated RI (above 0.8).
Figure 8: Grey scale USG image showing edema to us transplanted kidney with prominent pyramids and decreased cortical echogenicity may favors changes of Acute tubular Necrosis (ATN) or Acute Rejection (AR).
Rejection
Rejection can be classified according to the period of appearance as hyper acute (occurring within minutes), acute (occurring within days to weeks), late acute (occurring after 3 months), or chronic (occurring months to years after transplantation). Classification of renal allograft rejection by Banff classification of allograft pathology is routinely followed nowadays. It is based on a combination of histopathologic features coupled with molecular, serologic, and clinical parameters.
Acute Rejection (AR)
i. Incidence: up to 40% of patients in the early transplant period [9].
Figure 9: (A) Grey scale USG image shows enlargement of transplanted kidney, swelling of the medullary pyramids and echogenic sinus fat, (B) Spectral Doppler analysis shows elevated RI may favors changes of Acute Rejection (AR) or Acute Tubular Necrosis (ATN).
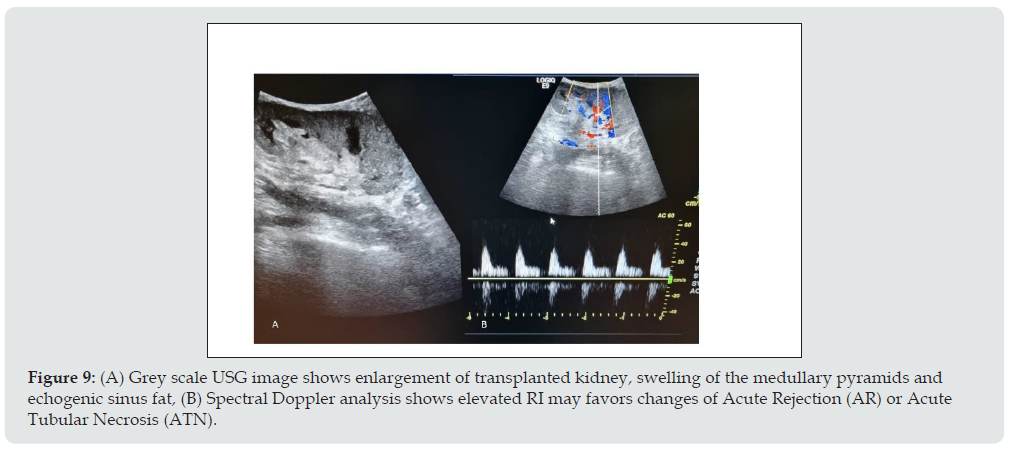
ii. Imaging appearance: Graft enlargement due to edema, Decreased cortical echogenicity, swelling of the medullary pyramids, echogenic sinus fat, edematous wall of pelvic calyceal system, focal hypoechoic areas in parenchyma which may favors infarct and collection in perigraft region due to necrosis or hemorrhage. PI and RI elevated in both ATN as well as in AR, but AR has high values of it. In severe cases, Power Doppler shows reduced, absent or reversed diastolic flow with elevation of the RI (Figure 9).
Chronic Rejection
Chronic rejection occurs in case of insufficient immunosuppression given to recipient to control the residual antigraft lymphocytes and antibodies.
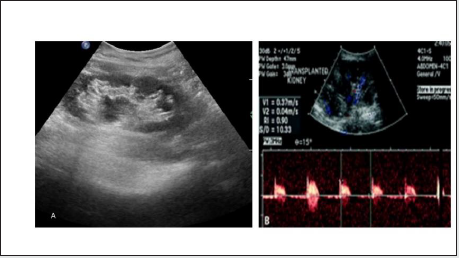
i. Imaging appearance: US appearance is not typical, ranging from normal to hyper echogenic, along with cortical thinning, reduced number of intrarenal vessels, and mild hydronephrosis (Figure 10). RI measurements are not reliable for this diagnosis. The diagnosis is made histologically.
Figure 10: (A) Grey scale USG image shows echogenic transplanted kidney with cortical thinning and minimal hydronephrosis, (B) Spectral Doppler image shows reduced diastolic flow and elevated RI may favors Chronic Rejection.
Drug Nephrotoxicity
Calcineurin inhibitors are key immunosuppression agents administered to avoid acute rejection, but they are nephrotoxic.
A. Imaging appearance: USG may show completely normal results or nonspecific findings such as graft swelling, increased or decreased renal echogenicity and loss of cortico-medullary differentiation. Doppler study may show a RI increase of 0.80. Findings of USG and Doppler study should be correlated with the serum drug levels. USG and Doppler findings of ATN and AR is almost similar, but both can be differentiated by time course of the findings. Clinical & biochemical correlation and serial measurements of RI and Pulsatile index (PI) would be further helpful to monitor the patient.
Infections and abscesses
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
Incidence and time of onset: More than 80% of renal transplant recipients have at least 1 episode of infection during the first year of post transplantation.
Figure 11: Grey scale USG image shows ill-defined hypoechoic lesion (A) in mid pole, (B) in upper pole of transplanted kidney posteriorly Suggestive of Abscess formation. First one is proved case of fugal etiology and second one is pyogenic abscess.
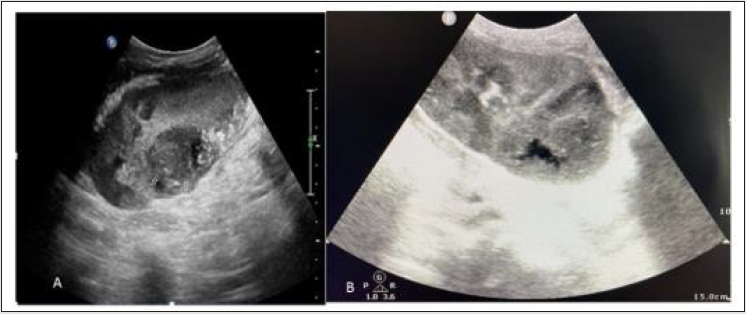
I. Imaging appearance: USG appearance is quite variable. Focal pyelonephritis appear as a focal hyperechoic or hypoechoic area, but this finding is nonspecific because it can represent infarction or rejection also (Figure 11). Abscess has varied appearance on USG like- heterogeneous, hypoechoic or cystic. Urothelial thickening may be seen. In febrile post renal transplant patient low level echoes in dilated collecting system may favors pyonephrosis. Fungus ball appears as focal rounded, weakly shadowing or echogenic structure in dilated pelvic calyceal system. In emphysematous pyelonephritis, gas in the parenchyma of the renal graft produces an echogenic line with distal reverberation artifacts. Papillary necrosis has no typical sonographic findings, and it subsequently leads to ureteric obstruction.
End stage disease
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
Nonfunctional renal grafts are left in place in abdominal cavity. Gradually graft becomes small and can have fatty replacement, hydronephrosis, infarcts, hemorrhage or calcification.
Vascular Complications
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
Vascular complications in post renal transplantation have significant negative influence of graft survival. They are infrequent, occur in approximately 1%–2%; [10] but can cause sudden loss of renal allograft. Selective catheter angiography is the gold standard for diagnosis; however, it is invasive and may cause various complications. Hence it is not used as a screening tool but reserved for patients with inconclusive results on the noninvasive screening tests. Noninvasive imaging like ultrasound, Doppler, scintigraphy, CT and MR angiography plays major role to evaluate them.
Renal artery thrombosis (RAT)
Incidence: Ranges from 0.5% to 3.5 % [11]
Imaging appearance: No evidence of any arterial or venous flow noted on color, spectral and power Doppler study (Figure 12). Doppler sonography had 100% sensitivity and specificity for diagnosis and hardly any other imaging study is required for diagnosis [12].
Figure 12: (A) Grey scale USG image, (B) Power Doppler image of transplanted kidney show enlargement and absent colour flow Suggest Vascular Thrombosis.

Focal Renal Infarction
Imaging appearance: A segmental infarct appears as a poorly marginated wedge shaped hypoechoic mass or a hypoechoic mass with a well-defined echogenic wall without colour flow (Figure 13). If the infarction is global, the kidney will appear hypoechoic and be diffusely enlarged.
Figure 13: Colour Doppler image of transplanted kidney shows absent colour flow (A) in upper and mid pole, (B) in upper pole favoring Segmental infarct.
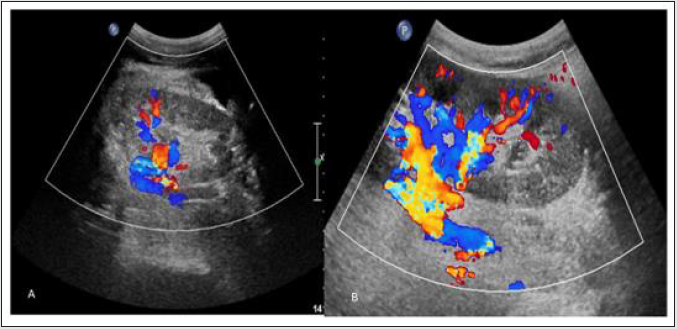
Renal Artery Stenosis (RAS)
Incidence: It has wide range varying from 1% to 23 % depending on the definition and diagnostic techniques used.
Site: usually at anastomotic site
Imaging appearance: On gray scale USG, there is lack of normal post-transplant hypertrophy. On color Doppler study appearance of focal color aliasing noted at stenotic segments. On spectral Doppler study, peak systolic velocity in main renal artery >300 cm/sec and Ratio of PSV in transplanted main renal artery and external iliac artery greater than or equal to 1.8 are highly suggestive of significant stenosis. Indirect criteria are low resistive index <0.56, Acceleration time >0.07 sec, Acceleration index <3 meter/ sec and Intrarenal tardus–parvus waveform (Figure 14).
Figure 14: Spectral Doppler image shows (A) elevated peak systolic velocity in main renal artery at anastomosis, (B) delayed systolic upstroke and rounding of systolic peak consistent with tardus parvus spectral waveform in case of transplanted renal artery stenosis.
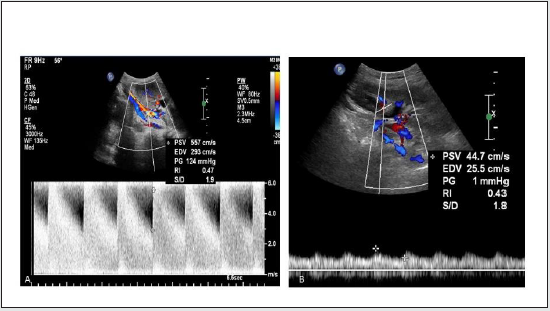
Limitation: Results are strongly depends on the operator’s individual experience and skill.
Rate of restenosis is less than 10 %. Doppler ultrasonography is the procedure of choice to evaluate graft perfusion before and after revascularization The term pseudo transplant renal artery stenosis (TRAS) refers to thrombosis or stenosis of iliac artery or aorta proximal to transplant renal artery.
Renal vein thrombosis: (RVT)
Figure 15: (A) Grey scale USG image shows swollen and hypo echoic transplanted kidney, (B) Colour Doppler image shows absent venous flow and spectral Doppler image shows absent and reversal of diastolic flow in renal artery favoring Renal vein thrombosis.
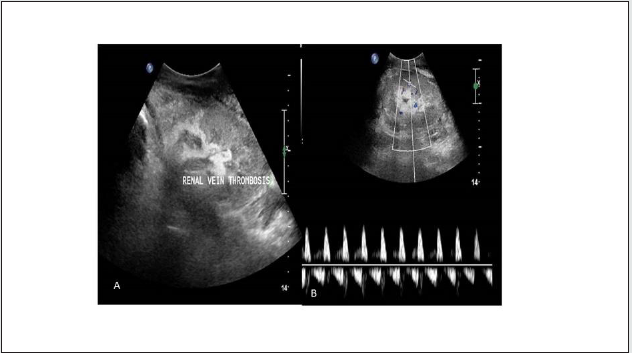
Incidence: Ranges from 0.9% to 4.5 % [13]
Imaging Findings: Graft appears swollen and hypoechoic.
Doppler shows absent venous flow. Renal arterial Doppler spectrum shows absent or reversal of diastolic flow due to increased resistance (Figure 15). Reversal flow in renal artery is nonspecific as it also seen in severe rejection and in acute tubular necrosis, its combination with absent venous flow is the diagnosis of renal vein thrombosis. Partial thrombosis also can occur near anastomosis or within the transplanted kidney (Figure 16).
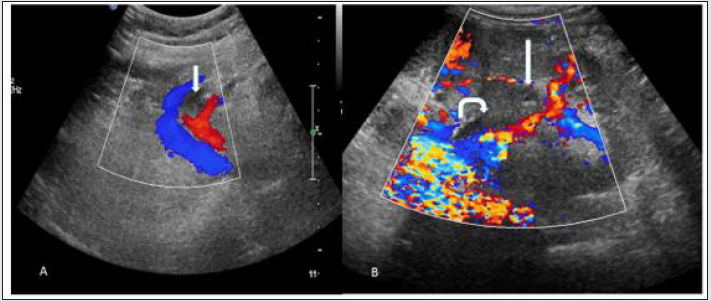
Figure 16: Spectral Doppler image shows (A) partial thrombosis in renal vein near anastomosis (white straight arrow), (B) partial thrombosis in main renal vein (white straight arrow) and adjacent renal pelvis showing DJ Stent (curved whited arrow) in two different cases. Intra renal venous flow is very well demonstrated and appears normal Suggest Partial renal vein thrombosis.
Extra parenchymal pseudo aneurysm
Incidence: Anastomotic pseudoaneurysm is a rare complication of renal transplantation occurring in 0.3%. [14]
Imaging findings : On gray scale ultrasound it appears as cystic lesion which shows color flow and to and fro spectral pattern on doppler study(Figure 17). Endovascular treatment with covered stent placement to exclude pseudoaneurysm can also be evaluated with USG and colour doppler (Figure 18).
Figure 17: (A)Grey scale USG image of transplanted kidney shows cystic lesion near anastomosis which shows color filling in Colour Doppler image(white arrow), (B) Colour Doppler image shows colour filled out pouching near anastomosis(white arrow) confirmed non thrombosed extra parenchymal pseudo aneurysm in two different cases.
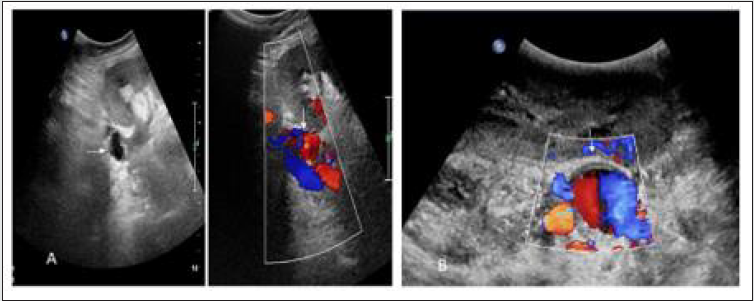
Figure 18: (A) Two echogenic line structure(Stent) in main anastomotic renal artery in gray scale USG image of transplanted kidney, (B) Colour doppler image shows partially colour filled pseudoaneurysm at anastomosis with stent suggest Imaging findings of endovascular treatment with covered stent placement.
Intra-parenchymal arteriovenous fistula and pseudoaneurysm
AVF occurs when both artery and vein are simultaneously lacerated, while pseudoaneurysm results when only artery is lacerated.
Incidence: 1-18% of the biopsies [15]
Time of onset: occur at time of biopsy. They depend on many factors – ultrasound guidance, needle caliber and imaging follow up.
Imaging appearance: colour Doppler study shows AVF as focal areas of disorganized flow adjoining the normal vasculature. Spectral waveforms show increased arterial and venous flow with high velocity and low resistance (Figure 19). Pseudoaneurysm appears as simple or complex cyst on B mode ultrasound and intracystic flow on colour Doppler mode (Figure 20).
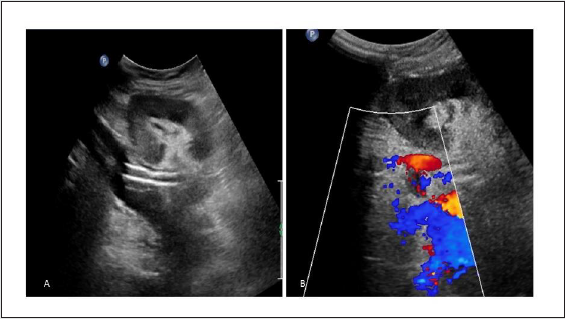
Figure 19: (A) focal colour aliasing at lower pole of transplanted kidney in colour doppler image, (B) Spectral Doppler evaluation demonstrates high velocity, low impedance waveform with increased diastolic flow Suggest arteriovenous fistula (AVF) in doppler evaluation after biopsy.
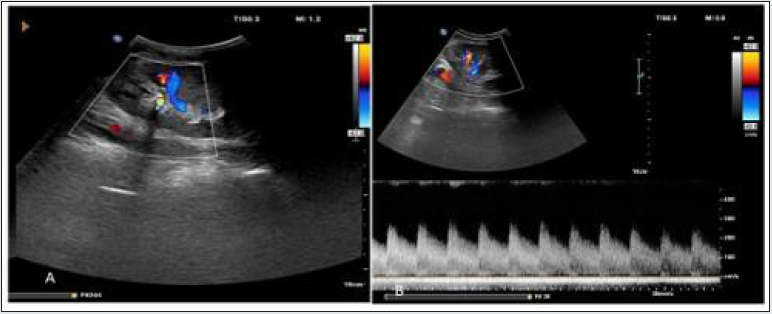
Figure 20: (A) Grey scale USG image shows cystic lesion in lower pole of transplanted kidney in grey scale USG image, (B) colour Doppler image shows intra cystic flow Suggest intra parenchymal pseudo aneurysm.

Neoplasms after renal transplantation
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
Post renal transplantation patients are at higher risk of development of neoplasms. Urologic tumour are 4 to 5 times more common in post renal transplantation recipients than normal population with significant exposure to cyclophosphamide immunosuppressant agent.
Renal cell carcinoma
Etiology: by means of transplanted organ or de novo development by immunocompromised status, patients on hemodialysis in case of chronic renal failure develop acquire renal cystic disease
Prevalence: 90 % occurring in native kidney and 10 % in transplanted kidney [16]
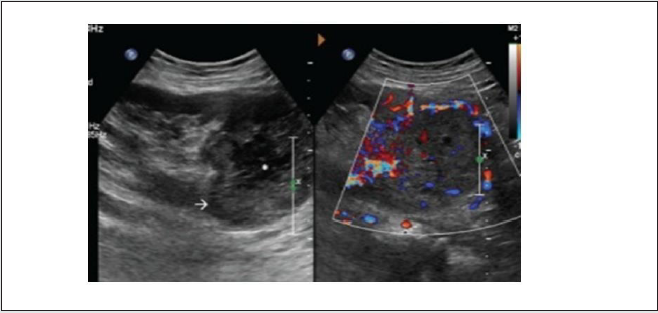
Imaging appearance: lesion appear heterogeneous with vascularity, similar picture as seen in native kidney [17] (Figure 21).
Figure 21: Gray scale ultrasound image of transplanted kidney shows (A) heterogeneous mass lesion at the upper pole (white arrow) with internal hypoechoic region (asterisk) suggesting necrosis, (B) the mass lesion showed internal as well as peripheral vascularity on color Doppler study. Suggest renal cell carcinoma in transplanted kidney.
Lymphomas
Incidence: 1 % of renal allograft recipients [18]
Time of onset: Post transplantation lymphoproliferative disorder is diagnosed at a median of 80 months after transplantation.
Imaging appearance: Lymphadenopathy at various sites but can also affect any solid organ or hollow viscera or transplant graft parenchyma itself. It appears as low or mixed reactive masses and tends to have a predilection for the renal hilum.
Recurrent Native disease
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
It depends on the primary disease before transplantation.
Imaging appearance: Imaging has no specific pattern in these situations apart from excluding the treatable cause of reduced renal function.
Abdominopelvic Complications
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
Renal graft is placed in extraperitoneal space via a peritoneal window in laparoscopic and robot assisted surgical techniques. So these cases are prone to same complications experienced by other surgical cases in whom peritoneum is exposed.
Renal Allograft Compartment Syndrome (RACS)
It is a rare syndrome, and it is under recognized cause of early transplant dysfunction or even loss. It may occur as a result of intracompartment hypertension and ensuring ischemia of renal graft [19]. Imaging appearance: absent or diffuse diminished cortical flow in transplant kidney at colour Doppler.
Fascial dehiscence and bowel or allograft evisceration
They tend to occur in perioperative period. Herniation of bowel through a transplant peritoneal defect may lead to compromise of intestine or transplant itself.
Limitations
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
The USG examination is examiner dependent and limited accessibility in obese patents impairs the evaluation and often leads misinterpretation. The RI index is also unspecific and influenced by many factors like site at which the RI is measures, increased intraabdominal pressure during forced inspiration and the pulse rate.
Summary
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
Kidney transplant is the treatment of choice for patients with end-stage renal disease. Improvements in surgical techniques and advanced immunosuppressive drugs have resulted in remarkable survival of patients and renal grafts. Still complications occur in both the early postoperative period and later. Kidney transplants follow up is common in radiology and sonography practice.
Ultrasonography and Doppler examination can accurately depict and characterize many of the potential complications of renal transplantation. It facilitates prompt and accurate diagnosis and thus guiding treatment.
References
- Abstract
- Introduction
- Surgical Technique
- Urologic Complications
- Peritransplant Fluid Collections
- Parenchymal Complication / Graft Dysfunction
- Infections and abscesses
- End stage disease
- Vascular Complications
- Neoplasms after renal transplantation
- Recurrent Native disease
- Abdominopelvic Complications
- Limitations
- Summary
- References
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K (1993) Comparison of survival probabilities for dialysis patients versus cadaveric renal transplant recipients. JAMA 270(11):1339 -1343.
- M. Cecka and P. I. Terasaki (1998) The UNOS scientific renal transplant registry. Clinical Transplants 1-16.
- Gogus C, Yaman O, Soygur T, Beduk Y, Gogus O (2002) Urological complications in renal transplantation: Long-term follow-up of the Woodruff ureteroneocystostomy procedure in 433 patients. Urol Int 69(2): 99-101.
- Kocak B, Baker TB, Koffron AJ, Leventhal JR (2010) Ureteral complications in the era of laparoscopic living donor nephrectomy: Do we need to preserve the gonadal vein with the specimen? J Endourol 24(2): 247-251.
- Cranston D, Nargund V.H (1996) Urological Complications after Renal Transplantation. Transplant. Rev 10(1): 24-33.
- Akbar SA, Jafri SZ, Amendola MA, Madrazo BL, Salem R, et al. (2005) Complications of renal transplantation. Radio Graphics 25(5): 1335-1356.
- Silver T. M, Campbell D, Wicks J. D, Lorber M. I, Surace P, et al. (1981) Peritransplant fluid collections. Ultrasound evaluation and clinical significance. Radiology 138(1): 145-151.
- B. Park, J. K. Kim, and K. S. Cho (2007) Complications of renal transplantation: ultrasonographic evaluation. Journal of Ultrasound in Medicine 26(5): 615-633.
- M. Rumack, S. F. Wilson, and J. W. Charboneau (2005) Diagnostic Ultrasound. J Ultrasound Med 24(5): 737-738.
- Kocak T, Nane I, Ander H, Ziylan O, Oktar T, et al. (2004) Urological and surgical complications in 362 consecutive living related donor kidney transplantations. Urol Int 72: 252-256.
- Rouvière O, Berger P, Béziat C, Garnier JL, Lefrançois N, et al. (2002) Acute thrombosis of renal artery: Graft salvage by means of intra-arterial fibrinolysis. Transplantation 73(3): 403-409.
- Sandhu JS, Sandhu P, Saggar K (2004) Sonographic evaluation of renal allograft. J Assoc Physicians India 52: 568-572.
- Giustacchini P, Pisanti F, Citterio F, De Gaetano AM, Castagneto M (2002) Renal vein thrombosis after renal transplantation: an important cause of graft loss. Transplant Proc 34(6): 2126-2127.
- Koo CK, Rodger S, Baxter GM (1999) Extra-renal pseudo aneurysm: An uncommon complication following renal transplantation. Clin Radiol 54: 755-758.
- Katsuhiro Kobayashi, Michael L. Censullo, Lucho L. Rossman, Polina N. Kyriakides, Barry D. Kahan, et al. (2007) Interventional Radiologic Management of Renal Transplant Dysfunction: Indications, Limitations, and Technical Considerations. RadioGraphics 27: 1109-1130.
- Vervloessem I, Oyen R, Vanrenterghem Y, Poppel Van. H, Debakker G, et al. (1998) Transitional cell carcinoma in a renal allograft. Eur Radiol 8(6): 936-938.
- Pandya VK, Sutariya HC (2016) Post-renal Transplantation de novo Renal Cell Carcinoma in a Middle-aged Man. Int J Organ Transplant Med 7(1): 51-56.
- Vrachliotis TG, Vaswani KK, Davies EA, Elkahammas EA, Bennett WF, et al. (2000) CT findings in posttransplantation lymphoproliferative disorder of renal transplants. AJR Am J Roentgenol 175: 183-188.
- Ball CG, Kirkpatrick AW, Yilmaz S, Monroy M, Nicolaou S, et al. (2006) Renal allograft compartment syndrome: an underappreciated postoperative complication. Am J Surg 191(5): 619-624.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




