
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Case Report(ISSN: 2641-1687) 
Presentation of Contained Ruptured External Iliac Artery Pseudoaneurysm following Transplant Nephrectomy Masquerading as Hemorrhagic Collection: An Imagine Surprise Volume 3 - Issue 5
Kajal Nitin Patel1*, Ketul K Pathak2, Subho Banerjee3 and Syed Jamal Rizvi4
- 1Associate Professor in Radiology, Institute of Kidney disease and research center, Gujrat University of transplantation sciences, India
- 2Assistant Professor, Gujrat University of transplantation science (GUTS), India
- 3Associate Professor in Nephrology, G. R. Doshi and K. M. Mehta Institute of Kidney Diseases and Research Centre, India
- 4Professor, Department of Urology, Gujrat University of transplantation science, India
Received: November 11, 2022; Published: November 23, 2022
Corresponding author: Kajal Patel, Associate Professor in Radiology, Institute of Kidney disease and research center, Gujrat University of transplantation sciences, India
DOI: 10.32474/JUNS.2022.03.000174
Abstract
The development of an external iliac artery pseudoaneurysm in the post renal transplantectomy bed is extremely rare. Estimated incidence is < 1 % and very few cases have been published in the medical literature. Our aim is to present a case of development of non-infected right external iliac artery pseudoaneurysm following renal transplant nephrectomy in early postoperative period. A 36-year-old man presented with blood discharge from the sinus tract of right iliac fossa, 3 months after transplant nephrectomy. USG was performed showing a small patent pseudoaneurysm arising from right external iliac artery with surrounding hemorrhagic fluid collection and sinus tract extending up to skin level in right iliac fossa. For confirmation and treatment planning CT angiography was performed yields same results. The patient underwent endovascular stenting of the right external iliac artery and lumen of pseudoaneurysm was treated successfully with an uneventful post-operative recovery. Formation of pseudoaneurysm following renal transplant nephrectomy is rare but may present significant morbidity and mortality. To our knowledge, this is the first case report of external iliac artery pseudoaneurysm presented with blood discharge from sinus tract; diagnosed with USG & Colour doppler and treated successfully.
Keywords: External iliac artery pseudoaneurysm; transplant nephrectomy; endovascular stent
Introduction
About 10 % of all renal transplants fail or become nonfunctional during the first year of transplantation and approximately 3% to 5% yearly thereafter [1]. A failed post-transplant kidney elicits an inflammatory response and is associated with increased morbidity (4.3–84.4%) and mortality (1.2–38%) whether maintained with low dose immunosuppression or not [2]. The incidence of vascular complications following transplant nephrectomy ranges from 0.9%- 14% [3]. According to McIntosh et al, only six cases of iliac artery pseudoaneurysm formation at site of post-transplant nephrectomy has been described up to 2005. In PubMed and google research, there were about ten cases of iliac artery pseudoaneurysm following renal transplant nephrectomy reported between 2006 and 2017 [4- 12]. Several different causes of iliac pseudoaneurysms have been described; infection or rejection of anastomotic remnant, which degenerate until forming a pseudoaneurysm. Diagnosis of the pseudoaneurysm with radiological imaging is necessary. Treatment options include open surgical repair of the leaking pseudoaneurysm or percutaneous endovascular covered stent placement. We report a case of external iliac artery pseudoaneurysm presented with blood discharge from sinus tract; diagnosed with USG & CT angiography and treated successfully with endovascular stent.
Study of a Patient
A 36-year-old male patient came to our institute for complain of altered renal function. He was suffering from chronic kidney disease and was on maintenance dialysis since 2012. He has O positive blood group. There was no history of hypertension, diabetes mellitus or any systemic illness. He has history of open right renal fossa live donor renal transplantation in 2014. The donor was his mother. This renal transplant had been complicated by Acute T+ B cell mediated rejection and the patient was on dialysis from 2016 onwards. Patient was considered as post renal transplant chronic kidney disease since 2018. Patient was stable up to sept. 2021 on maintenance dialysis twice a week. Then he suddenly developed fever and tenderness in right iliac fossa. His blood pressure was 126/86 mm/Hg. His Hematological and biochemical laboratory values showed elevated white cell counts of about 13,200 cells per cubic millimeter and serum creatinine of 11.2mg/dl. So, the patient was referred to our Radiology department for USG and Colour Doppler study for renal transplant evaluation. Renal graft showed changes of pyelonephritis and pyonephrosis with reduced perfusion.
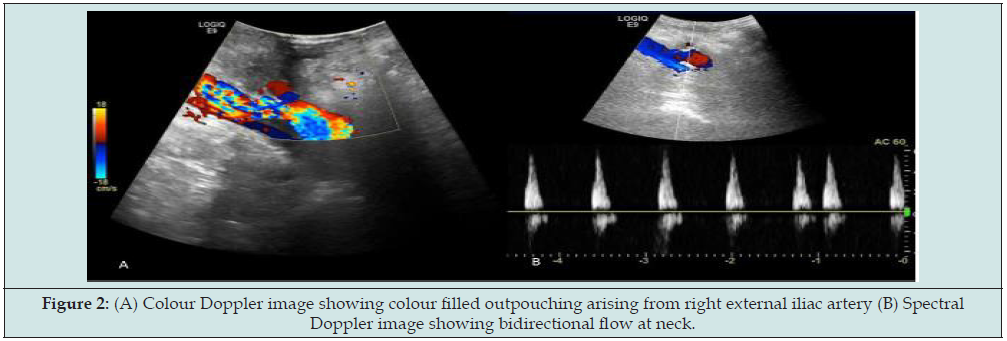
For further evaluation, the patient underwent CT Angiography; finding was necrosis with associated infection/pyonephrosis and non-visualization of transplanted artery and vein. So, the decision to transplant nephrectomy was taken. Transplant nephrectomy was done through retroperitoneal access. Histopathology findings were necrosis of graft with thrombosed vessels and no evidence of fungal infection. The patient presented to transplant surgeon 3 months later with blood discharge from the drain site suture with stable hemodynamics. So, the patient again referred to radiology department for USG evaluation of RIF for possibility of collection. But unfortunately, we found that there was about 5.0x3.4cm size organized hyperechoic collection (Figure 1) and 11x9mm size cystic area within it in basic USG scan. The cystic area shows swirling motion type bidirectional flow continue with right external iliac artery in colour Doppler study (Figure 2). Spectral waveforms demonstrate the classic to and fro flow at neck suggestive of external iliac artery pseudoaneurysm. For further evaluation CT angiography of iliac vessels was prescribed.
Figure 1: Grey scale USG image showing hypoechoic collection in right renal fossa adjacent to iliac vessels.
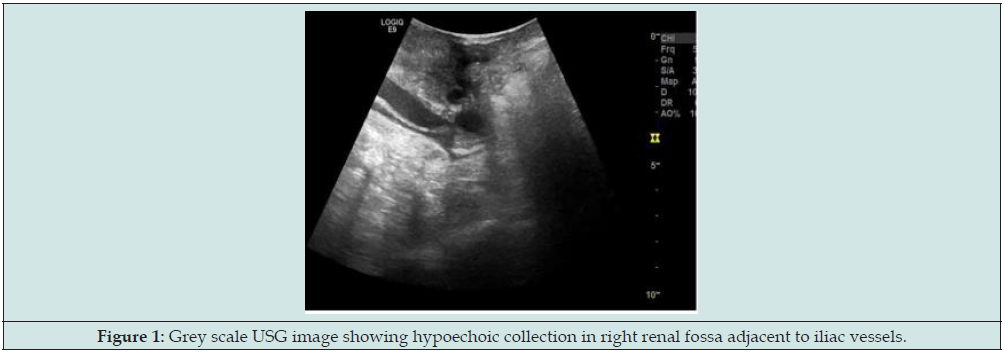
Figure 2: (A) Colour Doppler image showing colour filled outpouching arising from right external iliac artery (B) Spectral Doppler image showing bidirectional flow at neck.
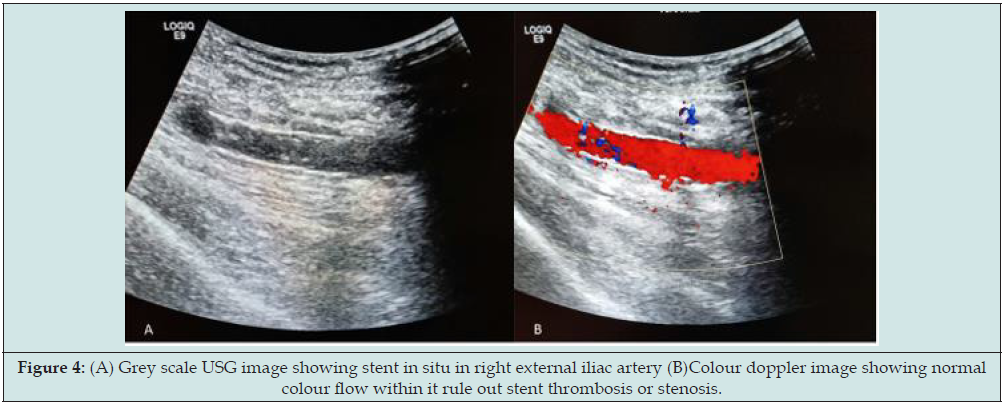
CT angiography findings were small pseudoaneurysm (measuring 11x8x6mm in size and neck measuring 2mm) was seen arising from right external iliac artery (Figure 3). Hemorrhage containing sinus tract (measuring 30mm in length and 4mm in diameter) was also demonstrated in right iliac fossa extending from skin to pseudoaneurysm within extraperitoneal compartment. So final diagnosis of contained ruptured right external iliac artery pseudoaneurysm following post-transplant nephrectomy was made. Patient was treated with endovascular stenting through right femoral approach. A 10x38mm size balloon mounted covered stent was placed in the right external iliac artery covering pseudoaneurysm. No filling of pseudoaneurysm was seen in completion angiography. There were no post-procedural complications and patient was discharged 2 days after the procedure. No pseudoaneurysm demonstrated in colour doppler study with patent iliofemoral vessels on 1 month follow up (Figure 4).
Figure 3: Sagittal image of Multislice CT Angiography showing contrast filled outpouching continuing with external iliac artery and elongated tract extending up to skin.
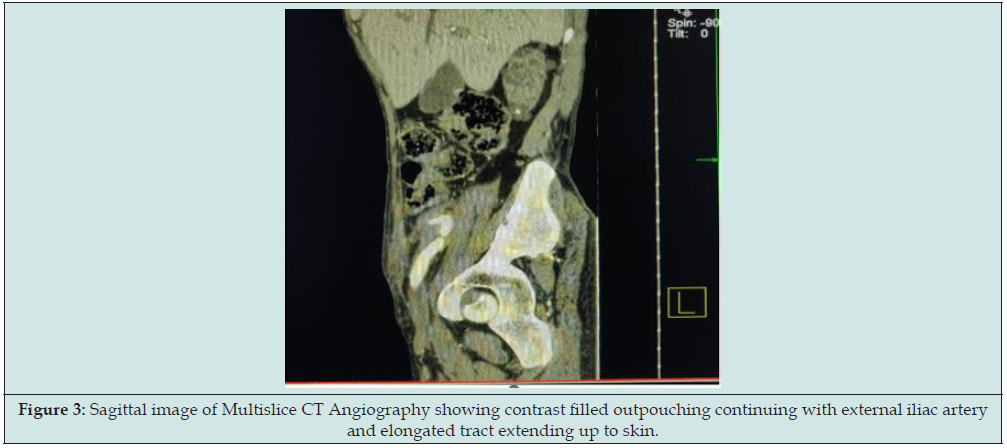
Figure 4: (A) Grey scale USG image showing stent in situ in right external iliac artery (B)Colour doppler image showing normal colour flow within it rule out stent thrombosis or stenosis.
Discussion
There are various indications for renal transplant nephrectomy. They are major graft related complications like arterial or venous thrombosis in renal transplant, septic pyonephrosis, acute rejection intractable to medical therapy, symptomatic chronic rejection, symptomatic extra or intra renal pseudoaneurysm with or without rupture, uncontrolled post biopsy bleeding, development of mass, persistent hematuria, recurrent urinary tract infection with renal transplant failure, recurrence of primary disease, lymphoma [13,14]. Infection is the most common complication of renal transplant nephrectomy followed by bleeding. Other infrequent complications are urinary fistulae, iatrogenic bowel injury, and obturator nerve or lateral cutaneous nerve injury. Vascular complications are uncommon, having been reported in 0.9–14% of cases, but are associated with poor outcomes. Formation of iliac artery pseudoaneurysm at a prior transplant nephrectomy site is a rare event with an estimated incidence < 1%. Donckier et al mentioned in their study that only two (0.6%) patients developed pseudoaneurysm on the donor side of the arterial anastomosis out of 352 kidney allograft nephrectomies [15].
Several risk factors like chronic rejection, local or systemic infection, bleeding, coagulopathy, atherosclerosis, iatrogenic trauma during nephrectomy leading anastomotic disruption of the Carrell’s patch, fibromuscular dysplasia, cystic medial necrosis and marfan syndrome are described in literature. There is an association of dyslipidemia and hypertension too. Chronic rejection of the residual donor tissue at vascular stump may result in erosion/ degeneration of stump with subsequent pseudoaneurysm. External iliac artery pseudoaneurysm is usually clinically silent. However, it may present as a pulsatile mass, fever, anaemia, unilateral leg swelling on the affected side and symptoms of rupture. There may be symptoms due to compression of neighboring structures like compression of adjacent nerves or veins, obstruction of the large intestine, and ureteral obstruction. Some patients may present with elevated inflammatory markers, erythropoietin resistance and hypoalbuminemia [16]. If rupture of pseudoaneurysm occurs then patient can enter into hemorrhagic shock associated with fever, abdominal pain, nausea and vomiting, genital or urinary symptoms.
Multiple imaging modalities have been used to identify the condition. Ultrasonography is routinely used; On B mode external iliac artery pseudoaneurysm appears as a simple or a complex cyst along the supplying artery. Concentric layers of hematoma are occasionally seen. The Color Doppler study shows intra-cystic swirling motion type blood flow and bidirectional flow at neck on spectral evaluation. Color Doppler allows a differential diagnosis of hematoma, urinoma and lymphocele. It is a relatively cheap, readily available, inexpensive, fast, portable, noninvasive modality with no ionizing radiation. However, it is difficult to evaluate deep visceral arteries and it is operator dependent. Multi slice Computed tomography angiography (CTA), Magnetic resonance angiography or catheter directed conventional angiography can be used to confirm the findings of ultrasound before embarking on treatment modality. Contrast enhanced Multislice CTA demonstrates a contrast material–filled sac arising from iliac artery; Wall of the pseudoaneurysm usually smooth and well delineated except in a mycotic pseudoaneurysm, whose wall is thickened, irregular, or ill defined. It also delineates partial thrombosis within the pseudoaneurysm.
Intermediate or high attenuation (hemorrhage) can be identified adjacent to the pseudoaneurysm which indicates pseudoaneurysm rupture. The advantage of CT scan is it allows visualization of the lesion from all angles and provides a global perspective on the entire vasculature, including adjacent vascular beds. The disadvantages of the CT scan are that it requires more contrast material than for angiography and endovascular therapy cannot be performed at the time of diagnosis. So conventional angiography carries the additional benefit of intervention. Degree of opacification of pseudo aneurysmal sac depends on the size of its neck and amount of thrombosis within its lumen. It may exhibit heterogeneous opacification owing to luminal thrombosis. To prevent rupture, to stop inflammatory response and to relieve compressive symptoms of adjacent organs treatment is necessary. Conventional open repair, endovascular repair and USG guided percutaneous thrombin injection in to pseudoaneurymal sac are the current therapeutic choices. Conventional surgical approach includes resection of pseudoaneurysm and even iliac artery ligation and subsequent extra-anatomical revascularization by insertion of prosthesis.
Due to high risk of infection and subsequent chance of anastomotic disruption and bleeding, anatomic revascularization should be avoided or deferred. However additional technical difficulties may arise to dissect a fibrotic surgical field as a result of previous operation which often leads to injury [17]. Due to multiple adhesions, open surgery did not allow preservation of peritoneal cavity, so patient must switch off from peritoneal dialysis to hemodialysis. Endovascular exclusion of pseudo aneurysmal sac with covered stent is currently the preferred method of choice. Endovascular approach is indicated in case of favorable anatomy and absence of compressive symptoms. It is a quick, safe and effective alternative to open surgery in case of contained rupture also. It offers long length (up to 15 cm) endoprostheses. No need for general anesthesia is required during the procedure. It has less intraoperative bleeding, lower incidence of postoperative complications and a lower mortality rate, when compared to open surgery.
The endo grafting decreases intimal hyperplasia also, so patent iliac arteries are available for good flow to lower limb arteries. However, the procedure requires assistance using costly imaging techniques in terms of radiation and contrast material. Long term follow up is also required in case of stent graft because long term patency, stability and durability of it is still not known. Our patient was presented with pyelonephritis and associated vascular thrombosis in renal transplant. So, the transplant nephrectomy was done. Infection and chronic rejection may be the causative factors for the development of pseudoaneurysm in our patient. Pseudoaneurysm may show contained rupture as in our case as patient presented with only complain of bloody discharge from drain site suture and imaging modality confirmed the diagnosis of contained rupture of iliac artery pseudoaneurysm with small hemorrhagic collection in iliac fossa. Our patient did not have any febrile illness and had normal inflammatory markers, hemoglobin and albumin level after nephrectomy and his condition was also stable and had negative blood culture. Also imaging findings were not favoring of infected etiology. So endovascular stent placement treatment was considered in our patient and thus avoided major reconstructive vascular surgery and associated morbidity also.
Conclusion
Vascular complications following renal transplant nephrectomy are rare. A high index of clinical suspicion of iliac artery pseudoaneurysm is necessary when there is a history of previous vascular surgical or radiological procedure through femoral asses or even after post renal transplant nephrectomy of whatever duration. Imaging modality such as colour Doppler and CT angiography should be used to diagnose the case. Endovascular exclusion with placement of covered stent is safe and effective treatment and is good alternative to open surgery even in case of contained rupture of iliac artery pseudoaneurysm.
Authors’ Contribution:
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Conflicts of interest:
There are no conflicts of interest.
Acknowledgements:
We are also thankful to Ms Jyotsna Suthar for literature search work.
References
- Lamb KE, Lodhi S, Meier-Kriesche HU (2011) Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant 11(3): 450-462.
- Akoh JA (2011) Transplant nephrectomy. World J Transplant 1(1): 4-12.
- Eng MM, Power RE, Hickey DP, Little DM (2006) Vascular complications of allograft nephrectomy. Eur J Vasc Endovasc Surg 32(2): 212-216.
- McIntosh BC, Bakhos CT, Sweeney TF, DeNatale RW, Ferneini AM (2005) Endovascular repair of transplant nephrectomy external iliac artery pseudoaneurysm. Conn Med 69(8): 465-466.
- Bracale UM, Porcellini M, del Guercio L, Bracale G (2013) Embolization of a symptomatic pseudoaneurysm developing after transplant nephrectomy. Intern Med 52(2): 291-292.
- Nuno Guimarães Rosa, Sónia Silva, Sofia Jorge, Patrícia Branco, João Inácio, et al. (2007) Iliac artery false aneurysm twelve years after allograft nephrectomy. Port J Nephrol Hypert 21(2): 105-107.
- Borges L, Oliveira N, Dias E, Cássio I (2014) Iliac artery pseudoaneurysm: a rare complication following allograft nephrectomy. BMJ Case Rep 3: bcr2013202596.
- Diller R, Holzen J, Senninger N, Kramer S (2006) Interventional stenting for ruptured iliac aneurysm following transplant nephrectomy. Transplant Proc 38(3): 718-720.
- Narayanan S, Manivannan M, Subrayan L, Balamurugan B (2017) Endograft Placement for Iliac Artery Pseudoaneurysm Following Graft Nephrectomy. Indian J Vasc Endovasc Surg 4(1): 32-34.
- Moosavi CA, Gujrathi SK, Friedman A, Fox D, Silberzweig JE (2008) Endovascular repair of symptomatic renal transplant site pseudoaneurysm. Vasc Endovascular Surg 42(6): 607-609.
- Clevert DA, Stickel M, Steitz HO, Kopp R, Strautz T, et al. (2007) Treatment of secondary stent-graft collapse after endovascular stent-grafting for iliac artery pseudoaneurysms. Cardiovasc Intervent Radiol 30(1): 111-115.
- Marcela Acosta-Silvaa, Efrén Martel-Almeidaa, Serguei De Varona-Frolova, Guido Volo-Péreza (2012) Kidney post-transplantectomy ruptured iliac pseudoaneurysm: emergency endovascular repair. Nefrologia 32(5): 694-696.
- Aparicio TF, López BM, Gómez BF, Villar GP, González RD, et al. (1996) Renal transplantectomy. Arch Esp Urol 49(10): 1079-1091.
- Durbán MM, Valor PC, Sebastian JN, García-Matres JM, Piñeiro LM, et al. (1989) Non-functioning renal graft: indications for transplant excision. Arch Esp Urol 42(9): 873-878.
- Donckier V, De Pauw L, Ferreira J, Hanquinet S, Hooghe L, et al. (1995) False aneurysm after transplant nephrectomy. Report of two cases, Transplantation 60(3): 303-304.
- Peel RK, Patel J, Woodrow G (2003) Iliac artery false aneurysm following renal allograft: presentation with non-specific inflammatory response and treatment by endovascular stent graft. Nephrol Dial Transplant 18(9): 1939-1940.
- Pérez P, Esteban C, Muchart J, Callejas JM (2004) Endovascular resolution of iliac artery pseudoaneurysm in a transplanted patient. Nefrologia 24(6): 596-599.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




