Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Research Article(ISSN: 2641-1687) 
Presentation and Outcomes of First-Degree Relatives Treated with Brachytherapy for Clinically Localized Prostate Cancer Volume 1 - Issue 2
Oren Factor*, Nelson Stone and Richard Stock
- Department of Radiation Oncology, Mount Sinai Hospital, New York, USA
Received: June 27, 2018; Published: July 03, 2018
Corresponding author: Oren Factor, PGY-3 Resident, Department of Radiation Oncology, Mount Sinai Hospital, 1184 Fifth Avenue 1st Floor, New York, USA
DOI: 10.32474/JUNS.2018.01.000107
Abstract
Purpose: To determine if first-degree relatives treated with definitive radiation for clinically localized prostate cancers have similar baseline characteristics and clinical outcomes.
Methods: From a prospectively assembled database, we identified a set of first-degree relatives with clinically localized prostate adenocarcinoma. Patients were treated with brachytherapy with or without external beam radiation therapy. Biochemical failure was the primary outcome. Toxicities were measured with the International Prostate Symptom Score (IPSS) and Mount Sinai Erectile Function Score (MSEF).
Results: We identified 41 patients including 22 pairs of first-degree relatives. One family included 3 separately treated brothers. The median follow up was 72 months. Eight pairs of first-degree relatives presented with the same Gleason score, representing 36% of pairs. Eleven pairs of relatives (50%) fell in the same NCCN risk category. There were 4 total biochemical failures, representing 9.8% of the cohort. In a single pair of brothers both patients experienced a biochemical failure. Therefore, in two pairs of relatives (9.1%) a different biochemical outcome was experienced. In the pair of brothers who both experienced biochemical failure, both brothers developed metastatic disease. One of the brothers died due to prostate cancer. Amongst pairs of first-degree relatives, 10 pairs (52%) experienced a concordant change in IPSS relative to the average. Fifty percent of pairs experienced concordant changes in MSEF score.
Conclusion: First-degree relatives treated with radiation therapy did not present similarly. Outcomes appeared to be concordant amongst relatives. There was no suggestion of worse outcomes in patients with a brother or father having prostate cancer.
Keywords:Prostate cancer, Brachytherapy, Family History, Biochemical Recurrence, Radiation Therapy
Abbrevations: IPSS: International Prostate Symptom Score, MSEF: Mount Sinai Erectile Function Score, SNPs: Single Nucleotide Polymorphisms, NCCN: National Comprehensive Cancer Network, ADT: Androgen Deprivation Therapy, EBRT: External Beam Radiation Therapy, BCR: Biochemical Recurrence, PSA: Prostate-Specific Antigen
Introduction
Prostate cancer is the most common cancer in men worldwide. In 2015 there was an estimated 1,600,000 cases and 366,000 deaths [1]. There are a number of kno¬wn risk factors for the development of prostate cancer including age, ethnicity, and genetic factors. Two large family studies have demonstrated the impact of family history on the development of prostate cancer. The Prostate¬ Cancer Data Base Sweden evaluated the risk of prostate cancer in 51,897 men who were brothers of 32,807 men with prostate cancer [2]. They found a significantly increased risk of prostate cancer development at age 65 and age 75 in men whose brother had prostate cancer. Similarly, a prospective study of 203,000 pairs of twins from European countries found a significant concordance of prostate cancer development in both monozygotic and dizygotic twins [3]. They estimated that approximately 57 percent of prostate cancer risk could be explained by genetic factors.
The heritable factors contributing to prostate cancer risk include two categories, rare deleterious variants from mutations, and more common variants or single nucleotide polymorphisms (SNPs). The rare variants, such as BRCA mutations, have a high penetrance and are often associated with more aggressive prostate cancer [4]. When considering more common genetic variants, genome wide association studies using SNPs have discovered over 100 loci associated with prostate cancer [5]. These loci are estimated to account for 33 percent of the familial prostate cancer risk [6-15]. Unlike BRCA and deleterious mutations, few SNP foci have been found to be associated with more aggressive prostate cancer or worse outcomes. [16]. Despite the inability in identifying loci associated with prostate cancer outcomes, a Swedish study looking at family history found a significant correlation between family history and outcomes. The study included 610 sons of men with prostate cancer. They found a significant correlation in prostate cancer specific death between fathers and sons [17]. We previously evaluated the effect of family history on outcomes in brachytherapy treated patients at our institution. We did not find a significant effect of positive family history on biochemical failure in our cohort [18]. Given the strong genetic component of prostate cancer risk and possibly prognosis, we aimed to discover if brothers and fathers with prostate cancer all treated with radiation therapy at our institution had similar baseline characteristics, treatment choices, outcomes, and toxicities.
Materials and Methods
We identified 41 first-degree relatives with clinically localized prostate adenocarcinoma treated with brachytherapy with or without external beam radiation therapy between January 1998 and January 2016 at a single institution. Patients with node positive or distant metastatic disease were excluded. We received institutional review board approval, and all consenting patients were enrolled in a dedicated prospective database. Prostate brachytherapy was performed using a real-time transrectal ultrasound-guided technique as described previously [19]. Patient’s treatments were based on their National Comprehensive Cancer Network (NCCN) risk group [20]. They were treated with: 125 I or 103 Pd implant alone (NCCN low risk); androgen deprivation therapy (ADT) and a full dose implant or a partial 103 Pd implant followed by external beam radiation therapy (EBRT) to 45 Gy (NCCN intermediate risk); trimodal therapy including ADT, partial 103 Pd implant, and 45 Gy of EBRT (NCCN high risk). Iodine-125 implants were generally prescribed to 160 Gy, full 103 Pd implants to 124 Gy, and partial 103 Pd implant to 100 Gy. EBRT was delivered with three-dimensional conformal radiation prior to 2003 and intensity-modulated RT thereafter. Dose per fraction was 180 cGy. Median EBRT dose was 4500 cGy.
Biochemical recurrence (BCR) was defined by the Phoenix criteria as a rise of 2 ng/mL or more above the prostate-specific antigen (PSA) nadir. Distant metastases were documented by cross sectional imaging (chest/abdomen or pelvis) and/or radionuclide bone scans. Prostate cancer death was defined as death in the presence of metastatic disease. The International Prostate Symptom Score (IPSS) was used to follow urinary toxicities. The Mount Sinai Erectile Function Score (MSEF) was used to follow sexual function. A MSEF score change from 2 or 3 to 0 or 1 was considered a significant change, representing a decline from erections at least adequate for penetration to erections inadequate for penetration. Descriptive statistics were used to analyze the baseline characteristics, biochemical outcomes, and toxicities among first-degree relatives in the cohort. Variables included pretreatment Gleason, PSA, T-stage, NCCN risk group, and treatment modality. Percentage of first-degree relatives having the same or different treatment outcome was calculated. For urinary toxicity the mean IPSS score for the entire cohort was calculated, and the percentage of relatives having a concordant change relevant to the mean was recorded. For sexual dysfunction the number of patients with a significant score change, as described above, was recorded, and the percentage of relatives with a concordant change was calculated.
Results
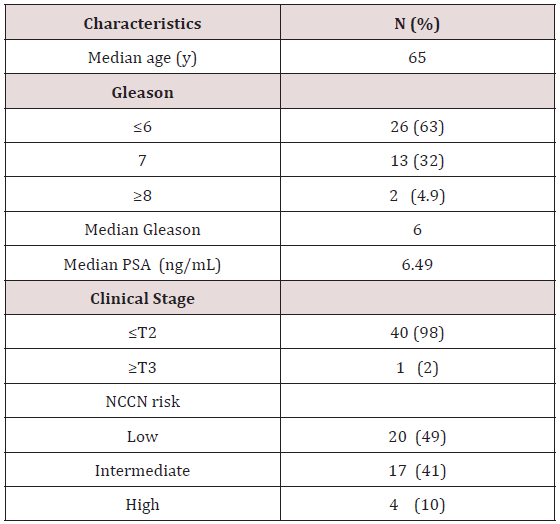
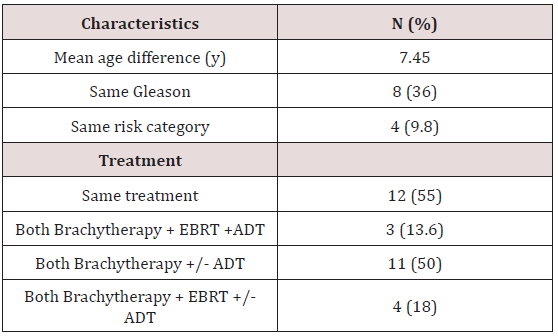
There were 41 patients identified, including 22 pairs of firstdegree relatives. We found two father-son pairs and twenty pairs of brothers. One family included 3 separately treated brothers. The median age was 65 years old, ranging from 50 to 83 years old. The median follow up for the entire cohort was 72 months. Baseline characteristics are demonstrated in Table 1. The median Gleason score was 6 and median PSA was 6.49. Twenty patients (49%) had low-risk disease, 17 patients (41%) intermediate-risk, and 11 patients (10%) had high-risk prostate adenocarcinoma. Baseline characteristics and treatment similarities amongst first degree relative pairs are seen in Table 2. Twelve pairs of firstdegree relatives (55%) received the exact treatment compromising EBRT+/- brachytherapy +/-ADT. In three pairs of relatives (13.6%) both relatives received trimodal therapy. In four pairs of relatives (18.1%) both patients received a combination of EBRT with brachytherapy with or without the addition of ADT. All but one patient received brachytherapy in his treatment.
NCCN= National Comprehensive Cancer Network
EBRT= External beam radiation therapy ADT= Androgen deprivation therapy.
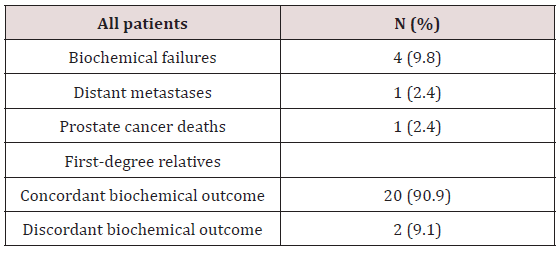
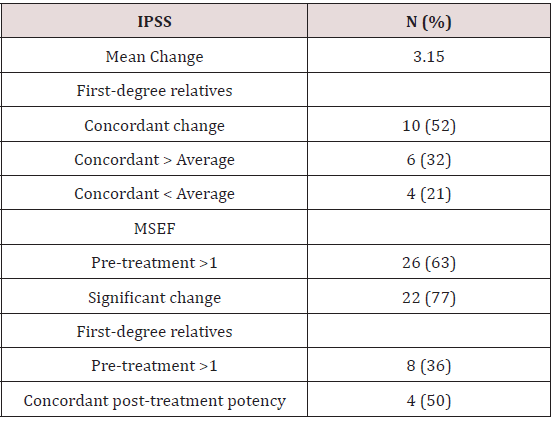
The average difference in age at diagnosis between first-degree relatives was 7.45 years ranging from 0 to 16 years. Eight pairs of first-degree relatives (36%) presented with the same Gleason score representing. Eleven pairs of relatives (50%) fell in the same NCCN risk category. Presentation comparisons amongst first-degree relatives are shown in Table 2. There were 4 total biochemical failures, comprising 9.8% of the cohort. In a single pair of brothers, both patients experienced a biochemical failure. Therefore, in two pairs of relatives (9.1%) a different biochemical outcome was experienced. While one relative experienced a biochemical recurrence, the other relative remained biochemically free of disease. In 20 pairs of relatives (90.9%) both relatives experienced the same biochemical outcome. In the pair of brothers who both experienced biochemical failure, both brothers developed metastatic disease. One of the brothers died due to prostate cancer. The brother who died presented with T3a, Gleason 6, and pre-treatment PSA 12.0 prostate adenocarcinoma. The surviving brother presented with T2b, Gleason 7, and pre-treatment PSA 3.77 prostate adenocarcinoma. Both brothers received trimodal therapy. No other patients developed metastatic prostate cancer or died from their disease. Disease control outcomes are shown in Table 3. The average change in IPSS score from baseline for all patients was 3.15. Four patients did not have an initial IPSS score recorded and were excluded from this analysis. Amongst pairs of first-degree relatives, 10 pairs (52%) experienced a concordant change in IPSS relative to the average. In 4 pairs (21%) both relatives experienced a change in IPSS score less than the average. In 6 pairs (32%) both relatives experienced an increase in score relative to the average change.
Twenty-six patients had a pre-treatment MSEF score >1. Twenty of these patients (77%) experienced a significant change in score. Twelve patients had an initial score of 0 or 1 and 3 patients did not have follow up MSEF data recorded. There were eight pairs of first-degree relatives in which both relatives had a pretreatment MSEF score of >1. Four of these pairs (50%) experienced concordant outcomes with both relatives either remaining potent or losing their potency. In the other 4 pairs one relative maintained a score >1 while the other relative experienced a significant decline in sexual function. Toxicity data is displayed in Table 4.
IPSS= International prostate symptom score MSEF= Mount Sinai erectile function.
Discussion
This is the first study to our knowledge to look at a cohort of first-degree relatives treated with radiation therapy at a single institution. Overall, there weren’t any obvious similarities between the initial presentation amongst the first-degree relatives since only 36% presented with the same Gleason score and 50% presented with the same NCCN risk category. Families with known genetic deleterious mutations, such as the BRCA mutation, have been known to present with more aggressive prostate cancer. These patients are at increased risk of cancer development as well as an earlier age at diagnosis, higher Gleason score, higher rates of lymph node involvement, increased distant metastatic disease at diagnosis, and increased prostate cancer specific mortality [4,21- 26].
Additionally patients with BRCA2 mutations have been found to have worst outcomes following initial treatment. A study looked at a cohort of patients with localized prostate cancer treated with surgery or radiation with or without a BRCA2 mutation. This study found worsened metastases free survival and cancer specific survival in the BRCA2 mutated population [26]. However, the large majority of patients with genetically influenced prostate cancer are not found to have deleterious mutations such as BRCA2. Given the rarity of these mutations and the less aggressive presentation of our cohort (median Gleason 6 and median PSA 6.49) it is more likely the genetic influence amongst patients in this cohort is caused by SNPs.
It remains an open question whether prostate cancer patients with positive family history without a known genetic mutation have worse treatment outcomes. In our study, which included patients with low, intermediate, and high risk prostate cancer, patients did well in terms of biochemical failure. Ninety percent of the cohort remained free from biochemical failure. There is no suggestion from our study of a positive first-degree relative family history having a negative impact on treatment outcomes. This finding is similar to our prior study, in which patients with a positive family history had similar outcomes to those patients without a positive family history [18] In the Swedish study by Hemminiki there was a suggestion of a concordance of outcomes between treated fathers and sons with prostate cancer [17] Similar to Hemminiki’s findings the pairs of relatives tended to have similar outcomes in terms of disease control with only two pairs of relatives, 9.1% experiencing an outcome different from their relative.
It has been suggested that variation to radiation sensitivity is an inherited genetic trait [27] It has been estimated that as much as 80% of the variation in normal tissue reactions between patients is genetic. [28] Therefore, it was possible that first-degree relatives may have had similar radiation sensitivities and toxicity outcomes. However, we did not see a clear correlation between relatives’ response to treatment in terms of toxicity. Fifty-two percent of pairs experienced a concordant change in IPSS relative to the mean and 48% experienced a discordant change. A majority of patients, 77%, experienced a significant decline in MSEF score. This study is limited by its small sample size as well as retrospective study design. With a larger cohort it is possible different correlations and treatment outcomes may have been detected. Unfortunately, a larger cohort of first-degree relatives all treated with the same modality would be difficult to obtain.
Conclusion
This is a unique study of first-degree relatives with prostate adenocarcinoma; all patients were treated with radiation therapy at the same institution. In our study, first-degree relatives did not present similarly. Outcomes appeared to be concordant amongst relatives. Patients tended to do well, but it remains an open question whether patients with genetically influenced prostate cancer not caused by a known mutation actually have worse treatment outcomes. Larger clinical studies, as well as a better understanding of the heritable basis for prostate cancer are needed.
References
- Fitzmaurice C, Allen C, Barber R (2017) Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 3(4): 524-548.
- Bratt O, Drevin L, Akre O (2016) Family History and Probability of Prostate Cancer, Differentiated by Risk Category: A Nationwide Population-Based Study. J Natl Cancer Inst 108(10).
- Mucci LA, Hjelmborg JB, Harris JR (2016) Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 315(1): 68-76.
- Agalliu I, Gern R, Leanza S, Burk RD (2009) Associations of high-grade prostate cancer with BRCA1 and BRCA2 founder mutations. Clin Cancer Res 15(3): 1112-1120.
- Al Olama AA, Kote-Jarai Z, Berndt SI (2014) A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet 46(10): 1103-1109.
- Amundadottir LT, Sulem P, Gudmundsson J (2006) A common variant associated with prostate cancer in European and African populations. Nat Genet 38(6): 652-658.
- Freedman ML, Haiman CA, Patterson N (2006) Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci USA 103(38): 14068-14073.
- Gudmundsson J, Sulem P, Manolescu A (2007) Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet 39(5): 631-637.
- Haiman CA, Patterson N, Freedman ML (2007) Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet 39(5): 638-644.
- Yeager M, Orr N, Hayes RB (2007) Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet 39(5): 645-649.
- Zheng SL, Sun J, Cheng Y (2007) Association between two unlinked loci at 8q24 and prostate cancer risk among European Americans. J Natl Cancer Inst 99(20): 1525-1533.
- Eeles RA, Kote Jarai Z, Giles GG (2008) Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet 40(3): 316- 321.
- Thomas G, Jacobs KB, Yeager M (2008) Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet 40(3): 310-305.
- Gudmundsson J, Sulem P, Steinthorsdottir V (2007) Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet 39(8): 977-983.
- Zheng SL, Sun J, Wiklund F (2008) Cumulative association of five genetic variants with prostate cancer. N Engl J Med 358(9): 910-919.
- Szulkin R, Karlsson R, Whitington T (2015) Genome-wide association study of prostate cancer-specific survival. Cancer Epidemiol Biomarkers Prev 24(11): 1796-1800.
- Hemminki K, Ji J, Försti A (2008) Concordance of survival in family members with prostate cancer. J Clin Oncol 26(10): 1705-1709.
- Peters C, Stock R, Blacksburg S (2009) Effect of family history on outcomes in patients treated with definitive brachytherapy for clinically localized prostate cancer. Int J. Radiation Oncol Biol Phys 73(1): 24-29.
- Stock RG, Stone NN, Wesson MF (1995) A modified technique allowing interactive ultrasound guided three dimensional transperineal prostate implantation. Int J Radiat Oncol Biol Phys 32(1): 219-225.
- National Comprehensive Cancer Network (NCCN) (2017) clinical practice guidelines in oncology.
- Mitra A, Fisher C, Foster CS (2008) Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br J Cancer 98(2): 502-507.
- Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T (2007) Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst 99(12): 929-935.
- Narod SA, Neuhausen S, Vichodez G (2008) Rapid progression of prostate cancer in men with a BRCA2 mutation. Br J Cancer 99(2): 371-374.
- Edwards SM, Evans DG, Hope Q (2010) Prostate cancer in BRCA2 germline mutation carriers is associated with poorer prognosis. Br J Cancer 103(6): 918-924.
- Castro E, Goh C, Olmos D (2013) Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 31(14): 1748-1757.
- Castro E, Goh C, Leongamornlert D (2015) Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol 68(2): 186-193.
- Barnett GC, West CM, Dunning AM (2009) Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer 9(2): 134-142.
- Turesson I, Nyman J, Holmberg E, Oden A (1996) Prognostic factors for acute and late skin reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys 36(5): 1065-1075.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...