Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Review Article(ISSN: 2641-1687) 
Neuroleptic Amyloidosis of The Kidneys Volume 3 - Issue 4
Volkov Vladimir Petrovich*
- Tver center of judicial examinations, Russia
Received: January 17, 2022; Published: June 08, 2022
Corresponding author: Volkov Vladimir Petrovich, Tver center of judicial examinations, Russia
DOI: 10.32474/JUNS.2022.03.000167
Abstract
The analysis of clinical data causes of death and pathoanatomic picture of renal amyloidosis in 14 deceased mentally ill patients receiving long-term antipsychotic therapy is presented.
Keywords: Symptomatic uremia; Intestinal dialysis; Ketoanalogues of essential amino acids.
Introduction
The problem of side effects of medicines is currently of great concern around the world [1]. Practically all pharmaceutical preparations with pronounced pharmacological activity, including psychopharmacological preparations, while having a therapeutic effect, at the same time can cause undesirable reactions, leading in some cases to severe complications and even death [2-4]. A special form of manifestation of the side effect of neuroleptics can be attributed to the medicinal (neuroleptic) amyloidosis of the kidneys described by us [5-7], which develops in patients with psychochronics with prolonged use of atypsychotic drugs. There are practically no observations of this kind, that is, amyloidosis, etiologically caused by the use of drugs in general and neuroleptics in particular, in the available literature. We can only cite publications on the development of β2M—amyloidosis in chronic hemodialysis, dating back to the late 1980s [8.9], and an even earlier description of amyloidosis as a complication of vaccine therapy [10]. At the same time, antipsychotics by their pharmacobiological properties may well be amyloidogenic agents [11]. As noted above, in 2005-2011 we described kidney amyloidosis as a complication of prolonged antipsychotic therapy [12]. Medical histories and autopsy materials from the archive of the Regional Clinical Psychiatric Hospital No. 1 named after M.P. Litvinov (Tver) were studied for more than 50 years (from January 1952 to August 2011). Micropreparations were again stained congo red and studied in polarized light. The autoclave method of T. Kitamoto (1986) was used to verify the form of amyloidosis [13].
The Main criteria for the Diagnosis of Neuroleptic Amyloidosis were
1) long-term neuroleptic therapy of the underlying mental illness; 2) absence of somatic “amyloidogenic” pathology; 3) morphological and histochemical properties of amyloid, characteristic of secondary AA-amyloidosis. In the material originally collected by us, 12 observations of renal amyloidosis were presented, which was a complication of long-term antipsychotic therapy. Recently, information has been received about two more cases of this kind. The following is a generalized analysis of the currently available proprietary data on this issue. So, among the 14 deceased psychiatric hospital patients who received various antipsychotic drugs during their lifetime as part of the therapeutic standard, there were 8 men and 6 women aged 19 to 67 years. The average age of men was 42 years, women — 55 years. The period from the beginning of treatment with neuroleptics to the appearance of persistent urinary syndrome is from 40 to 296 months, on average, about 15 years. It is interesting to note that the earliest observation refers only to 1972, while the widespread clinical use of aminazine in the hospital began in 1956 [14]. This fact indirectly indicates that a sufficiently long period of exogenous drug exposure is required for the development of drug amyloidosis.
The manifestation of nephropathic amyloidosis in 13 patients began with an isolated urinary syndrome. Only 1 49-year-old patient immediately developed a complete nephrotic syndrome with a rapid increase in edema. However, 8 of the 13 mentioned patients subsequently also developed complete (edematous) nephrotic syndrome. The period of occurrence of edema after the detection of persistent changes in urine is from 4 to 8 years. In 3 patients, edema was initially transient and only eventually became permanent.
Persistent proteinuria was observed in all patients, but its level was different. In 1 patient, the protein content in the urine was only 0.0033%, in 3 it did not exceed 0.33%, in 2 — 1.65%. Most often, the maximum level of proteinuria was kept within 3.3% (5 patients), sometimes reaching 6.6% (3 patients). The daily loss of protein was determined only in 5 people. It was 2.4-3.3 g. Such disorders are quite typical for renal amyloidosis [15]. A constant phenomenon was a small persistent leukocyturia. The number of leukocytes in the friction field ranged from 1-2 to 15-30 (in one-time single analyses in a 67-year-old patient). Hematuria was observed in 9 patients, was unstable and very moderate. The number of red blood cells was usually from single to 2-4 in the friction field. Only occasionally in some patients, hematuria reached 10-15 cells in the friction field. In 9 patients, transient small cylindruria was noted. The cylinders are usually hyaline and granular, occasionally waxy. The number of them in the friction field is up to 2-3, sometimes 5-6. The described features of urinary sediment are considered typical for urinary syndrome in renal amyloidosis [16].
In clinical blood tests, moderate hypochromic anemia was detected in all patients. From the very beginning of treatment with neuroleptics, the constantly accelerated ESR (up to 25 mm / hour on average) attracts attention, which indirectly leads to certain shifts in homeostasis. Moreover, in recent years of the disease in the presence of persistent urinary syndrome (that is, essentially, kidney amyloidosis), the average value of ESR was about 45 mm / hour. A similar phenomenon in this pathology is known from the literature [17]. Changes in blood biochemical parameters are significant and typical for nephrotic syndrome. So, Hypercholesterolemia was recorded in 9 out of 11 examined patients. The maximum cholesterol level was 17.2 mmol/l, the average value was 8.58 mmol/l. Blood fibrinogen was detected in 7 patients and in all cases was elevated. Its average level is 6.58 g/l with fluctuations from 4.44 to 9.32 g/l.
Protein metabolism was also seriously disrupted. Studies were conducted in 7 patients. All had pronounced hypoproteinemia. By the time of death, the total protein content in the blood had dropped to 40-44 g/l in 5 patients and only 1 was only slightly below normal (62.7 g/l). At the same time, all the examined patients had significant dysproteinemia. Thus, the average content of albumins was 44%, globulins — 56%. A /G—the replacement coefficient was reduced (on average — 0.82). The study of globulin fractions showed a relatively normal content of alpha globulins and the rise of beta and especially gamma fractions. The average values of these indicators are as follows: 17.5, 14.8 and 23.7%, respectively. Nitrogen excretion function of the kidneys was preserved for a long time. The level of residual nitrogen, urea and creatinine in the blood of almost all patients remained within normal limits. Only in 3 patients in the last month before death, the blood content of urea and creatinine increased slightly (up to 8.5-15.8 mmol/l and 125-135 mmol/l, respectively). At the same time, the Rehberg test conducted in 2 patients showed a decrease in glomerular filtration (up to 60.2 and 75 ml/min) and tubular reabsorption (up to 85- 86%).
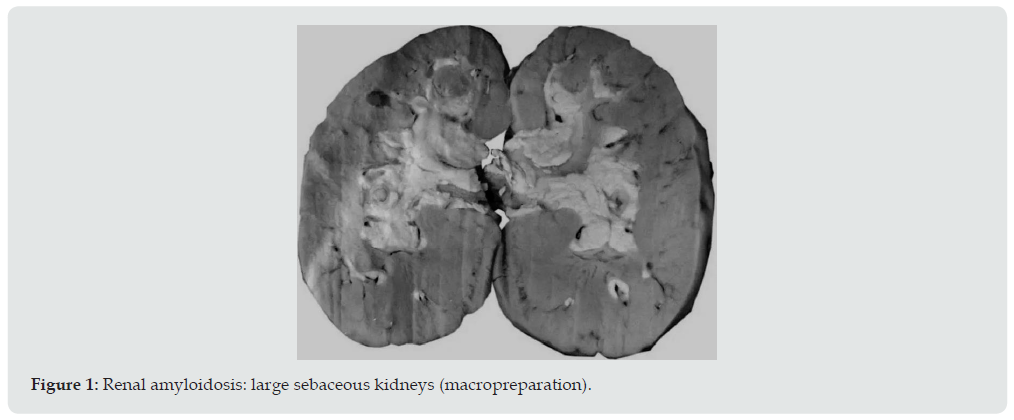
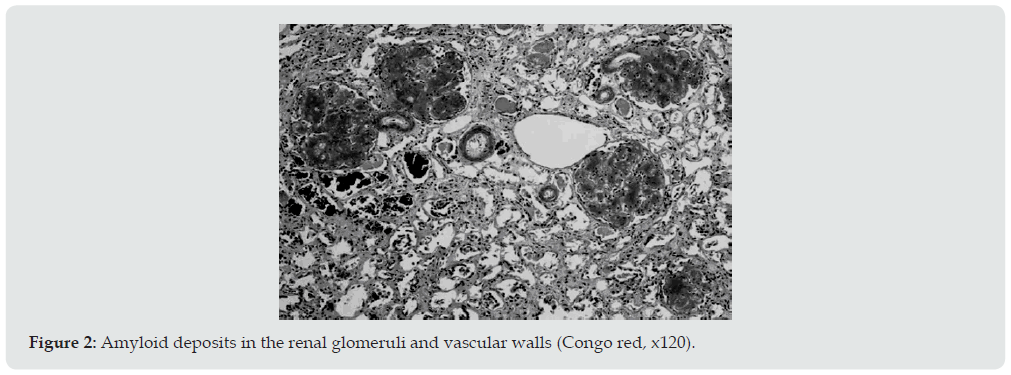
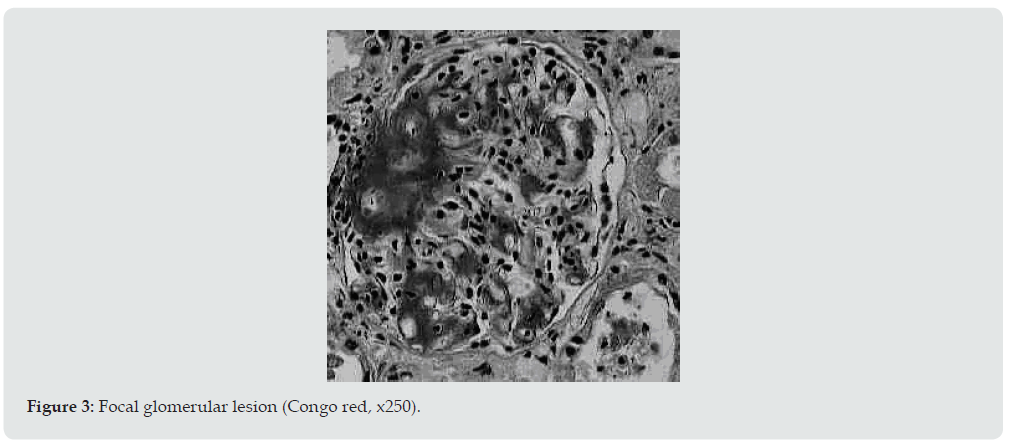
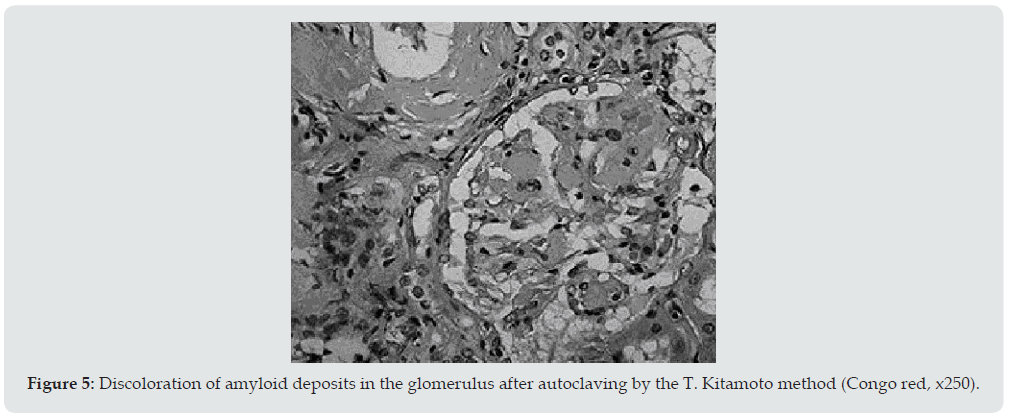
Ultrasound examination of the kidneys was performed in 6 patients, and in 4 of them repeatedly. Diffuse changes in the renal parenchyma were noted in all conclusions. Blood pressure in 13 patients was constantly within the normal range or even slightly lowered, which is characteristic of kidney amyloidosis. Only 1 of the examined 2 months before death had arterial hypertension with figures 160/110-190/100 mmHg. It is interesting that the same patient, the only one of all, did not have pronounced hypo— and dysproteinemia. In addition, shortly before his death, his urea and creatinine levels increased moderately. Summing up the description of clinical data, it can be stated that 4 patients died in the proteinuric stage of renal amyloidosis, 9 — in nephrotic, 1 patient had the transition of the latter to the azotemic stage. The death of patients occurred from various causes. Six died during the period of advanced nephrotic syndrome from increasing heart failure. A similar mechanism of death in renal amyloidosis is well known [18]. Three died from acute pneumonia and coronary pathology, one from hemorrhagic stroke and drug toxicoderma. The section revealed a typical picture of kidney amidoidosis [19]. In 11 of the deceased, these were the so-called large sebaceous kidneys (Figure 1), their mass ranged from 480 to 960 g. In 3 cases, the kidneys were amyloid wrinkled (weight 300-340 g). In 4 cases, in addition to the kidneys, the spleen was affected by amyloidosis, and in 1 of them the liver was also affected. Microscopically, congophilic amyloid masses were found in the capillary loops of the glomeruli and in the vessel wall (Figure 2). The intensity of amyloid deposition in glomeruli ranged from focal (Figure 3) to total lesion (Figure 4). At the same time, the severity and prevalence of the process correlated with the clinical stages, as well as with the duration of antipsychotic therapy. So, with a treatment period of more than 15 years, a total lesion of the vast majority of glomeruli was detected in all cases, and with a shorter period — only in 1 out of 6. In polarized light, the characteristic dichroism of amyloid was revealed in the form of a light greenish glow. During denaturation of amyloid by means of temperature action (autoclaving of sections before painting the congo red at a temperature of 130 ° C) by the method of T Kitamoto (1986) [20], its tinctorial properties were lost within 30 minutes (Figure 5), which is inherent in the AA-type of amyloid.
Figure 5: Discoloration of amyloid deposits in the glomerulus after autoclaving by the T. Kitamoto method (Congo red, x250).
Conclusion
Thus, in the described observations after prolonged neuroleptic therapy, a fairly typical clinic for the development of nephropathic amyloidosis was noted. As a rule, it manifested as an isolated urinary syndrome, which subsequently turned into a complete nephrotic syndrome with edema, increasing hypo- and dysproteinemia, hypercholesterolemia and hyperfibrinogenemia in more than half of the patients. At the same time, arterial hypertension and severe azotemia were usually absent. Morphologically, the process in the kidneys has been verified as secondary nephropathic AAamyloidosis. Based on these observations, we can confidently assume that medicinal neuroleptic amyloidosis of the kidneys is not a myth, but a reality.
References
- Zmushko EI, Belozerov ES (2001) Medical complications. Quick reference. St. Petersburg, Peter, USA pp.448.
- Petkov V, G Majdrakov, P Popkhristov (1973) The main mechanisms of undesirable effects of drugs in Drug disease. Medicine and Physical Culture pp. 15-78.
- Ryzhenko IM, Zaichenko AV, Kudina AV (2008) Side effects of antipsychotic drugs and their prevention. Pharmacist 1: 41-43.
- Shchekina EG, Drogovoz SM (2008) Interrelation of side effects and mechanisms of action of psychotropic drugs. Pharmacist 5: 58-61.
- Volkov V.P (2005) Medicinal amyloidosis: myth or reality. Verkhnevolzhsky Med J 4(5): 33-37.
- Volkov V.P (2011) On the issue of neuroleptic amyloidosis. Sixth National Congress of therapists p. 42.
- Volkov VP, Denisova IO (2007) Medicinal amyloidosis is a reality. Verkhnevolzhsky Med J 5(1): 40-42.
- Goveric PD, Casey TT, Stone WJ, DiRaimondo CR, Prelli FC, et al. (1985) Beta—2 microglobulin is an amyloidogenic protein in man. J Clin Invest 76(6): 2425-2429.
- Bruckner FE, Burke M, Pereira RS, Eisinger AJ, Bending M, et al. (1987) Synovial amyloid in chronic haemodialysis contains beta 2 microglobulin. Ann Rheum Dis 46(8): 634-637.
- Linke RP, Hampl H, Bartel—Schwarze S, Eulitz M (1987) Beta 2-microglobulin, different fragments and polymers thereof in synovial amyloid in long—term haemodialysis. Biol Chem Hoppe Seyler 368(1): 137-144.
- Linke RP, Hampl H, Lobeck H, Ritz E, Bommer J, et al. (1989) Lysine-specific cleavage of beta 2-microglobulin in amyloid deposits associated with haemodialysis. Kidney Int 36(4): 675-681.
- Reimann HA, Eklund CM (1935) Long continued vaccine therapy as a cause of amyloidosis. Am J Med Sci 190(1): 88-91.
- Rameyev VV, Kozlovskaya LV, Rameeva AS, Novikov AV (2021) The analysis of secondary AA-amyloidosis current etiology and its influence on the approaches for diagnosis and treatment. Ther Arch 93(6): 672-678.
- Kitomoto T, Tashima T, Tateishi J (1986) Novel histochemical approaches to the prealbumin—related senile and familial forms of systemic amyloidosis. Am J Pathol 123(3): 407-412.
- Zykova LD (1989) Methods of verification of forms of amyloidosis by a pathologist. Arch Pathol 51(1): 63-64.
- Kuvshinov VA (1974) History of Kalinin Regional Psychiatric Hospital No. 1 named after M.P. Litvinov. Abstracts of scientific and practical conference dedicated to the 90th anniversary of the hospital named after Dr. M.P. Litvinov p. 4-6.
- Majdrakov G, Majdrakov G, Popov N (1976) Amyloidosis of the kidneys. Kidney diseases. Medicine and Physical Culture pp. 648-659.
- Shulutko BI (1983) Pathology of the kidneys: Clinical and morphological examination. Leningrad, Medicine pp. 296.
- Serov VV, Shamov IA (1977) Amyloidosis. Moscow Medicine, USA pp. 266.
- Rameyev VV, Kozlovskaya LV, Sarkisova IA (2006) Amyloidosis: problems of its diagnosis and treatment. Clinician 4: 35-41.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...