Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Research Article(ISSN: 2641-1687) 
Hypercalcemia: A review and Update Volume 4 - Issue 1
Anthony Kodzo-Grey Venyo1* and Olawumi Adaramodu2
- *1Educational Supervisor Certificate (RCP London) And Accreditation, North Manchester General Hospital, Department of Urology, United Kingdom
- 2St Georges Medical Centre, St George’s Drive, United Kingdom
Received: December 29, 2022; Published: January 17, 2023
Corresponding author: Anthony Kodzo-Grey Venyo, Educational Supervisor Certificate (RCP London) And Accreditation, North Manchester General Hospital, Department of Urology, United Kingdom
DOI: 10.32474/JUNS.2023.04.000179
Abstract
Hypercalcemia is a relatively common clinical problem. Calcium is the most abundant cation in the human body and plays a significant role in neural transmission, enzyme activity, myocardial function, coagulation and other cellular functions. Most of the calcium is found in the bones as calcium phosphate, while a small percentage is located in the cells and extracellular fluids. In the serum, about 45% of calcium is bound to proteins, 45% exists as free or ionized calcium in the active form, and 10% is bound to anions. Systemic acidosis decreases calcium binding to albumin, increasing serum levels, while alkalosis causes the opposite effect. Serum calcium concentrations are highest in neonates and infants, reduce during childhood and adolescence, and does tend to become stable at adult values by 17 years of age. Hypercalcemia is serum calcium concentration two standard deviations above the mean values. Normal serum concentrations of total calcium generally range between 8.5 and 10.5 mg/dL (2.12 to 2.62 mmol/l) and ionized calcium between 4.65-5.30 mg/dL (1.16-1.31 mmol/l). Decreased Parathyroid hormone and decreased 1,25(OH)2D should accompany hypercalcemia unless Parathyroid hormone or 1,25(OH)2D is causal. Primary hyperparathyroidism and malignancy account for 90% of the cases of hypercalcemia. Treatment should be aimed at the underlying disorder. However, if serum calcium exceeds 12 to 14mg/dL (3 to 3.5mmol/l), acute hydration and agents that inhibit bone resorption are required. Under selected conditions, calcimimetics, calciuresis, glucocorticoids, or dialysis may be needed.
Keywords: Hypercalcemia; epidemiology; diagnosis; treatment; incidence; survival; mortality
Introduction
Most of the calcium is found in the bones as calcium phosphate, while a small percentage is located in the cells and extracellular fluids. In the serum, about 45% of calcium is bound to proteins, 45% exists as free or ionized calcium in the active form, and 10% is bound to anions [1,2]. Hypercalcemia is defined by a serum calcium value above the upper limit of the normal range, defined as greater than 2 SDs above the population mean [3]. Hypercalcemia affects approximately 1% of the general population and around 2% of patients with cancer [4-6]. The annual incidence of hypercalcemia is 0.09% to 0.6%, varying by the population screened [7,8]. Approximately 90% of people with hypercalcemia have primary hyperparathyroidism (PHPT) or malignancy. However, hypercalcemia may be due to a large number of disorders. Optimal management requires identifying the etiology of hypercalcemia. Majority of health care establishments within the developed countries have facilities to quickly and fully investigate the cause of hypercalcemia; however, it would be envisaged that some small health care establishments in remove areas within some developing countries may not have facilities to quickly and thoroughly investigate for the cause of hypercalcemia. This review which is divided into two parts (A) which has summarized the causes, pathophysiology, presentation, diagnosis, and treatment of hypercalcemia with a focus on severe hypercalcemia and has highlighted the role of the interprofessional team in its management; and (B) Miscellaneous Narrations and Discussions related to some case reports and case series as well as studies related to hypercalcemia.
Methods
Internet databases were searched, including Google, Google Scholar, Yahoo, Bing, Pub line and PUBMED. The search words used included: hypercalcemia, and: epidemiology, diagnosis, treatment, bisphosphonates, incidence, survival, prognoses, and mortality without year restriction. The National Library for Health and the National Guidelines Clearing House were also searched for relevant guidelines and reviews. Fifty-one (51) references were identified which were used to write the article which has been divided into two parts: (A) Overview and (B) Miscellaneous narrations from some case reports, case series, and some studies related to hypercalcaemia.
Results
Overview
General statements
The normal range of serum calcium does vary between 2.12– 2.60 mmol/L. Calcium in the blood is normally transported partly bound to plasma proteins (about 45%), notably albumin, partly bound to small anions such as phosphate and citrate (about 10%) and partly in the free or ionized state (about 45%). Calcium is highly bound to plasma proteins, therefore if albumin is low, the corrected calcium should be used.
Hypercalcemia may be associated with a spectrum of clinical manifestations, ranging from few or no symptoms in patients with mild chronic hypercalcemia to severe obtundation and coma. The degree of hypercalcemia, along with the rate of rise of serum calcium concentration, often determines symptoms and the urgency of therapy. The therapeutic approach should reflect these differences.
a) Mild hypercalcemia: Patients with asymptomatic or mildly symptomatic (e.g., constipation) hypercalcemia (calcium <12 mg/dL [3 mmol/L]) do not require immediate treatment.
b) Moderate hypercalcemia: Patients with a serum calcium of 12 to 14 mg/dL (3 to 3.5 mmol/L) may not require immediate treatment, because that degree of hypercalcemia may be well tolerated chronically. However, an acute rise to these concentrations may cause marked changes in sensorium, which requires more aggressive measures.
c) Severe hypercalcemia: Patients with a serum calcium concentration >14 mg/dL (3.5 mmol/L) require more aggressive treatment, regardless of symptoms.
The reference range for ionized calcium varies with the assay, and therefore, the ionized calcium thresholds for intervention depend on the assay. In an ionized calcium assay with a normal range of 4.8 to 5.6 mg/dL (1.2 to 1.4 mmol/L), mild, moderate, and severe hypercalcemia may be defined as follows (13):
a) Mild – Ionized calcium 5.6 to 8 mg/dL (1.4 to 2 mmol/L)
b) Moderate – Ionized calcium 8 to 10 mg/dL (2 to 2.5 mmol/L)
Clinical Manifestations of Acute Cystitis
c) Severe – Ionized calcium 10 to 12 mg/dL (2.5 to 3 mmol/L)
Causes
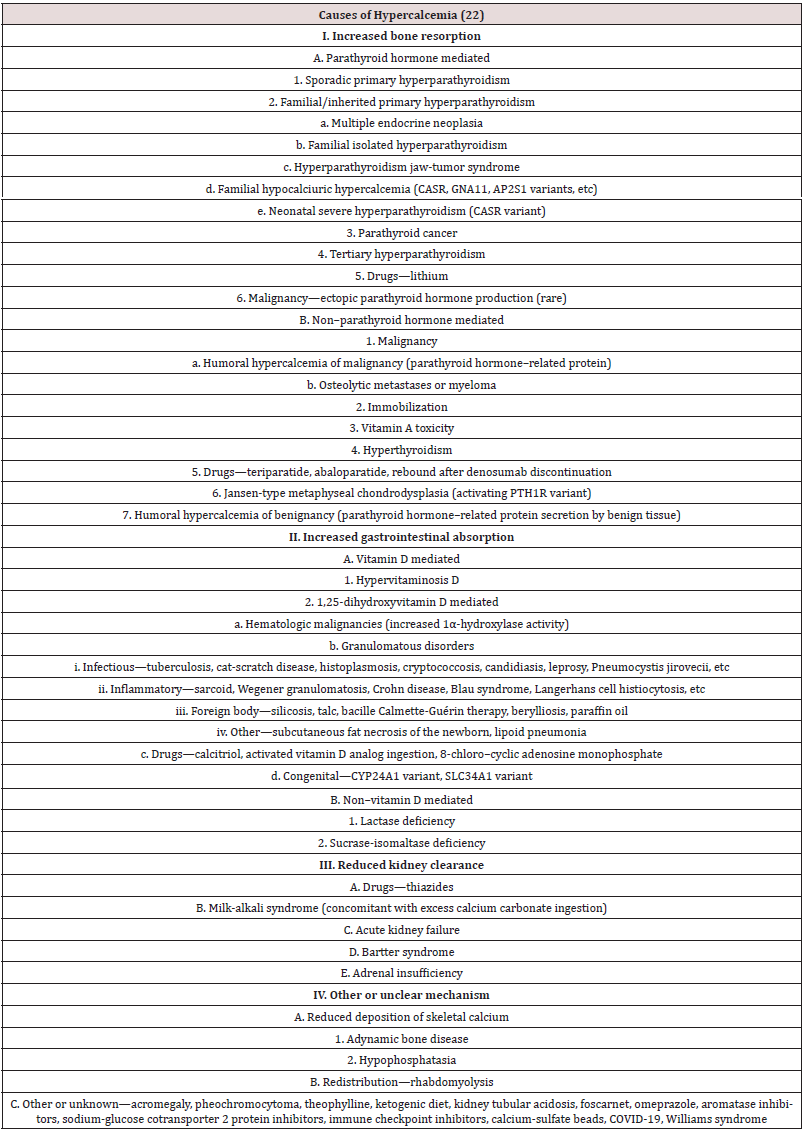
Hypercalcemia is a relatively common clinical problem. Among all causes of hypercalcemia, primary hyperparathyroidism and malignancy are the most common, accounting for greater than 90 percent of cases. Therefore, the diagnostic approach to hypercalcemia typically involves distinguishing between the two. It is usually not difficult to differentiate between them. Malignancy is often evident clinically by the time it causes hypercalcemia, and patients with hypercalcemia of malignancy usually have higher calcium concentrations and are more symptomatic from hypercalcemia than individuals with primary hyperparathyroidism. Although hypercalcemia in otherwise healthy outpatients is usually due to primary hyperparathyroidism and malignancy is more often responsible for hypercalcemia in hospitalized patients, other potential causes of hypercalcemia must be considered. Various causes of hypercalcaemia exist as illustrated in Table 1. Other potential causes include Addison’s disease, Medications such as thiazide diuretics, vitamin D, lithium, calcium-containing antacids (Table 2).
Epidemiology
The prevalence of hypercalcemia in the general population is approximately 1% to 2%. Most of the cases (90%) of hypercalcemia are due to primary hyperparathyroidism and malignancy-associated hypercalcemia. The prevalence of primary hyperparathyroidism in the general population ranges from 0.2% to 0.8% and increases with age. Overall, 2% of all cancers are associated with hypercalcemia, but in the pediatric age group, the prevalence is about 0.4% to 1.3%.
Pathophysiology
Calcium concentration is regulated by plasma membrane calcium receptor, PTH and its receptor, calcitonin and its receptor, and by the actions of vitamin D on kidneys, bone, and intestines. PTH mobilizes calcium directly by enhancing bone resorption, and indirectly, by stimulating one alpha-hydroxylase which increases vitamin D3 production, in turn, leading to increased absorption of calcium from the gut and increased bone resorption. Primary hyperparathyroidism is due to a solitary adenoma or diffuse hyperplasia of the gland. In this condition, there is an abnormal set point in the relation between calcium and PTH levels and calcium independent PTH secretion. Familial hypocalciuric hypercalcemia is inherited autosomal dominant pattern and is due to an inactivating mutation in the calcium-sensing receptor gene. Granulomatous lesions cause ectopic vitamin D production. Transient neonatal hypercalcemia can rarely be seen in infants born to mothers with hypoparathyroidism. Hypercalcemia from hyperparathyroidism is mild and could go one for years and be asymptomatic. Hypercalcemia from malignancy is associated with rapidly increasing calcium levels.
Manifestations of hypercalcemia
Symptoms of hypercalcemia are usually seen when serum calcium levels are more than 12 mg/dl. Irrespective of the etiology, the broad signs and symptoms can be summarized as “groans, bones, stones, moans, thrones and psychic overtones”.
Groans: Gastrointestinal symptoms like pain, nausea, and vomiting. Hypercalcemia can lead to peptic ulcer disease and pancreatitis.
Bones: Bone related complications like bone pain. Hypercalcemia can lead to osteoporosis, osteomalacia, arthritis and pathological fractures.
Stones: Renal stones causing pain.
Moans: Refers to fatigue and malaise.
Thrones: Polyuria, polydipsia, and constipation (sitting on the toilet as you are sitting on a throne).
Psychic overtones: Lethargy, confusion, depression and memory loss.
Microscopy histopathology examination findings
It has been iterated that in cases of acute cystitis, histopathology examination of the urinary bladder or urothelium of the urethra tend to demonstrate neutrophils [3].
Severe hypercalcemia inhibits neuromuscular and myocardial depolarization leading to muscle weakness and arrhythmias. Cardiovascular effects include prolonged PR interval, short QT interval, widened QRS complex, and bradycardia. Increased thirst with polydipsia and polyuria is seen initially, progressing to nephrolithiasis and nephrocalcinosis in chronic cases. Neurologic features include impaired concentration, altered mental status ranging from confusion to irritability. Levels of more than 14 mg/dL can cause encephalopathy and levels above 15 mg/dL is a medica emergency. Severe acute abdominal pain should be a clue to evaluate for pancreatitis. Osseous changes can lead to bone pain, gait abnormalities, and fractures and are seen radiologically as subperiosteal and endosteal bone resorption. In infants and young children, hypercalcemia can cause poor weight gain and failure to thrive.
Investigations and Evaluation
Most cases of hypercalcemia are detected through routine testing. Work up for etiology includes obtaining serum PTH, calcitonin, Vitamin D, ionized calcium, phosphorus, magnesium, alkaline phosphatase levels, renal functions, and urinary calciumcreatinine ratio. Sensitive and specific immunochemiluminometric assays should be used to measure parathyroid hormone (PTH) (i-PTH). Hyperparathyroidism is characterized by high calcium, high i-PTH levels with low phosphorous levels. Familial hypocalciuric hypercalcemia has inappropriately normal PTH levels with high magnesium and low calcium creatinine clearance. Other causes of hypercalcemia typically have low PTH levels. Patients with hypercalcemia and hypervitaminosis D have high serum concentrations of calcidiol, whereas patients with a granulomatous disease have high levels of calcitriol. In patients with hypercalcemia associated with malignancy, the PTH-related peptide levels are elevated. Imaging is indicated to identify nephrolithiasis and nephrocalcinosis- all articles. Neonates and infants who are found to be hypercalcemic in the presence of dysmorphic features should prompt evaluation for Williams or Murk Jansen syndrome. Imaging studies are done to rule out sarcoidosis or lung cancer. A mammogram is used to rule out breast cancer and a CT scan is done to rule out renal cancer. The parathyroid glands are investigated with ultrasound and MRI.
ECG features of hypercalcemia include:
a) T wave flattening or inversion
b) Mild prolongation of the QRS and PR intervals
c) ST-elevation
d) The presence of J wave at the end of the QRS complex.
Differential Diagnoses
The differential diagnoses include: Hypermagnesemia, Hyperparathyroidism, Hyperphosphatemia
Treatment
Treatment for hypercalcemia should be aimed at lowering the serum calcium concentration and, if possible, correcting or decreasing impact of the underlying disease. Patients with mild or moderate hypercalcemia that have no symptoms do not need immediate treatment. They should avoid exacerbating factors (dehydration, calcium intake >1000 mg/d, immobilization). If feasible, thiazide diuretics should be discontinued. Vitamin D supplements should be discontinued until serum measurements are available. If vitamin D levels are low and hypercalcemia is not likely vitamin D mediated, vitamin D supplements may be resumed. Chronic moderate hypercalcemia that is mildly symptomatic (such as fatigue) may not require immediate treatment. However, if nausea, vomiting, dehydration, or altered mental status are present, therapy for severe hypercalcemia should be instituted. Definitive management of mild hypercalcemia requires treatment of the underlying cause (Table 1), including discontinuation of contributing medications if clinically indicated. Most patients with chronic, mild hypercalcemia have Sporadic primary hyperparathyroidism PHPT. In the US, most patients with PHPT are “asymptomatic” because they do not manifest any of the classic features of PHPT, namely symptoms of hypercalcemia, nephrolithiasis, or osteitis fibrosa cystica.
Treatment of Severe or Symptomatic Hypercalcemia
Severe hypercalcemia is life-threatening. Therefore, treatment is often required before the cause is known (Table 1). Most patients with severe hypercalcemia are severely dehydrated. Therefore, initial treatment of severe hypercalcemia includes hydration with saline. Similarly, increased bone resorption is either responsible for or substantially contributes to most etiologies of hypercalcemia, and prompt treatment with intravenous bisphosphonates such as zoledronic acid, pamidronate, and/or calcitonin is usually indicated. The approach should be modified based on clinical factors. Hydration alone might be sufficient when the cause is known and readily reversible (e.g., milk-alkali syndrome). In contrast, hydration alone is typically insufficient with hypercalcemia of malignancy. Glucocorticoids may be an initial adjunctive therapy in those with known or highly suspected vitamin D–mediated hypercalcemia and can be used as an additional therapy in refractory lifethreatening hypercalcemia of any cause. Once acute hypercalcemia is controlled and the cause identified, treatment can be tailored to pathophysiological mechanism.
The administration of calcitonin plus saline hydration should result in substantial reduction in serum calcium concentrations within 12 to 48 hours. The bisphosphonate will be effective by the second to fourth day and provide a more sustained effect, thereby maintaining control of hypercalcemia. Volume expansion with isotonic saline — Most patients presenting with severe hypercalcemia have marked intravascular volume depletion. Hypovolemia exacerbates hypercalcemia by impairing the renal clearance of calcium. Isotonic saline for 24 to 48 hours corrects possible volume depletion due to hypercalcemia-induced urinary salt wasting and, in some cases, vomiting. The rate of saline infusion depends upon several factors, including the severity of hypercalcemia, the age of the patient, and presence of comorbid conditions, particularly underlying cardiac or renal disease. A reasonable regimen, in the absence of oedema, is the administration of isotonic saline at an initial rate of 200 to 300 mL/hour that is then adjusted to maintain the urine output at 100 to 150 mL/hour.
In the absence of renal failure or heart failure, loop diuretic therapy to directly increase calcium excretion is not recommended, because of the availability of drugs (e.g., bisphosphonates) that inhibit bone resorption, which is primarily responsible for the hypercalcemia, as well as the potential for fluid and electrolyte complications (e.g., hypokalaemia, hypomagnesemia) and volume depletion resulting from a massive saline infusion and furosemideinduced diuresis. Nevertheless, saline therapy can lead to fluid overload in patients who cannot excrete the administered salt because of impaired renal function or heart failure. In individuals with renal insufficiency or heart failure, careful monitoring and judicious use of loop diuretics (after intravascular volume has been repleted) may be required to prevent fluid overload.
Saline therapy rarely normalizes the serum calcium concentration in patients with more than mild hypercalcemia. Concurrent treatment with bisphosphonates with or without calcitonin is typically required to treat moderate to severe hypercalcemia. Calcitonin — Calcitonin should be administered intramuscularly or subcutaneously; nasal administration of calcitonin is not efficacious for treatment of hypercalcemia. The initial dose is 4 units/kg. The serum calcium is repeated in four to six hours. If a hypocalcaemia response is noted, then the patient is calcitonin sensitive, and the calcitonin can be repeated every 12 hours for a total duration of 24 to 48 hours. If the response is not satisfactory, the dose may be increased to 8 units/kg every 6 to 12 hours (total duration of therapy 24 to 48 hours).
The efficacy of calcitonin is limited to the first 48 hours, even with repeated doses, indicating the development of tachyphylaxis, perhaps due to receptor downregulation. Because of its limited duration of effect, calcitonin is most useful in symptomatic patients with calcium >14 mg/dL (3.5 mmol/L), when combined with hydration and bisphosphonates (or denosumab, in bisphosphonate-refractory patients). Pharmacologic doses of calcitonin reduce the serum calcium concentration by increasing renal calcium excretion and, more importantly, by decreasing bone resorption via interference with osteoclast function. Calcitonin is safe and relatively nontoxic (other than mild nausea and the rare hypersensitivity reaction). Although a relatively weak agent, it works rapidly, lowering the serum calcium concentration by a maximum of 1 to 2 mg/dL (0.3 to 0.5 mmol/L) beginning within four to six hours. Thus, it is useful in combination with hydration for the initial management of severe hypercalcemia.
Bisphosphonates — Bisphosphonates are relatively nontoxic compounds, and they are more potent than calcitonin and saline for patients with moderate or severe hypercalcemia. As a result, they have become the preferred agents for management of hypercalcemia due to excessive bone resorption from a variety of causes, including malignancy-related hypercalcemia. Their maximum effect occurs in two to four days, so that they are usually given in conjunction with saline and/or calcitonin, which reduce calcium concentration more rapidly.
Although bisphosphonates are most commonly used to treat established hypercalcemia, they have also been given to prevent hypercalcemia and adverse skeletal events, particularly in patients with metastatic cancer to bone. The use of bisphosphonates to improve outcomes for patients with cancer is discussed separately.
Choice of drug and dosing — Zoledronic acid (ZA; 4 mg IV over 15 minutes) is preferred over pamidronate (60 to 90 mg over 2 hours) because it is superior to pamidronate in reversing hypercalcemia related to malignancy. Ibandronate and clodronate are less commonly used options. Alendronate and risedronate are potent, third-generation bisphosphonates that can be given orally; however, neither is used for the treatment of severe or acute hypercalcemia.
a) Zoledronic acid – In a pooled analysis of two separate phase III trials involving a total of 275 patients with tumourinduced hypercalcemia, a single dose of ZA (either 4 or 8 mg) normalized the corrected serum calcium concentration in 87 to 88 percent of patients, compared with only 70 percent of those receiving pamidronate (90 mg) (40). In addition, the median duration of serum calcium control was longer for those receiving ZA (32 to 43 versus 18 days).
b) Although renal events were reported more frequently with ZA than with pamidronate in trials evaluating chronic use of these drugs to treat patients with metastatic bone disease, there was no difference in the frequency of grade 3 or 4 renal toxicity with either drug. The efficacy of the 4 and 8 mg ZA doses were similar, but the 4 mg dose was recommended because there was greater renal toxicity with the 8 mg dose (5.2 versus 2.3 percent with 4 mg) and higher all-cause mortality (33 versus 19 percent).
c) Pamidronate – A number of observational studies and some randomized trials have demonstrated the efficacy of IV pamidronate for the treatment of hypercalcemia due to excessive bone resorption from a variety of causes, including malignancy, acute primary hyperparathyroidism, immobilization, hypervitaminosis D, and sarcoidosis.. Early trials showed pamidronate (60 mg over 24 hours) was more effective in ameliorating hypercalcemia of malignancy than IV etidronate (70 versus 41 percent) or clodronate. Subsequent trials showed that shorter infusion times (two to four hours) were safe and effective, maintaining normocalcemia for two or more weeks. The maximal calcium response occurs at 90 mg IV (31). However, some clinicians vary the usual initial dose of pamidronate according to the degree of hypercalcemia: 60 mg if the serum calcium concentration is up to 13.5 mg/dL (3 to 3.4 mmol/L) and 90 mg for higher levels. Serum calcium concentrations begin to decrease in one or two days. Doses should not be repeated sooner than a minimum of seven days.
d) IV pamidronate is well tolerated, with a low incidence of fever being the main side effect. A less favourable response may be seen in patients with humoral hypercalcemia of malignancy, a paraneoplastic syndrome typically resulting from autonomous production of parathyroid hormone-related protein (PTHrP) by the tumour. Such patients may have a better response to ZA.
e) Ibandronate – Ibandronate effectively treats hypercalcemia of malignancy. In combined trials with over 320 patients, ibandronate doses of 2 mg IV administered over two hours normalized serum calcium in up to 67 percent of patients, and doses up to 6 mg were safe and well tolerated. The frequency of response was significantly higher with 4 or 6 mg than with 2 mg (76 to 77 versus 50 percent), but the duration of response was not dose dependent. Ibandronate appears to be as effective as pamidronate. Ibandronate (2 or 4 mg IV) was directly compared with pamidronate (15 to 90 mg IV) in a randomized trial involving 72 patients with hypercalcemia of malignancy. The number of patients responding to both agents was similar (77 and 76 percent) for ibandronate and pamidronate, respectively) but the median time until the serum calcium began to rise again was significantly longer with ibandronate (14 versus 4 days). However, four days is an unusually short duration of effect for pamidronate and may reflect inadequate dosing or the small size of the clinical trial.
f) Clodronate – Clodronate, a first-generation bisphosphonate, is a relatively weak inhibitor of bone resorption compared with the newer agents. Oral clodronate is available outside the United States. In randomized trials of patients with multiple myeloma or metastatic breast cancer, the administration of oral clodronate to decrease skeletal complications was associated with fewer episodes of severe hypercalcemia. An IV bisphosphonate is often preferred at the onset of therapy, with oral clodronate being used for maintenance therapy. The poor oral bioavailability of clodronate, the size of the tablets, and the need to take them on an empty stomach with nothing to eat for one hour afterward increases the risk of noncompliance.
Side effects and precautions
Although IV bisphosphonates are generally well tolerated, side effects may include flu-like symptoms (fever, arthralgias, myalgia, fatigue, bone pain), ocular inflammation (uveitis), hypercalcemia, hypophosphatemia, and impaired renal function, including proteinuria. Repetitive use of bisphosphonates has been associated with risk of developing osteonecrosis of the jaw and atypical femur fractures (in patients who require long-term therapy). These side effects associated with long-term therapy may be of limited relevance in the management of acute hypercalcemia where the use of these drugs may not be repeated on a regular basis. The incidence of adverse effects of bisphosphonates varies somewhat with the indication for use, due in part to the higher doses used in cancer patients compared with those with osteoporosis. Dosing in renal impairment — In patients with impaired renal function (creatinine >4.5 mg/dL), caution should be adopted when using IV bisphosphonates to treat hypercalcemia. Adequate hydration with saline and treatment with a reduced dose and/or slower infusion rate (2 to 4 mg ZA over 30 to 60 minutes, 30 to 45 mg pamidronate over 4 hours, 2 mg ibandronate over 1 hour) may minimize risk. The renal tubular toxicity is related to the rate of infusion. As mentioned in the preceding section, bisphosphonates have potential nephrotoxicity. However, in clinical trials of ZA for the treatment of hypercalcemia of malignancy, patients with serum creatinine concentrations as high as 4.5 mg/dL (400 micromol/L) were eligible for participation. In addition, there are case reports of successful use of ibandronate and pamidronate for patients with renal failure and multiple myeloma, renal insufficiency (creatinine ≥1.5 mg/dL [133 micromol/L]), and in haemodialysis patients with severe hypercalcemia.
Bisphosphonate contraindications or refractory hypercalcemia
For patients in whom bisphosphonates are contraindicated (e.g., due to severe renal impairment, bisphosphonate allergy), or in patients with hypercalcemia refractory to zoledronic acid, denosumab is an option and can be administered concurrently with calcitonin and saline hydration. Haemodialysis should be considered in patients who have serum calcium concentrations in the range of 18 to 20 mg/dL (4.5 to 5 mmol/L) and neurologic symptoms but are hemodynamically stable and in those with severe hypercalcemia complicated by renal insufficiency or heart failure, in whom hydration cannot be safely administered.
Denosumab dosing
For patients that have contraindications to bisphosphonates should be treated with an initial dose of 60 mg subcutaneously. Careful monitoring of serum calcium levels is necessary in patients with renal insufficiency because there is a higher risk of hypocalcaemia with denosumab than with bisphosphonates. Patients with vitamin D deficiency may be more likely to develop hypercalcemia after denosumab administration. If the serum 25-hydroxyvitamin D returns below normal, cautious treatment with vitamin D should be commenced (e.g., 400 to 800 international units daily). Denosumab, unlike bisphosphonates, is not cleared by the kidney, and as a consequence, there is no restriction of its use in patients with chronic kidney disease, for whom bisphosphonates are used with caution or contraindicated. In case reports of patients with hypercalcemia of malignancy and severe renal impairment (serum creatinine 2.5 to 5.7 mg/ dL), denosumab 60 mg subcutaneously improved serum calcium within two to four days and, in one case, was associated with improvement in renal function. Thus, denosumab may have a role in the treatment of hypercalcemia associated with marked renal impairment or renal failure. However, since the renal failure may be due to acute hypercalcemia, avoiding IV bisphosphonates may be unwarranted, especially as denosumab is associated with a higher risk of hypercalcemia than bisphosphonates.
Refractory hypercalcemia
Patients with hypercalcemia refractory to zoledronic acid should be treated with an initial dose of 120 mg subcutaneously. If after approximately two to seven days there is little improvement, another dose of 120 mg should be administered. Thereafter, if the underlying cause of hypercalcemia persists (e.g., malignancy), longterm therapy (monthly) is required. There are an increasing number of case reports and case series of denosumab for the management of chronic hypercalcemia of malignancy, particularly in patients with persistent hypercalcemia after treatment with bisphosphonates. In one study, 33 patients with hypercalcemia of malignancy with persistently elevated serum calcium levels corrected for albumin >12.5 mg/dL (3.1 mmol/L) after treatment with bisphosphonates were treated with denosumab 120 mg subcutaneously weekly for three to four weeks and then monthly thereafter. Within 10 days, 21 patients (64 percent) had serum calcium levels <11.5 mg/dL (2.9 mmol/L).
Dialysis
Haemodialysis with little or no calcium in the dialysis fluid and peritoneal dialysis (though it is slower) are both effective therapies for hypercalcemia and are considered treatments of last resort. Dialysis may be indicated in patients with severe malignancyassociated hypercalcemia and renal insufficiency or heart failure, in whom hydration cannot be safely administered. The use of haemodialysis for patients with hypercalcemia but without renal failure may require alterations in the composition of conventional dialysis solutions in order to avoid an exacerbation or induction of other metabolic abnormalities, particularly hypophosphatemia. As an example, haemodialysis with a dialysis solution enriched with phosphorus (final phosphorous concentration of 4 mg/dL) resulted in rapid correction of all abnormalities in one patient in whom medical therapy had failed to reverse hypercalcemia, mental status changes, and hypophosphatemia due to primary hyperparathyroidism.
Preventing Recurrence
Follow-up therapy is aimed at preventing recurrence of hypercalcemia.
a) In patients with hypercalcemia of malignancy, progressive hypercalcemia will inevitably accompany tumour progression, and therefore, the underlying disease causing the hypercalcemia should be treated, if at all possible. Many patients with malignancy may also have metastatic bone disease and will receive intravenous (IV) zoledronic acid (ZA) or pamidronate every three to four weeks as part of their treatment to prevent skeletal complications. As a result, recurrent hypercalcemia will be prevented.
b) In patients with renal insufficiency and history of hypercalcemia, calcium intake should be limited to 1000 mg per day (total diet plus any supplements). Excessive vitamin D supplements (ergocalciferol or cholecalciferol) should be avoided.
c) In patients with normal renal function and history of hypercalcemia, excessive amounts of calcium and vitamin D supplements should also be avoided.
Disease-Specific Approach
The modalities described above apply in varying degrees to patients with all causes of hypercalcemia. Other disease- specific approaches are summarized briefly:
a) Hyperparathyroidism: Hyperparathyroidism is the most common outpatient cause of mild hypercalcemia. The treatment is typically parathyroidectomy or monitoring for complications of primary hyperparathyroidism.
b) Parathyroid carcinoma is a rare cause of hyperparathyroidism but may cause more severe hypercalcemia than most parathyroid adenomas. Patients typically present with marked hypercalcemia and very high parathyroid hormone concentrations or a neck mass. The primary treatment of parathyroid carcinoma is surgery. When the tumour is no longer curable by surgical intervention, treatment becomes focused on the control of hypercalcemia with medical therapy, which can include bisphosphonates, calcimimetic agents, or denosumab. The calcimimetic agent cinacalcet reduces the serum calcium concentration in patients with severe hypercalcemia due to parathyroid carcinoma and in haemodialysis patients with an elevated calcium-phosphorous product and secondary hyperparathyroidism. Calcimimetics have also been evaluated in patients with primary hyperparathyroidism but are not standard therapy.
c) Granulomatous diseases Patients with lymphoma, sarcoidosis, or other granulomatous causes of hypercalcemia have enhanced intestinal calcium absorption due to increased endogenous calcitriol production. The major modalities of therapy are a low-calcium diet, glucocorticoids, and treatment of the underlying disease. Glucocorticoids (e.g., prednisone in a dose of 20 to 40 mg/day) will usually reduce serum calcium concentrations within two to five days by decreasing calcitriol production by the activated mononuclear cells in the lung and lymph nodes and by reducing intestinal calcium absorption.
d) Hypervitaminosis D Hypercalcemia associated with excess administration or ingestion of vitamin D is primarily due to increased absorption of dietary calcium. It is sometimes seen in combination with excess calcium intake and in individuals with renal insufficiency. High doses of vitamin D also have been shown to increase bone resorption, by activating osteoclasts. Vitamin D actions on the distal nephron will increase the tubular reabsorption of calcium, which further worsens hypercalcemia.
e) Calcitriol Hypercalcemia due to ingestion of calcitriol as treatment for hypoparathyroidism, or for the hypocalcemia and hyperparathyroidism of renal failure, usually lasts only one to two days because of the relatively short biologic half-life of calcitriol. Thus, stopping the calcitriol, increasing salt and fluid intake, or perhaps hydrating with intravenous (IV) saline may be the only therapy that is needed.
f) Vitamin D Hypercalcemia caused by vitamin D or calcidiol lasts longer, so that more aggressive therapy such as glucocorticoids (e.g., prednisone in a dose of 20 to 40 mg/day) and zoledronic acid (ZA) or pamidronate may be necessary.
g) Familial hypocalciuric hypercalcemia Hypercalcemia is typically not treated in patients with familial hypocalciuric hypercalcemia (FHH), because the elevation in serum calcium is typically mild and produces few, if any, symptoms.
Prognosis
Hypercalcemia, when it occurs following a benign disorder, has a good prognosis but when the cause is secondary to malignancy the prognosis is poor. Patients with hypercalcemia associated with malignancy are often symptomatic and need frequent hospitalizations. When hypercalcemia is due to ectopic production of PTH related protein, most patients die within a few months. The osteolytic metastatic lesions cause fractures, nerve compression, and paralysis. Complications that could arise from hypercalcemia include Depression, Kidney stones, Bone pain, Constipation, Pancreatitis, Renal failure, Gastric ulcers, Paraesthesia, Syncope and arrhythmias, altered mental status, Pearls and Other Issues.
Enhancing Healthcare Team Outcomes
Hypercalcemia is not an uncommon presentation in patients admitted to the hospital. The severity of the symptoms is related to the speed of development and the concentration of calcium. This metabolic problem can present in many ways, and only an interprofessional approach can help reduce the morbidity and mortality of the disorder. Without treatment, the condition carries a very high morbidity. Besides, physicians, pharmacists and nurses play a vital role in the management of patients with hypercalcemia. The nurse plays a vital role in patient hydration and monitoring of vital signs. The pharmacist has to educate the patient on the different drugs available to reduce calcium and their adverse effects. The pharmacist should check the medications to ensure that the cause is not due to a drug. In addition. The patient should be educated about compliance with the bisphosphonates. All clinicians and nurses should educate the patients on maintaining hydration, changes in diet and limiting calcium intake. The patient and family should be told about the symptoms of hypercalcemia and when to seek assistance. Physical therapy should be involved to ensure that the patient remains mobile and active. If the patient has a metastatic disease associated with hypercalcemia, the hospice, palliative care, and pain teams should be involved to ensure that the quality of life is not compromised. Finally, a dietitian should be consulted to educate the patient on what type of foods to avoid [9,10]. With such a team approach, it is hoped that the patient outcomes will improve [11].
Summary and Recommendations from The Overview
The diagnostic approach to hypercalcemia typically involves clinical evaluation and laboratory testing to distinguish between primary hyperparathyroidism and malignancy, which together account for greater than 90 percent of cases. The remaining 10 percent of patients with hypercalcemia may have one of many causes (Table 1) that must be systematically considered, and Serum calcium should be corrected for albumin and an elevated concentration should be confirmed by repeat Sampling.
a) Clinical evaluation, including duration of hypercalcemia, presence or absence of symptoms, family history, and medications, may help determine the aetiology of hypercalcemia.
b) Measurement of intact parathyroid hormone (PTH) is important to distinguish PTH-mediated from non-PTH mediated causes of hypercalcemia. A frankly elevated PTH concentration or a PTH value in the upper half of the normal range in the setting of hypercalcemia is likely the result of primary hyperparathyroidism.
c) PTH concentrations below 20 pg/mL in the setting of hypercalcemia are usually not consistent with primary hyperparathyroidism and indicate the need for evaluation for other causes of hypercalcemia (This evaluation should include measurement of PTH-related peptide (PTHrp) and vitamin D metabolites. If the diagnosis is still not clear, other tests should be considered, including thyroid-stimulating hormone (TSH), serum protein electrophoresis (SPEP), urine protein electrophoresis (UPEP), and vitamin A.
The outcomes depend on the cause of hypercalcemia. When the condition is due to hyperparathyroidism, symptoms tend to be mild but are prolonged. The highest morbidity is due to bone wasting. Mild hypercalcemia does not lead to mortality. However, hypercalcemia from a malignant cause carries a grave prognosis. These patients need aggressive treatment and also require frequent admission to the hospital. Finally, the morbidity from the malignancy-associated hypercalcemia can be severe leading to many symptoms and a very poor quality of life. [12,13].
Miscellaneous Narrations and Discussions from some Case Reports, Case Series and Some Studies related to Hypercalcaemia
Hicks et al. iterated that subcutaneous fat necrosis of the newborn (SCFN) alone is an uncommon condition, as well as its association with hypercalcemia had at the time of the report of their case been reported in 19 neonates since 1926. They additionally stated that the two occur in full-term to postterm newborns with perinatal complications that are associated wtth delivery. Hicks et al. furthermore stated that erythematous to violaceous, firm, subcutaneous nodules do tend in hypercalcemia of the newborn to appear approximately 1 week to 4 weeks pursuant to the delivery of the baby, which also tends to precede the development of signs and symptoms of hypercalcemia. Hicks et al. additionally iterated that even though SCFN and hypercalcemia are rare complications in neonates with perinatal problems, death due to the sequelae of hypercalcemia occurred in 3 of the 19 reported patients. Hicks et al. [14] recommended that a neonate who develops skin lesions that are consistent with SCFN should be followed for the possible onset of hypercalcemia and treated in a timely fashion.
Rey et al. [15] stated the following: Hypercalcemia is a rare pathology in pregnancy, but an important one to recognize in the effort to reduce fetal and neonatal morbidity and mortality. The difficulty arises from the nonspecific presentation of hypercalcemia. Symptoms may be confused with nausea and other discomforts frequently observed in pregnancy, and total calcium levels are modified by decreased albumin values. Further, physicians involved in pregnancy management are often reluctant to use radiological imaging for investigations and therapeutic options appear limited. They would present three cases of hypercalcemia to illustrate some of these challenges and discuss optimal management. For convenience, we have included calcium conversion and correction factors. Case Histories Patient 1 A 40-year-old woman on her fourth pregnancy and one live birth (G4P1) was admitted at 33 weeks’ pregnancy with known sickle-cell disease, bone pain, and polyuria. Prior to pregnancy, she had had several episodes of acute thoracic syndrome and multiple hospitalizations for bone pain, cholestasis, chronic articular pain, retinopathy, and a kidney infarct. The sicklecell disease was being treated with erythrocytapheresis every 8 weeks and, since pregnancy, every 6 weeks. In the last year, the patient’s serum calcium levels had fluctuated from normal to high (maximum 2.73 mmol/L; normal range [NR]: 2.2–2.5 mmol/ L), with no further investigations.
During an admission for a sickle-cell crisis and hyponatremia at 28 weeks’ ª 2016 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial- NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial, and no modifications or adaptations are made. 1001 gestation, hypercalcemia was noticed at 2.68 mmol/L (ionized calcium: 1.44 mmol/L; NR: 1.14–1.35 mmol/L). The parathyroid hormone (PTH) level was 7.8 pmol/L (NR: 1.3–6.8 pmol/L). A parathyroid adenoma was diagnosed on cervical ultrasound. The medical team considered it important to reduce the patient’s serum calcium levels because hypercalcemia was thought to be responsible for the polyuria, with ensuing dehydration and sickle-cell crisis. Despite aggressive saline infusion, followed by furosemide, and then administration of calcitonin at 10 IU/kg twice daily for 48 h, serum calcium levels remained unchanged. Surgery was not considered because of the advanced pregnancy and active sicklecell disease. The patient had an in-depth discussion with the multidisciplinary team, and she agreed to receive cinacalcet 30 mg twice daily; three days later, it was increased to 60 mg twice daily, in spite of significant nausea requiring medication.
Calcium levels dropped progressively to 2.04 mmol/L 10 days after starting treatment. Cinacalcet was stopped prior to the patient’s scheduled erythrocytapheresis, as the procedure usually induced symptomatic short-term hypocalcemia. Two days later, the calcium level was 2.26 mmol/L and labor was induced. The patient gave birth vaginally to a normal 2650-g boy at 35.6 weeks’ gestation. The newborn’s calcium levels were normal over the following 10 days. Cinacalcet and calcitonin were stopped after delivery. Five months postpartum, the mother had surgery to remove the parathyroid adenoma. Patient 2 A 30-year-old woman (G1) was transferred to our center at 36 weeks’ gestation for symptomatic urolithiasis, acute renal insufficiency, and hypercalcemia. She had had multiple episodes of nephrolithiasis in the previous year, but investigations were postponed when she became pregnant. The patient’s bloodwork revealed a creatinine level of 132 lmol/L (NR: 53–97 lmol/L), and total calcium of 2.97 mmol/L (corrected calcium: 3.33 mmol/L; measured ionized calcium: 1.68 mmol/L). The patient was taking prenatal vitamins daily, vitamin D tablets (1000 IU), and calcium carbonate tablets as needed. Aggressive intravenous hydration with isotonic saline was started, along with calcitonin 4 IU/kg twice daily. Over the next few days, the pain decreased, and creatinine and calcium levels improved to 89 lmol/L and 2.43 mmol/L, respectively. However, the patient developed diabetes insipidus and calcium levels again increased to 2.82 mmol/L, despite a doubling of the calcitonin dose.
PTH was measured at 29.3 pmol/L, vitamin D levels were normal, and bilateral nephrocalcinosis was detected, along with a large renal staghorn calculus without hydronephrosis. A diagnosis of primary hyperparathyroidism was made. Considering that the pregnancy was at 37.5 weeks and that calcitonin has a timedlimited efficacy, labor was induced. The patient gave birth vaginally, with no complications, to a healthy 2496-g girl. The baby’s calcium levels were normal over the next 10 days. Immediately after delivery, the mother’s calcitonin was stopped, and she received 60 mg of intravenous pamidronate as there was uncertainty about the timing of an eventual surgery. A sestamibi parathyroid scan showed a single parathyroid adenoma. Finally, the woman had resection of the adenoma on postpartum day 7. Thereafter, serum calcium levels remained normal, and the creatinine returned to prepregnancy values. Patient 3 A 26-year-old woman (G1) was admitted at 14 weeks’ gestation for nausea and vomiting. She had no medical problems except for fibroma discovered at 8 weeks’ gestation. On admission, the patient complained of nausea, vomiting, constipation, weight loss, and severe arthralgia in the wrists, hands, knees, and back. Initial bloodwork revealed normocytic anemia (hemoglobin: 81 g/L; NR: 120–160 g/L) and elevated calcium levels (total calcium: 3.64 mmol/L; measured ionized calcium: 2.05 mmol/L).
Parathyroid hormone was undetectable, but parathyroid hormone-related peptide (PTHrP) was elevated at 46 pg/ L (NR < 15 pg/L). Vitamin D concentrations were slightly elevated at 388.4 pmol/L (NR: 63–228 pmol/L). The angiotensin-converting enzyme level was normal. Bone marrow aspirate, breast ultrasound, bone series, and thoracic magnetic resonance imaging (MRI) were normal. A pelvic ultrasound and abdominal MRI showed a 9-cm fibroma and bilateral nephromegaly, with a hyperechoic cortex and diminished corticomedullary differentiation. On admission, the patient was administered intravenous saline with electrolyte replacement and calcitonin (8 IU/kg four times a day), followed by furosemide, ondansetron, dimenhydrinate, and hydromorphone. We then discussed the possible use of cinacalcet with the patient and after reflection, she agreed to start cinacalcet 30 mg daily which was increased to twice daily 2 days Table 1. Correction and conversion to SI units. Calcium(Ca) mg/dL = Ca mmol/L 9 4 Corrected Ca (mmol/L) = Ca mmol/L + (0.02 x [40-albumin g/L]) Parathyroid hormone (PTH) pg/mL = PTH pmol/L 9 0.1061 PTHrP pg/mL = PTHrP pmol/L 9 1 1002ª 2016 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd. Hypercalcemia in pregnancy E. Rey et al. later, but without clinical or biochemical improvement (serum calcium: 3.76 mmol/L). In view of the persistent hypercalcemia and symptoms, the source of the PTHrP was thought to be either the placenta or fibroma and we opted, after discussion with the patient, to terminate the pregnancy.
The patient underwent curettage and immediately after she developed mild symptomatic pancreatitis which resolved over the next week. Intravenous pamidronate 90 mg was administered and calcium levels and PTHrP normalized 2 days after. Calcitonin and cinacalcet therapy were stopped. Her clinical status improved, with the exception of persistent arthralgia. A positron emission tomography (PET) scan showed increased uptake at the knees, elbows, ulnocarpal joints, fingers, and kidneys in a nondiagnostic pattern. The histopathological study of the placenta showed trophoblastic and stromal cells with strong immunohistochemical reactivity for PTHrP. Six weeks after termination, an abdominal ultrasound showed normal kidneys and the persistence of a 9-cm fibroma. A myomectomy was performed by median laparotomy, without complications. The arthralgia that had persisted since pregnancy termination resolved within 24 h. The fibroma weighed 325 g, and no malignancy was detected. Immunohistochemical staining for PTHrP was positive on more than 50% of cells of the fibroma and showed strong nuclear and moderate cytoplasmic positivity. It was impossible to determine whether the site of maximal PTHrP secretion had been the placenta or the fibroma.
Vanaja et al. studid a patient who was admitted to hospitali with hypercalcemia and a history of chronic vitamin A ingestion in order to investigate the rarely reported association between elevated serum calcium and vitamin A toxicity. Vanaja et al. reported that the clinical presentation, which was marked by profound weight loss, a psychiatric disturbance, total body alopecia, erosive dermatitis, and liver disease, was compatible with hypervitaminosis A. The diagnosis of vitamin A toxicity was established based upon the finding of elevated serum total vitamin A levels and the component due to retinyl esters. Vanaja et al. excluded other etiologies for hypercalcemia. Vanaja et al. [16] stated that in view of these results and the well-known effects of vitamin A on bone metabolism, they had concluded that the most likely etiology of the hypercalcemia in their reported patient was vitamin A toxicity. Cappellani et al. reported a 17-year-old lady who was referred to the Endocrine Unit of the University Hospital of Pisa for further evaluation of hypercalcemia that was associated with undetectable/low parathormone (PTH) levels. Her clinical history was documented as unremarkable except for her previous admission to the local Emergency Unit for renal colic 3 years earlier; and she then had an abdominal ultrasound which demonstrated unilateral kidney stones. On that occasion, she was treated with analgesics and hydration and no further investigations were undertaken.
One year later she underwent extracorporeal shockwave lithotripsy (ESWL), for the recurrence of renal colic pains. At that time, she had routine blood tests which demonstrated hypercalcemia, with her serum calcium level recorded as 12.4 mg/dL; [reference range 8.4-10.2], hypercalciuria of 390 mg/24h, [reference range 100-300], and undetectable serum PTH level of less than 4 pg/mL; (NV 8-40) and a 25-hydroxyvitamin D [25(OH)D) level of 37.4 ng/mL. Her family history was noted to be unremarkable with the exception of nephrolithiasis in her sister. During her admission, her physical examination was normal, with no evidence of major bone abnormalities. The results of her biochemistry laboratory tests confirmed hypercalcemia, hypercalciuria, and low/undetectable PTH levels; and her bone turnover markers were slightly above the upper limit of adult reference range (see Table 2). The results of her routine biochemistry tests were normal. She had chest X-ray and abdominal and neck ultrasound scans which were unremarkable. Cappellani et al. [17] were of the opinion that her long lasting hypercalcemia, the negative medical history beyond nephrolithiasis, and the normal radiology imaging studies made unlikely the hypothesis or postulate of paraneoplastic hypercalcemia. She underwent further assessment which revealed elevated serum levels of 1,25(OH)2D which had suggested vitamin D-dependent hypercalcemia.
A granulomatous disease was considered to be ruled out upon the basis of her having normal serum concentration of angiotensin converting enzyme and the absence of specific signs at chest X-rays. In view of the young age of the patient and the family history of nephrolithiasis, biochemical tests were performed in her firstdegree relatives. Total and ionized serum calcium, phosphate, PTH, and 1,25(OH)2D levels were found to be in the normal range in both parents, who were found to have a low vitamin D status. Interestingly, in her siblings PTH concentration was found to be in the low-normal range and 1,25(OH)2D at the upper normal limit or slightly elevated (Table 3). The latter findings, together with the biochemical profile of the patient, had suggested to the authors that the hypercalcemia might be due to an impairment of the CYP24A1 catabolic pathway. The genetic analysis in the proband was made utilizing High Resolution Melting Analysis (HRMA) [18] and this further confirmed utilizing gene amplification and sequencing [19], that demonstrated a known homozygous PV (c.428_430delAAG, rs777676129, p.Glu143del) in the CYP24A1 gene (Figure 1A). The same heterozygous variant was identified in the parents and the siblings (Figure 1B). The parents had excluded consanguinity, even though they were noted to have come from the same small village.
In order to complete the biochemical profile of vitamin D metabolites, liquid chromatography tandem mass spectrometry (LC-MS/MS) examination was run upon stored serum samples of all the family members. They reported that serum samples had been prepared by immuno-extraction and derivative with 4-[2-(6,7-dimethoxy-4-methyl-3,4-dihydroquinoxalinyl)ehtyl]- 1,2,4-triazoline-3,5-dione (DMEQ-TAD), as reported. [20] Cappellani et al. observed that the proband exhibited low 24,25(OH)2D3 (0.42 ng/mL) and elevated 25(OH)D3:24,25(OH)2D3 ratio (118; cut-off >80) which had confirmed the diagnosis of impaired CYP24A1 function. A more rigorous chromatographic method [21] was also utilized to assay the same sample (25(OH)D3:24,25(OH)2D3 ratio = 3117; cut-off>140), which also suggested inappropriately low levels of 24,25(OH)2D3 in the proband. The other family members, who had presented as heterozygous variants, had exhibited essentially normal serum 24,25(OH)2D3 concentrations and 25(OH)D3:24,25(OH)2D3 ratios (Table 3&Figure 2). In view of the mild hypercalcemia, Cappellani et al. did not advise pharmacologic treatments that are aimed at modulating 1,25(OH)2D metabolism and they recommended maintenance of adequate hydration and avoidance of unprotected excessive sunlight exposure. Follow-up assessment up to 24 months that had been undertaken had shown that the lady was in an overall stable condition, with serum calcium concentration slightly above the upper normal limit and she had ultrasound scan of her renal tract which did not show any recurrent nephrolithiasis.
Figure 1A: (a) Sequences of the CYP24A1 exon 2 obtained by proband and her parents. CYP24A1 gene amplification and sequencing were performed as reported. Sequences of the exon 2 obtained by proband and her parents were shown. Arrows indicate the position of c.428_430delAAG heterozygous and homozygous variant in the parents and the proband, respectively.

Figure 2: LC-MS/MS chromatogram of dihydroxylated vitamin D metabolites. Serum samples were prepared by immunoextraction and derivatized with DMEQ-TAD. Peaks corresponding to 6S and 6R isomers of DMEQ-TAD adducts of 24,25(OH)2D3 and 1,25(OH)2D3 were observed using the multiple reaction monitoring (MRM) transition of mass/charge (m/z) 762.3- ->468.1+484.1, from which serum concentrations of these metabolites were determined. The figure reveals the dramatically reduced concentration of 24,25(OH)2D3 in the proband II.3 as well as elevated 1,25(OH)2D3, in comparison to unaffected family members.

Nowadays vitamin D (25-OHD) deficiency is supposed to be a global epidemic condition. Guerra et al. stated the following:
a) Expectedly, measurement of vitamin D intake had exponentially increased within Brazil within the decade preceding 2016.
b) Even though the benefit of vitamin D to general health has remained debatable, its indiscriminate utilization potentially could lead to enhance the incidence of vitamin D intoxication, which is regarded as a rare disorder
Guerra et al. reported a case of a 70-year-old diabetic man who had chronic renal disease (blood creatinine of 1.6 mg/dL) which had progressed suddenly to acute kidney injury (blood creatinine of 5.7 mg/dL) that was associated with hypercalcemia and high blood levels of vitamin D. Vitamin D and calcitriol medicaments were discontinued and his hypercalcemia was managed by hydration followed by furosemide. Subsequently, he received disodium pamidronate and he did not undergo dialysis. It took about 14 months to normalize 25-OHD levels and his serum creatinine returned to basal levels only after 24 months. Guerra et al. [22] stated the following:
a) The indicated labeling dosage was 2000 IU, but most likely the vitamin D manipulated preparation was higher as the vitamin D blood levels were very high.
b) Even though uncommon, vitamin D intoxication was becoming more frequent as the patients utilize frequently manipulated preparations which could be subject to errors in the manufacturing and labeling of the tablets or capsules.
c) Their report case had alerted them to the potential increase in the incidence of severe vitamin D intoxication due to the frequent utilization of this secosteroid as a nutritional supplement.
d) Contemporaneously, it is necessary to improve regulation on the nutrient supplement market.
Pilz et al. stated the following:
a) Pathogenic mutations of CYP24A1 do lead to an impaired catabolism of vitamin D metabolites and hence it should be considered in the differential diagnosis of hypercalcemia with low parathyroid hormone concentrations.
b) Diagnosis of pathogenic mutations of CYP24A1 is based upon a reduced 24,25-dihydroxyvitamin D to 25-hydroxyvitamin D ratio and is confirmed by the undertaking of genetic analyses.
c) Pregnancy is associated with an upregulation of the active vitamin D hormone calcitriol, and it could thus particularly trigger hypercalcemia in affected patients.
Pilz et al. published a case report and a narrative review of pregnant women with CYP24A1 mutations in 13 women with 29 pregnancies in which they outlined the laboratory and clinical characteristics during pregnancy and postpartum and the applied treatment approaches. Pilz et al. [23] stated that generally, pregnancy triggered hypercalcemia in the affected women and obstetric complications were frequently reported. They also stated the following:
a) Conclusions on drugs to treat hypercalcemia during pregnancy are extremely rare and they do not show clear evidence of efficacy.
b) Strictly avoiding vitamin D supplementation does seem to be effective in the prevention of or reduction in the degree of hypercalcemia.
c) Their case of a 24-year-old woman who had manifested with hypercalcemia in the 24th gestational week had delivered a healthy baby and her hypercalcemia resolved while she was breast-feeding her baby.
d) Pathogenic mutations of CYP24A1 mutations are rare; nevertheless, it should be considered in the context of vitamin D supplementation during pregnancy.
Malekar-Raikar et al. reported a report a case of primary hyperparathyroidism in a pregnant as well as they reported the obstetric and neonatal outcomes, and they also reviewed the relevant literature. Malekar-Raikar et al. reported a 29-year-old primigravida patient who was successfully treated for primary hyperparathyroidism (PHP) with minimally invasive resection of a parathyroid adenoma in the second trimester of her pregnancy. A healthy baby girl was delivered at 37-week gestation with an unremarkable neonatal course. Malekar-Raikar et al. iterated that to the best of their knowledge, their reported case was the second case report in the literature that utilized intraoperative PTH during a parathyroidectomy in a pregnant woman. Malekar-Raikar et al. [24] made the following conclusions:
a) Primary hyperparathyroidism is a rare life-threatening condition which could manifest during pregnancy.
b) The diagnosis could be difficult to establish during pregnancy, considering the nonspecific symptoms related to hypercalcemia.
c) Nevertheless, a better understanding of the condition, improved diagnostic studies, and well-organized multidisciplinary management decisions could significantly reduce the morbidity and mortality associated with the disease during pregnancy.
d) They had reported the case in order to highlight the value of early diagnosis and appropriate management of PHP during pregnancy.
Roux et al. stated the following:
a) Denosumab is an anti-RANKL antibody which is commonly utilized for the treatment of osteoporosis; in oncology, bisphosphonates and denosumab had become the standard therapy options for the management as well as prevention of skeletal complications in patients who have myeloma and solid tumours.
b) In recent years, excessive bone remodelling after the discontinuation of denosumab had raised concerns.
c) Many cases of hypercalcemia had been reported following the discontinuation of high-dose denosumab (120 mg every 4 weeks), mainly in children.
They were reporting a new case of severe refractory hypercalcemia in a 54-year-old woman who had received high-dose denosumab for 5 years as an adjuvant treatment for her carcinoma of the breast. She was at the time of the report of her case in remission and undergoing treatment with anastrazole, which is an aromatase inhibitor. Rouz et al. [25] stated that the peculiarities of their reported case included: the presence of associated bone pain with subperiosteal bone resorption on hand X-rays, and diffuse, long bone diaphyseal uptake on a bone scan. They furthermore iterated the following:
a) Hyperparathyroidism had been ruled out, and existing evidence had suggested that their observed high-level of bone remodelling could be due to a rebound hyperactivation of the RANKL pathway.
b) In addition to rehydration, repeated use of intravenous bisphosphonates was required to control the patient’s recurrent hypercalcemia.
c) In view of the fact that hypercalcemia is a serious metabolic complication, a gradual dose reduction should be taken into consideration when interruption of high dose denosumab therapy is planned.
Nakajima et al. [26] reported that hypercalcemia with concomitant elevation of serum parathyroid hormone (PTH) and PTH-related protein (PTHrP) levels was found in a patient who had advanced gastric carcinoma and multiple liver metastases. The most common features that were found included evidence of hypercalcemia that was associated with hypersecretion of PTHrP and physiological suppression of PTH secretion in the syndrome of humoral hypercalcemia of malignancy (HHM). They also stated the following:
a) Even though, they had initially made a diagnosis of primary hyperparathyroidism that was concomitant with HHM due to gastric cancer, diagnostic imaging studies, such as echography, CT, sestamibi scintigraphy, and autopsy findings, did not demonstrate any evidence of any parathyroid tumours or ectopic parathyroid glands in the mediastinum.
b) Both primary and metastatic tumour cells showed positive immunohistochemistry staining with PTH-specific antibody as well as PTHrP-specific antibody on immunohistochemical examination.
c) PTH concentration in the cytosolic fraction of the metastatic tumour was found to be elevated in comparison with that from a control patient who did not have any calcium metabolic disorders in vitro.
d) The aforementioned findings had indicated that PTH secreted ectopically by gastric cancer cells, not by parathyroid glands, caused hypercalcemia in their reported patient.
Kurt and Naber [16] stated that urinary tract infection (UTI) has been classified as uncomplicated if it does occur in a patient who has a structurally and functionally normal urinary tract and that acute uncomplicated cystitis tends to be observed mainly in women. They also stated the:
e) To their knowledge, their reported case was the first case report of PTH-secreting gastric carcinoma cells.
Eller-Vainicher et al. reported a 35-year-old oriental woman, who was 32 weeks pregnant, and who was admitted to a hospital with suspected preeclampsia. Subsequently, she developed stupor and lethargy. Her serum biochemical assessment showed severe hypercalcemia of 21 mg/dl with an undetectable parathyroid hormone (PTH) as well as markedly elevated PTH related peptide (PTHrP) levels of 26 pmol/l, normal values!1.1 pmol/l). The patient was treated with intravenous fluid administration, which had resulted in an unsatisfactory reduction in her serum calcium level. In view of this, a caesarean section was undertaken with her consent to deliver the baby. Her serum calcium levels promptly normalized after delivery of her baby with undetectable PTHrP levels. She delivered a healthy infant who only presented with transient mild jaundice and slightly prolonged QT interval with serum calcium level of 7.8–8.4 mg/dl (corrected for albumin levels). In the ensuing days, the patient developed a transient ‘hungry bone’ syndrome with serum calcium 6.7 mg/dl, phosphorous 2.1 mg/dl, and PTH 100.4 pg/ml). Eller-Vainicher et al. [27] made the following conclusions:
a) The pregnant patient had presented with PTHrPassociated hypercalcemia, presumably of placental origin.
b) Delivery of her baby had emanated in prompt reduction of her serum calcium levels and a transient ‘hungry bone’ syndrome.
Newman et al. stated the following:
a) Hepatocellular carcinoma (HCC) in non-cirrhotic livers is a rare or an uncommon finding and HCC could present insidiously in patients.
b) Another uncommon finding in HCC, and one of poor prognosis, is the presence of paraneoplastic diseases such as hypercalcemia.
Newman et al. reported a a case of a 66-year-old previous healthy Filipina woman who following her routine laboratory evaluation was found to have hypercalcemia as the first sign of an advanced HCC without underlying cirrhosis. In view of the patient’s relative lack of symptoms, healthy liver function, lack of classical HCC risk factors, and unexpected hypercalcemia, the diagnosis of a paraneoplastic syndrome caused by a noncirrhotic HCC was unanticipated or considered as a provisional diagnosis. Newman et al. stated also the following:
a) It is not known how often hypercalcemia is found in association with HCC in a non-cirrhotic liver.
b) Nevertheless, paraneoplastic manifestations of HCC, particularly hypercalcemia, could be correlated with poor prognosis.
c) For their reported patient, her initial management had included attempts to lower her serum calcium levels via zoledronate, which was not completely effective.
d) Tumour resection was then attempted; nevertheless, the patient expired due to complications from advanced tumour size.
Newman et al. [28] concluded that hypercalcemia of malignancy could be found in association with non-cirrhotic HCC and it should be considered on the differential diagnosis during clinical work-up of patients.
Stefanko et al. stated the following:
a) Subcutaneous fat necrosis of the newly born baby is an uncommon disorder, and even though usually benign, its associated hypercalcemia could emanate into complications such as failure to thrive and renal failure.
b) Several sources had recommended screening for hypercalcemia for 6 months following resolution of skin lesions; however, little data are available to support this recommendation.
Stefanko et al. undertook a study which examined the existing published literature to better guide practitioners regarding screening evaluations of asymptomatic patients with subcutaneous fat necrosis. Stefanko et al. [29] undertook a systematic review of the literature was utilizing a PubMed English literature search. Stefanko et al. collected case reports and case series regarding the presence of hypercalcemia and associated complications, birth history, and age of onset/ resolution of skin lesions and laboratory abnormalities. Stefanko et al. found out that about half (51%) of infants that had been reported had hypercalcemia. Majority of the patients that amounted to 77% of the patients had developed detectable hypercalcemia within 30 days of skin lesion onset, and 95% of the patients had developed detectable hypercalcemia within 60 days of skin lesion onset. Stefanko et al. also observed the following:
a) Hypercalcemia was identified in only 4% of patients > 70 days following the onset of their skin lesions.
b) Seventy-six percent of the patients had resolution of their hypercalcemia within 4 weeks of detection.
c) Hypercalcemia was found to be more prevalent in fullterm versus pre-term infants (P-value = 0.054), and higher birthweight was significantly found to be associated with an increased risk of developing hypercalcemia (P-value = 0.022).
d) Even though gestational age trended toward significance, the only statistically significant clinical feature predicting the development of hypercalcemia was noted to be higher birthweight.
Stefanko et al. [29] also iterated that current recommendations for laboratory monitoring are not evidence-based, and that their study had provided interim data to guide practitioners until prospective, randomized controlled trials are conducted. Shimonodan et al. stated that osteopathy is one of the common initial symptoms of acute lymphocytic leukemia (ALL) in children and adolescents, but multiple osteolysis accompanied by hypercalcemia had rarely been observed. Shimonodan et al. [30] treated a 14-year-old female who had multiple osteolytic lesions as well as hypercalcemia at initial onset of her ALL. Shimonodan et al. in their reported case we examined some humoral factors, which were known to be associated with hypercalcemia in malignancies. With regard to the results of their reported case, Shimonodan et al. [30] reported the following:
Stefanko et al. [29] also iterated that current recommendations for laboratory monitoring are not evidence-based, and that their study had provided interim data to guide practitioners until prospective, randomized controlled trials are conducted. Shimonodan et al. stated that osteopathy is one of the common initial symptoms of acute lymphocytic leukemia (ALL) in children and adolescents, but multiple osteolysis accompanied by hypercalcemia had rarely been observed. Shimonodan et al. [30] treated a 14-year-old female who had multiple osteolytic lesions as well as hypercalcemia at initial onset of her ALL. Shimonodan et al. in their reported case we examined some humoral factors, which were known to be associated with hypercalcemia in malignancies. With regard to the results of their reported case, Shimonodan et al. [30] reported the following:
b) Other humoral factors related to hypercalcemia were not identified.
c) The patient’s ALL relapsed in the bone marrow 3 months after achieving complete remission, and hypercalcemia and elevation of serum PTHrP were also noted.
d) A second remission could not be achieved, and hypercalcemia had continued.
e) The patient received allogeneic bone marrow transplantation. The serum calcium level had dropped to normal range after the conditioning therapy.
f) Before engraftment, nevertheless, the patient died of infection.
Shimonodan et al. [30] made the following conclusions:
Shimonodan et al. [30] made the following conclusions: a) Their reported case had suggested that blast producing PTHrP might be associated with multiple osteolytic lesions and hypercalcemia.
b) PTHrP expressed within the lymphoblasts might, in itself, confer a survival advantage to lymphoblasts and contribute to the refractory nature of the disease.
Koldkjær Sølling et al. stated that Denosumab is utilized for treatment of osteoporosis. Koldkjær Sølling et al. reported a case report of hypo-parathyroid hypercalcemia and increased bone turnover that was associated with discontinuation of treatment for 10 years with denosumab. They iterated the following:
a) There is a need for evidence-based guidelines on discontinuation of long-term denosumab treatment in order to avoid side effects and preserving anti-fracture efficacy.
b) Denosumab is commonly utilized as an anti-resorptive agent for the treatment of osteoporosis.
c) Following discontinuation of denosumab; nevertheless, bone resorption increases again, and the bone mass gained during therapy tends to be rapidly declining. Hence, treatment with denosumab is considered to be reversible.
Koldkjær Sølling et al. reported a case report of asymptomatic hypo-parathyroid hypercalcemia in a patient who had discontinued long-term treatment with denosumab. Koldkjær Sølling et al. summarized the results as follows:
A 67-year-old female who had osteoporosis was treated with denosumab 60 mg subcutaneously every 6 months from 2004 to 2014. She had received her last injection in May 2014. The results of her routine biochemistry in November 2014 had shown increased s-ionized calcium (I-Ca) 1.64 mmol/L (1.18-1.32 mmol/L) and suppressed p-parathyroid hormone (PTH) 1.6 pmol/L (1.6-6.9 pmol/L). The patient was extensively and thoroughly examined, but no underlying disease was found. In January 2015, the patient began treatment with alendronat 70 mg weekly. In April 2015, her serum levels of type 1 collagen C-terminal cross-linked telopeptide, procollagen type 1 N-terminal propeptide and bone-specific alkaline phosphatase were still noted to be markedly elevated. From then on, I-Ca and PTH had normalized, and her bone turnover markers (BTM) had decreased.
Koldkjær Sølling et al. [31] made the following conclusions:
a) In their case report, they had described increased BTMs and hypercalcemia that was associated with discontinuation of 10 years treatment with denosumab.
b) The increase in BTMs was assumed to be temporary and normalization was expected.
c) In view of the fact that denosumab is commonly utilized, there is an urgent need for evidence-based guidelines on discontinuation of long-term treatment, avoiding side effects and preserving anti-fracture efficacy.
Garbim et al. stated the following
a) Hypercalcemia is a rare condition in childhood; the most common causes of hypercalcaemia include: primary hyperparathyroidism, malignancy, prolonged immobilisation, thyrotoxicosis, thiazide diuretic, supplements containing calcium, milk-alkali syndrome, vitamin D intoxication, infections and idiopathic.
Garbim et al. reported three cases of severe hypercalcemia of unusual causes in children. The first patient had high fever, poor general condition, weight loss as well as myalgia. Extensive preliminary investigation did not identify the aetiology of her disease; nevertheless, a review of the medical history of the patient revealed prolonged contact with pet bird and a positive serology for Chlamydia which had confirmed the diagnosis of psittacosis. The second patient was documented to have a generalized lymphadenopathy and hepatosplenomegaly with fever a month preceding the manifestation of the patient to the clinicians. Paracoccidioides brasiliensis was identified in the myelogram of the patient; the patient showed partial improvement with utilization of co-trimoxazole, with subsequent emergence of multiple osteolytic lesions. Examination of a smear of gastric lavage was found to be positive for Mycobacterium tuberculosis and the patient was treated with rifampicin, isoniazid, ethambutol and pyrazinamide, with improvement of the patient’s clinical condition. The third patient was treated for hypercalciuria and idiopathic hypomagnesiuria with daily utilization of cholecalciferol; the patient had a two kilograms of weight loss in the preceding two months. No cause of hypercalcemia could be identified in the laboratory workout assessment of the patient. The capsules of cholecalciferol were analysed and presented an amount of 832,000 IU of vitamin D per capsule. Garbim et al. [32] also stated the following:
a) Acute hypercalcemia in childhood could be due to exogenous vitamin D intoxication, as well as due to infectious causes.
b) The possible causal relationship between psittacosis and the occurrence of hypercalcemia alert to the need for detailed investigation of the epidemiological antecedents.
Marx stated the following:
a) Familial hypocalciuric hypercalcemia (FHH) does tend to cause lifelong hypercalcemia which even persists following sub-total parathyroidectomy.
b) Symptoms of familial hypocalciuric hypercalcaemia are usually mild.
c) Past recommendations had often been for monitoring of the condition and against surgical or pharmacologic treatments.
Marx reviewed publications related to FHH, calcium-sensing receptors (CaSRs), and calcimimetics. Marx summarized the results as follows:
a) FHH does reflect heterozygous germline mutation of CASR, GNA11, or AP2S1. These mutations inactivate the CaSRs in the parathyroid cell. Thereby, they do shift the serum calcium set point to higher values and cause hypercalcemia.
b) Calcimimetic medicaments do enhance the effects of calcium on the CaSRs and thereby they do inhibit the parathyroid cell.
c) Calcimimetic drugs are indicated in adults who have primary hyperparathyroidism without a good surgical option.
d) Calcimimetic safety and efficacy had not been established in children who are younger than age 18 years.
e) Recent case reports had described treatment of FHH with calcimimetics. Successful treatment was classified as combinations of subjective improvements and decreases of serum calcium levels, but not necessarily into the normal range.
Treatment was reported to be successful in 14 of 16 cases that amounted to in 88% of the cases treated.
Marx [33] made the following conclusions:
a) Deductions based upon these case reports do have limitations. For example, failures of therapy might not have been reported.
b) Cost of the medicament might be rate limiting.
c) Calcimimetics could be offered to adults who have FHH and those in whom the serum calcium level is higher than (>) 0.25 mM (1 mg/dL) beyond the upper limit of normal or with possible symptoms of hypercalcemia.
d) Calcimimetics could now be offered to more adults who are afflicted by FHH.
Visnyei et al. stated the following:
a) Body contouring injections by non-licensed providers have tended to be frequently sought out by a subset of the maleto- female transgender community.
b) Even though short-term side effects such as pulmonary embolism and injection site infection had been well known, long-term consequences of such practices had been less well studied.
Visnyei et al. reported the case of a 40-year-old African American male-to-female transgender patient who had presented to their institution with hypercalcemia and acute renal failure secondary to body contouring injections with industrial strength silicone by non-licensed providers, a decade preceding her consultation visit. Her clinical assessment work-up had demonstrated an extensive granulomatous inflammatory process within her injection area which had resulted in electrolyte abnormalities and kidney injury. The results of the patient’s laboratory tests and symptoms did respond well to long-term corticosteroid treatment and correlated with treatment adherence.
Visnyei et al. [34] made the following conclusions:
a) Affected patients could at times manifest with unusual clinical symptoms many years pursuant to their silicone injections.
b) In a constantly growing transgender community which often have tended to utilize non-licensed providers for silicone injections, the medical community would likely face an increasing number of patients who have developed longterm side effects of such practices. Therefore, it is important for physicians to recognize such cases promptly and in order to commence potentially life-saving treatment for such patients.
Ghaznavi et al. stated the following:
a) Primary hyperparathyroidism (PHPT) and Familial Hypocalciuric Hypercalcemia (FHH) do tend to result in different maternal and foetal complications associated with pregnancy.
b) Calcium to creatinine clearance ratio (CCCR) has tended to be commonly utilized to help distinguish these two conditions.
c) Physiological changes in calcium handling during pregnancy and lactation could alter CCCR, making it a less useful tool to distinguish PHPT from FHH.
Ghaznavi et al. reported two cases as follows:
Case 1: A 25-year-old female had manifested with hypercalcemia and an inappropriately normal PTH. Her CCCR was 0.79% prior to her pregnancy and her CCCR rose to 1.99% in her second trimester. The proband’s mother and neonate had asymptomatic hypercalcemia. Genetic analysis that was undertaken revealed a CaSR mutation which was consistent with FHH.
Case II: A 19-year-old female had manifested with a history of nephrolithiasis who did undergo an emergency caesarean section at 29 weeks of her gestation for her severe preeclampsia. During her delivery, she was diagnosed as having hypercalcemia with an inappropriately normal PTH and a CCCR of 2.67%, which reduced to 0.88% during her lactation. She underwent parathyroidectomy which cured her hypercalcemia. Pathology examination of the excised parathyroid gland confirmed a parathyroid adenoma.
Ghaznavi et al. [35] made the following conclusions:
a) These reported cases had illustrated the influence of pregnancy and lactation upon renal calcium indices, such as the CCCR.
b) In order to avoid diagnostic error of women who have hypercalcemia during pregnancy and lactation, they would recommend routine screening testing of calcium biochemistry of first-degree relatives and genetic testing of select patients.
Cengiz et al. [36] stated the following:
a) Sarcoidosis is a systemic granulomatous disease primarily which tends to involve the lungs, lymph nodes, skin, eyes and nervous system; liver involvement by sarcoidosis has tended to be asymptomatic in majority of cases.
b) Nevertheless, once the patient does develop clinical symptoms, liver disease is usually progressive and may necessitate orthotopic liver transplantation.
c) There are occasional reports of asymptomatic recurrent sarcoidosis developing within the liver allograft.
Cengiz et al. reported a case of early recurrence of sarcoidosis within the liver allograft which was diagnosed upon pathology examination of biopsy specimen in a patient who had presented with severe hypercalcemia, kidney dysfunction, and increase in size of the patient’s abdominal lymph nodes. The liver chemistry tests were found to be within normal limits. The patient responded well to steroid treatment by normalizing the serum calcium and creatinine levels and reduction in the lymph node size. Cengiz et al. [36] stated that up to the time of their case report, there had been no report in the literature of symptomatic recurrence of hepatic sarcoidosis following orthotopic liver transplantation.
Sternlicht and Glezerman [37] stated the following:
a) Hypercalcemia of malignancy does affect up to one in five cancer patients during the course of their disease.
b) Hypercalcemia of malignancy tends to be associated with both liquid malignancies, commonly multiple myeloma, leukemia, and non-Hodgkin’s lymphoma and solid cancers, particularly breast and renal carcinomas as well as squamous cell carcinomas of any organ.
c) The clinical presentations of hypercalcemia tend to be generally constitutional in nature and they tend not to be specific to the inciting malignancy. Such physical presentations could range from malaise to lethargy and confusion. Constipation and anorexia tend to be common. Acute kidney injury (AKI) is likely the most frequently encountered presentation of end organ damage. Symptomatology of this condition has been closely linked to both the absolute elevation of serum calcium levels and the rapidity of calcium rise.
d) Most of them tend to be humoral in etiology and they tend to be related to parathyroid hormone-related protein (PTHrP).
e) Approximately 20% of cases tend to be the result of direct bone metastasis with extra-renal 1,25-dihydroxyvitamin D (calcitriol) and ectopic parathyroid hormone production which likely has tended to account for less than 1% of cases.
f) The diagnosis of hypercalcemia of malignancy has tended to be confirmed either by an elevated PTHrP or by evidence of bone metastasis in the appropriate clinical setting.
g) Treatment has tended to be predicated upon the patient’s symptoms and absolute serum calcium level. Interventions tend to be aimed at lowering the serum calcium concentration by inhibiting bone resorption and increasing urinary calcium excretion, the former has tended to be achieved via utilization of bisphosphonate therapy and the latter with aggressive hydration. Novel treatments for refractory disease do include: denosumab, a monoclonal antibody against the receptor activator of nuclear factor κB ligand, and the calcimimetic cinacalcet.
h) Finally, anti-PTHrP antibodies had been successfully deployed in animal models of disease.
i) Despite the efficacy of the above treatment options, hypercalcemia of malignancy does tend to portend an ominous prognosis, which has tended to be indicative of advanced and often refractory cancer with survival on the order of months.
Doyle and Malcolm reported the case of a 28-year-old woman who had manifested with hypercalcemia (total calcium =4.11 mmol/L), elevated parathyroid hormone (PTH) 24.6 pmol/L, normal parathyroid hormone-related peptide 7.8 pg/mL, and a 63 mm × 57 mm, poorly differentiated neuroendocrine carcinoma (small-cell type) pancreatic mass with liver metastases. Her hypercalcemia was acutely managed by means of intravenous fluids, pamidronate and calcitonin. She underwent investigations for multiple endocrine neoplasia type 1 and parathyroid adenoma. Her identified neuroendocrine tumor was treated with utilization of Cisplatinum / Etoposide chemotherapy. Doyle and Malcolm summarized the results as follows: The pancreatic mass measured 56 mm × 49 mm and her metastases had decreased in size with chemotherapy and her serum calcium levels normalized. Eight months later, her serum calcium level had increased to 3.23 mmol/L, her PTH increased to 48.2 pmol/L, and the pancreatic mass increased in size to 67 mm × 58 mm. She patient was given a trial of cinacalcet but she was not able to tolerate it. Chemotherapy was recommenced and this resulted in a decrease in the size of the pancreatic mass to 49 mm × 42 mm, a reduction in PTH levels to 16.6 pmol/L, and her serum calcium levels to 2.34 mmol/L. Doyle and Malcolm [38] made the following conclusions and recommendations:
a) Ectopic PTH secreting tumors should be taken into consideration when there is no parathyroid related cause for an elevated PTH.
b) Recognition of the association between PTH and hypercalcemia of malignancy might lead to an earlier detection of an undiagnosed malignancy.
Mishra and Newman, stated the following:
c) Severe hypercalcemia has tended to be a life-threatening condition.
d) Atypical parathyroid adenoma and parathyroid carcinomas have tended to be uncommon causes which could be difficult to differentiate.
Mishra and Newman reported a case of a 36-year-old man who had a very high serum calcium due to a possible atypical parathyroid adenoma versus parathyroid carcinoma. With regard to the patient, a serum calcium level of 23.2 mg/dl was noted during his admission. He was initially treated with intravenous hydration, pamidronate, and salmon calcitonin so as to lower his calcium levels. He also underwent a surgical en bloc resection of his parathyroid mass. Pathology examination of the excised parathyroid gland specimen showed a mixed picture which was consistent with possible atypical adenoma versus parathyroid carcinoma. Nevertheless, in view of the possible involvement of the recurrent laryngeal nerve, parathyroid carcinoma was considered to be more likely. Also, post-operatively, the patient developed hungry bones syndrome and his calcium was replaced vigorously. He at the time of the reporting of his case had continued to be on calcium, vitamin D, and calcitriol supplementation. Mishra and Newman reviewed the literature in order to identify previous studies pertaining to parathyroid adenomas and parathyroid cancer.
Mishra and Newman [39] made the following conclusions related to their reported case and literature review:
a) They had thereby concluded that hypercalcemia does require very careful monitoring especially following an operation.
b) Also, it could be very difficult to differentiate between atypical parathyroid adenomas and parathyroid carcinomas as in their case and no clear-cut guidelines had been detailed out to differentiate the two based on histology.
Hygum et al. stated the following:
a) Hypercalcemia is the commonest oncology metabolic emergency, but which is very rarely observed in patients who have gastrointestinal stromal tumour, which is a rare mesenchymal malignancy of the gastrointestinal tract.
b) They had reported a case of hypercalcemia that was caused by elevated levels of activated vitamin D in a patient who had gastrointestinal tumour.
c) Prior to their case report, only one paper had reported an association between hypercalcemia, gastrointestinal stromal tumours and elevated levels of vitamin D.
Hygum et al. reported an otherwise healthy 70-year-old Caucasian woman, who was previously treated for duodenal gastrointestinal stromal tumour, and who was diagnosed as having liver metastasis, and relapse of her gastrointestinal stromal tumour that was confirmed by biopsy. During her presentation, the patient was noted to have suffered from severe symptoms of hypercalcemia. Hygum et al. stated that the commonest causes of hypercalcemia, hyper-parathyroidism, parathyroid hormone-related peptide secretion from tumour cells, and metastatic bone disease, were all dismissed as the aetiology. They undertook an analysis of vitamin D subtypes which demonstrated normal levels of both 25-OH Vitamin D2 and 25-OH Vitamin D3; however, her level of activated vitamin D, 1,25 OH Vitamin D3, also referred to as calcitriol, was elevated.
Hygum et al. [40] made the following conclusions:
a) The fact that plasma calcitriol had decreased after commencement of her oncology treatment and the finding that her hypercalcemia did not recur during treatment had supported the conclusion that elevated calcitriol was a consequence of the gastrointestinal stromal tumour.
b) They recommend that gastrointestinal stromal tumours should be added to the list of causes of humoral hypercalcemia in malignancy, and they would postulate that gastrointestinal stromal tumour tissue might have high activity of the specific enzyme 1α-hydroxylase, which could lead to increased levels of calcitriol and secondarily hypercalcemia.
Nemr et al. stated that Calcitriol-mediated hypercalcemia had been reported in malignant lymphomas and granulomatous diseases but not in lung carcinoma. Nemr et al. reported a patient who had squamous cell lung carcinoma with hypercalcemia and elevated calcitriol levels. Nemr et al. reported a 60-year-old Caucasian male patient who had stage IIIB squamous cell lung cancer and who developed hypercalcemia at 14.8 mg/dL two years after receiving chemotherapy and radiotherapy where the results of his laboratory tests showed a serum intact PTH: 7 pg/mL, PTHrP: 30 pmol/L, 1,25-hydroxyvitamin D (calcitriol): 76 pg/mL, and 25-hydroxyvitamin D levels: <4 ng/mL. His Calcitriol levels were elevated despite undetectable 25-hydroxyvitamin D levels.
Nemr et al. [41] stated the following:
a) There were no previously reported lung cancer cases with elevated calcitriol as an etiology of hypercalcemia.
b) They believed that the elevated calcitriol levels in their reported case were due to a PTHrP-independent mechanism, possibly from either ectopic production of calcitriol in tumor cells or from increased activity of 1-alpha hydroxylase in the same cells.
c) The patient had died before they could assess the effects of prednisone therapy.
d) Studies are required in order to investigate the cellular source of calcitriol and its role in hypercalcemia in patients with lung cancer.
Abdullayev et al. stated the following:
a) Neonatal severe primary hyperthyroidism is an extremely uncommon disorder which occurs in the first six months of life.
b) Early recognition and prompt surgical intervention are of vital importance in order to achieve survival and in order to avoid neurological sequel.
c) Hypotonia, lethargy, respiratory distress, and growth and developmental delay do tend to occur in association with elevated serum parathormone levels and hypercalcemia.
d) Definitive therapy does entail total parathyroidectomy.
Abdullayev et al. reported a patient who had Neonatal severe primary hyperparathyroidism, and who successfully had undergone total parathyroidectomy. The patient had been followed up with medical treatment until he was seven months old, with no adequate clinical response to medical treatment. The patients’ parathormone levels rapidly declined following total parathyroidectomy, and the parathormone level fell to zero after removal of the ectopic tissue with a second surgery, and the patient was discharged with full recovery. Abdullayev et al. stated the following:
a) Sestamibi scintigraphy might not always demonstrate an ectopic parathyroid gland. In such conditions, confirmation of parathyroid glands excised with total parathyroidectomy by frozen biopsy would not be sufficient to terminate the surgery.
b) Intraoperative parathormone monitoring would be particularly important at this point.
c) The finding of persistently elevated parathormone levels should suggest a remnant parathyroid tissue at the surgical site or an ectopic parathyroid gland which needs to be excised.
Abdullayev et al. [42] made the following conclusions:
a) Neonatal severe primary hyperparathyroidism represents a life-threatening condition.
b) Early surgery is lifesaving in cases in whom medical therapy have failed to control the disease.
Camponovo et al. stated the following:
a) Denosumab discontinuation tends to be associated with a rapid increase in bone resorption and a decrease in bone mineral density.
b) Spontaneous vertebral fractures could occur as a side effect of the rebound of bone resorption.
c) Cases of rebound-linked hypercalcemia had also been reported, moderate in women with osteoporosis and breast cancer and severe in children who had been receiving oncological doses of denosumab.
Camponovo et al. reported the case of an adult woman who had primary hyperparathyroidism and moderate hypercalcemia, who was treated with denosumab for osteoporosis, and who had developed severe hypercalcemia and spontaneous vertebral fractures (SVFs) after denosumab discontinuation. Camponovo et al. reported an 86-year-old woman who had densitometric osteoporosis and who was treated for 3 years with 60 mg of subcutaneous denosumab every 6 months. It was known that she had primary hyperparathyroidism, with a serum albumincorrected calcium of 2.82 mmol/l (NV 2.15–2.5) at the end of denosumab effect. Nine months following her last denosumab injection, she was hospitalized due to worsening of her overall health. Her clinical evaluation demonstrated severe hypercalcemia (calcium 3.35 mmol/l). The very high values of her bone turnover markers (BTMs) had indicated a rebound effect due to denosumab discontinuation. She had an X-ray which revealed multiple new SVFs. Following injection of denosumab 60 mg, her serum calcium level rapidly decreased and her BTMs were dramatically reduced. The undertaking of a surgical approach by means of minimally invasive parathyroidectomy did allow for definite resolution of hyperparathyroidism and hypercalcemia. Camponovo et al. [43] concluded that their case had suggested that hypercalcemia could be a side consequence of denosumab discontinuation, which could become severe when other causes of hypercalcemia, such as primary hyperparathyroidism, are present.
Lamy et al. stated the following:
a) Denosumab inhibits resorption of bone, does increase bone mineral density, and does reduce fracture risk.
b) Denosumab had been approved for the treatment of osteoporosis and the prevention of bone loss with regard to some oncological situations.
c) Discontinuation of Denosumab is associated with a severe bone turnover rebound (BTR) and a rapid loss of bone mineral density.
d) The clinical consequences of the BTR observed after denosumab discontinuation are unknown.
Lamy et al. reported 9 women who had presented 50 reboundassociated vertebral fractures (RAVFs) following denosumab discontinuation. The undertaking of a broad biological and radiological assessment had excluded other causes than osteoporosis. Lamy et al. stated that these 9 cases were unusual and disturbing for several reasons including:
a) First, all vertebral fractures (VFs) were spontaneous fracture, and majority of the patients had a high number of VFs (mean = 5.5) in a short period of time.
b) Secondly, the fracture risk was low for majority of these women.
c) Thirdly, their VFs occurred rapidly following their last denosumab injection (9–16 months).
d) Fourth, vertebroplasty was found to be associated with a high number of new VFs.
e) All the observed VFs had seemed to be related to denosumab discontinuation and unlikely to the underlying osteoporosis or osteopenia.
f) They had postulated that the severe BTR is involved in microdamage accumulation in trabecular bone and thus promotes VFs.
Lamy et al. [44] made the following conclusions:
a) Studies are urgently needed in order to ascertain (1) the pathophysiological processes involved, (2) the clinical profile of patients at risk for RAVFs, and (3) the management and/or treatment regimens following denosumab discontinuation.
b) Health care authorities, physicians, and patients need to be aware of this RAVF risk.
c) Denosumab injections should be scrupulously undertaken every 6 months but not indefinitely.
Koldkjær Sølling et al. stated that Denosumab tends to be commonly used as an anti-resorptive agent for the treatment of osteoporosis and that following discontinuation of denosumab; nevertheless, bone resorption does tend to increase again, as well as the bone mass that is gained during the treatment tends to be rapidly declining. Koldkjær Sølling et al. reported a 67-year-old woman who had osteoporosis who was treated with denosumab 60 mg subcutaneously every 6 months from 2004 to 2014. She was reported to have received her last injection in May 2014. She had a routine serum biochemistry in November 2014 which showed increased s-ionized calcium (I-Ca) 1.64 mmol/L (1.18–1.32 mmol/L) and suppressed p-parathyroid hormone (PTH) 1.6 pmol/L (1.6–6.9 pmol/L). The patient was extensively examined; nevertheless, no underlying disease was found during her examination. In January 2015, the patient was stated to have commenced treatment with alendronat 70 mg weekly. In April 2015, her serum levels of type 1 collagen C-terminal cross-linked telopeptide, procollagen type 1 N-terminal propeptide and bone-specific alkaline phosphatase were found to be still markedly elevated. From then on, her I-Ca and PTH had normalized, and her bone turnover markers (BTM) had decreased.
Koldkjær Sølling et al. made the following conclusions:
a) In their reported case, they had described increased BTMs, and hypercalcemia associated with discontinuation of 10 years treatment with denosumab.
b) The increase in BTMs was assumed to be temporary and normalization was expected.
c) In view of the fact that denosumab is commonly utilized, there is an urgent need for evidence-based guidelines on discontinuation of long-term treatment, avoiding side effects and preserving anti-fracture efficacy.
Ma et al. stated the following:
a) The association between primary hyperparathyroidism (PHPT) and acute pancreatitis had rarely been reported.
b) They were describing the process of acute pancreatitis mediated PHPT which had been induced by hypercalcemia in a male patient.
c) Hypercalcemia induced by undiagnosed PHPT might be the causative factor in recurrent acute pancreatitis.
Ma et al. reported a case of hypercalcemia-induced acute pancreatitis which was caused by a functioning parathyroid adenoma in a 57-year-old man. The man had initially experienced a series of continuous gastrointestinal symptoms which included abdominal distension, abdominal pain, nausea, vomiting, electrolyte disturbance, renal dysfunction, and acute pancreatitis. In view of his prolonged hypercalcemia, the patient subsequently underwent surgical resection of his parathyroid adenoma. Two weeks pursuant to his surgery, his serum calcium, amylase, and lipase concentrations were recorded as normal. The patient had a good recovery after a series of other relevant treatments. Ma et al. [45] concluded that acute pancreatitis as the first presentation is an uncommon clinical symptom that is caused by PHPT-induced hypercalcemia. Marstrand, et al. reported a 47-year-old Caucasian man who had severe hypercalcemia, genetic FHH, and initially normal parathyroid scintigraphy and who was referred for endocrine evaluation due to him having non-specific symptoms. His biochemical evaluation showed revealed serum ionized calcium and PTH. His calcium–creatinine clearance ratio was low. The results of all his other biochemical measures were normal, including kidney function. His Genetic evaluation was re-undertaken and this confirmed FHH. He had a new parathyroid scintigraphy, which showed a significant single adenoma which had corresponded to his lower left gland. The patient underwent parathyroidectomy, and a parathyroid adenoma was excised. A reduced level of hypercalcemia was found to have persisted due to his FHH.
Marstrand, et al. [46] made the following conclusions:
a) The correct diagnosis of the underlying cause of hypercalcemia is important in order to ensure the right treatment.
b) Patients who have FHH should avoid operative treatment, and PHPT should be differentiated from MEN1 in order to ascertain whether surgery should include parathyroidectomy with removal of one adenoma or 3.5 hyperplastic parathyroid glands.
Królewicz et al. stated the following:
a) Hypercalcemia (HCM) tends to be caused predominantly primary hyperparathyroidism (PHPT) or malignancy.
b) The incidence of hypercalcaemia does vary from 0.17% to 4.74%.
c) The numerous manifestations of hypercalcaemia include renal symptoms.
Królewicz et al. undertook a study in order to assess the incidence and aetiology of hypercalcemia in patients who had been hospitalized at the Department of Nephrology of the Warsaw Military Institute, as well as to evaluate its impact upon renal function. Królewicz et al. undertook a cross-sectional study of patients who had been admitted into the Nephrology Department of the Warsaw Military Institute between January 2017 and December 2018 and who were retrospectively screened for presence of HCM, that was defined as total calcium level or corrected calcium level in case of hypoalbuminemia >10.2 mg/dl, that was measured at least twice. Each patient’s medical history as well as other laboratory test findings were subsequently analysed so as to establish the aetiology of the hypercalcemia.
Królewicz et al. summarized the results as follows:
a) Among 3062 hospitalisations of 1993 patients, within the Department, 96 patients were found to have had elevated serum calcium level of which 36 were identified as hypercalcaemic which amounted to 1,81% of the patients.
b) The median serum calcium level was 11.9 mg/dl (IQR: 11.25-13.46) with 22.24 mg/dl being the maximum observed value.
c) They had found that malignancy and medicaments having hypercalcaemizing effect were the commonest aetiologies that had been identified, both being found in 9 cases that amounted to 25% of cases.
d) Other causes of HCM had included sarcoidosis, multiple myeloma, which were analysed separately from other malignancies, PHPT and hypercalcaemic hypocalciuria.
e) In 7 cases, they could not establish the aetiology of the HCM aetiology, it was therefore, considered to have remained idiopathic.
f) Acute kidney injury (AKI) had developed in 20 patients that amounted to 56% of the cases, and in this group serum calcium levels were found to be significantly higher than in non-AKI patients (median: 12.85 mg/dl (IQR:11.82-14.65) versus 11.25 mg/dl (IQR:10.75-11.93); p=0.0039). Furthermore, chronic kidney disease (CKD) patients had manifested significantly lower serum calcium values in comparison with non-CKD patients (median: 11.47 mg/dl (IQR: 10.8-12.6) vs 13.01 mg/dl (IQR:11.9-16.08; p=0.0131).
Królewicz et al. [47] made the ensuing conclusions:
a) Hypercalcemia is a rare disorder among Nephrology Department patients, of which the primary aetiology is malignancy and medications having hypercalcaemizing effect.
b) Renal injury is dependent upon the severity of the hypercalcemia.
Bentata et al. stated the following:
a) The Covid-19 pandemic has had dramatic consequences on the progression of many pathologies, especially neoplastic pathologies.
b) The orientation of hospital activities toward the care of patients who have SARS-Cov2 infection had caused significant delays in the diagnosis and treatment of many other pathologies.
c) What about severe hypercalcemia? The aim of their reported study was to ascertain the clinical and biological manifestation, aetiologies, mortality, as well as the impact of the Covid-19 pandemic upon severe hypercalcemia.
Bentata et al. undertook a retrospective study for 84 months that spanned between September 2014 and September 2021, within the Nephrology Unit in University Hospital Mohammed VI, Oujda, Morocco. Included in the study were all adult patients who were diagnosed as having severe hypercalcemia which had been defined as corrected total serum calcium of >3.5 mmol/l or > 14.0 mg/dl) and who had benefited from one or more haemodialysis sessions. Bentata et al. summarized the results as follows:
a) 66 episodes of severe hypercalcemia were noted to have occurred in 64 patients.
b) The mean age of the patients was 57 ± 15 years and 57.6% were female.
c) The mean corrected serum calcium at the time of admission of the patients was 16.9 ± 2.1 mg/dl and 33.3% had more than 18.0 mg/dl.
d) Malignancies were recorded as to have represented 80.4% of all aetiologies.
e) Acute kidney injury was found in 69.7% of the patients.
f) The delta-drop in serum calcium 48 hours following commencement of medical treatment was 4.64 ± 1.63 mg /dl.
g) Mortality was noted in 14% of all cases.
h) Electrocardiographic (ECG) abnormalities were found in 58.3%, 87.5% and 85.7%, respectively, in group 1 (14.0-16.0 mg/dl), group 2 (16.1-18.0 mg/dl), and group 3 (> 18.0 mg/dl) (p = 0.04).
i) The mean serum potassium value was 5.1 ± 1.3, 4.0 ± 1.0, and 3.7 ± 0.7 respectively, in group 1 (14.0-16.0 mg/dl), group 2 (16.1-18.0 mg/dl), and group 3 (> 18.0 mg/dl) (p < 0.001). Newly diagnosed neoplasia, severe hypercalcemia (> 16.0 mg/ dl), and mortality have been observed in 15.4% vs. 23.7% (p = 0.31), 25% vs. 50% (p = 0.03), and 35.7% vs. 52.6% (p = 0.13) respectively, in the patients before and during the Covid-19 pandemic.
Benata et al. [48] concluded that the Covid-19 pandemic had caused an increase in both the incidence and severity of hypercalcemia and the haemodialysis which was practiced in this context had remained efficient and safe.
Conclusions And General Summary
Some summations that had been documented related to hypercalcaemia include the following:
a) Hypercalcaemia is a common clinical condition which can afflict patients of all ages both male and female [49].
b) Hypercalcaemia is a common disorder which has tended to be normally caused by primary hyperparathyroidism (PHPT) or malignancy.
c) A proportion of cases of hypercalcaemia manifests as an emergency, which has tended to be associated with a significant mortality.
d) Emergency management of hypercalcaemia has tended to be based upon utilization of intravenous rehydration with normal saline but when this form of treatment is found not to be adequate, bisphosphonate therapy tends to be utilized used; over recent years the novel anti-resorbtive agent denosumab has been demonstrated provide a useful role in the treatment of hypercalcemia.
e) It has been estimated that up to 10% of all cases of PHPT manifesting under the age of 45 years do tend to have an underlying genetic predisposition; nine potentially causative genes are now recognized, and these may be screened during routine clinical practice.
f) Although parathyroidectomy is the only curative treatment for PHPT, parathyroidectomy has tended to be indicated in a minority of cases.
g) Many cases of hypercalcaemia can be adequately and effectively managed conservatively and guidance from the 4th international workshop on the management of asymptomatic PHPT has recently been updated in a consensus statement.
h) Hypercalcaemia does account for about 0.6% of all acute medical admissions.
i) Presence of hypercalcaemia the general population is up to 1/1,000. Hypercalcaemia is most commonly caused by primary hyperparathyroidism or malignancy although there are many other causes.
j) The classical symptomatic manifestation of hypercalcaemia is seen relatively rarely within the developed world, the commonest manifestation of hypercalcaemia is the asymptomatic detection of raised levels of serum calcium upon biochemical testing.
k) The management of hypercalcaemia has tended to depend upon the manifestation as well as upon the underlying cause; additionally, severe hypercalcaemia with levels of serum calcium higher than 3.5 mmol/L does necessitate emergency management, [50] and a significant proportion of cases of asymptomatic mild hypercalcaemia has tended to be due to primary hyperparathyroidism (PHPT) and a number of cases of mild asymptomatic hypercalcaemia has tended to be amenable to conservative management.
Key Points
a) Nine potentially causative genes have recently been implicated in primary hyperparathyroidism (PHPT).
b) Guidance upon the management of asymptomatic PHPT was recently updated in the 4th international consensus guidelines.
c) Cinacalcet has been licensed for PHPT and its role is recognised in the 4th international consensus guidelines.
d) Denosumab is understood to have a role in the emergency management of hypercalcaemia.
e) Pre-operative parathyroid imaging is increasingly being undertaken by utilization of SPECT CT Scan.
Additionally, it is worth taking note of the fact that ectopic production of calcium that would lead to hypercalcaemia may emanate from ectopic lesions that do not involve the parathyroid gland, for example the pancreas, as well as lesions involving other organs of the body.
Conflict of interest
None.
References
- Sadiq NM, Naganathan S, Badireddy M (2022) Hypercalcemia. StatPearls Publishing.
- Goltzman D, Feingold KR, Anawalt B, Boyce A, Chrousos G, et al. (2019) Approach to Hypercalcemia. Endotext.
- Minisola S, Pepe J, Piemonte S, Cipriani C (2015) The diagnosis and management of hypercalcaemia. BMJ 350: h2723.
- Palmér M, Jakobsson S, Akerström G, Ljunghall S (1988) Prevalence of hypercalcaemia in a health survey: a 14-year follow-up study of serum calcium values. Eur J Clin Invest 18(1): 39-46.
- Gastanaga VM, Schwartzberg LS, Jain RK, Pirolli M, Quach D, et al. (2016) Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med 5(8): 2091-2100.
- Balasubramanian P, Majumdar S K (2021) Albumin-corrected calcium and the prevalence and categories of hypercalcemia in hospitalized patients with 1-year follow-up of undiagnosed cases. Endocr Pract 27(4): 279-285.
- Dent DM, Miller JL, Klaff L, Barron J (1987) The incidence and causes of hypercalcaemia. Postgrad Med J 63(743): 745-750.
- WALSER M (1961) Ion association. VI. Interactions between calcium, magnesium, inorganic phosphate, citrate and protein in normal human plasma. J Clin Invest 40(4): 723-7 30.
- Hoyoux C, Lombet J, Nicolescu CR (2017) Malignancy-Induced Hypercalcemia-Diagnostic Challenges. Front Pediatr 5: 233.
- Wood L, Jacobs P (1989) Myeloma--the integral role played by the professional nurse. Curationis 12(3-4): 67-71.
- Curtin M, Piggott RP, Murphy EP, Munigangaiah S, Baker JF, et al. (2017) Spinal Metastatic Disease: A Review of the Role of the Multidisciplinary Team. Orthop Surg 9(2): 145-151.
- Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B (2017) Bisphosphonates in multiple myeloma: an updated network meta-analysis. Cochrane Database Syst Rev 12(12): CD003188.
- Reid L, Muthukrishnan B, Patel D, Crane M, Akyol M, et al. (2018) Presentation, diagnostic assessment and surgical outcomes in primary hyperparathyroidism: a single centre's experience. Endocr Connect 7(10): 1105–1115.
- Hicks MJ, Levy M L, Alexander J, Flaitz C M (1993) Subcutaneous Fat Necrosis of the Newborn and Hypercalcemia: Case Report and Review of the literature. Paediatric Dermatol 10(3): 271-276.
- Rey E, Jacob CE, Koolian M, Morin F (2016) Hypercalcemia in pregnancy - a multifaceted challenge: case reports and literature review. Clin Case Rep 4(10): 1001-1008.
- Ragavan VV, Smith JE, Bilezikian JP (1982) Vitamin A toxicity and hypercalcemia. Am J Med Sci 283(3): 161-164.
- Cappellani D, Brancatella A, Kaufmann M, Minucci A, Vignali E, et al. (2019) Hereditary Hypercalcemia Caused by a Homozygous Pathogenic Variant in the CYP24A1 Gene: A Case Report and Review of the Literature. Case Rep Endocrinol 2019: 4982621.
- De Paolis E, Minucci A, De Bonis M, Scaglione GL, Gervasoni J, et al. (2018) A rapid screening of a recurrent CYP24A1 pathogenic variant opens the way to molecular testing for Idiopathic Infantile Hypercalcemia (IIH). Clin Chim Acta 482: 8-13.
- Ferraro PM, Minucci A, Primiano A, De Paolis E, Gervasoni J, et al. (2017) A novel CYP24A1 genotype associated to a clinical picture of hypercalcemia, nephrolithiasis and low bone mass. Urolithiasis 45(3): 291-294.
- Kaufmann M, J. Gallagher J C, Peacock M, Schlingmann M K, Konrad M, et al. (2014) Clinical Utility of Simultaneous Quantitation of 25-Hydroxyvitamin D and 24,25-Dihydroxyvitamin D by LC-MS/MS Involving Derivatization With DMEQ-TAD. J Clin Endocrinol Metab 99(7): 2567-2574.
- Kaufmann M, Morse N, Molloy BJ, Cooper DP, Schlingmann KP, et al. (2017) Improved screening test for idiopathic infantile hypercalcemia confirms residual levels of serum 24, 25‐(OH) 2D3 in affected patients. J Bone Miner Res 32(7): 1589-1596.
- Guerra V, Vieira Neto OM, Laurindo AF, Paula FJ, Moysés Neto M (2016) Hypercalcemia and renal function impairment associated with vitamin D toxicity: case report. J Bras Nefrol 38(4): 466-469.
- Pilz S, Theiler-Schwetz V, Pludowski P, Zelzer S, Meinitzer A, et al. (2022) Hypercalcemia in Pregnancy Due to CYP24A1 Mutations: Case Report and Review of the Literature. 14(12): 2518.
- Malekar-Raikar S, Sinnott BP (2011) Primary hyperparathyroidism in pregnancy-a rare cause of life-threatening hypercalcemia: case report and literature review. Case Rep Endocrinol 2011: 520516.
- Roux S, Massicotte MH, Huot Daneault A, Brazeau-Lamontagne L, Dufresne J (2019) Acute hypercalcemia and excessive bone resorption following anti-RANKL withdrawal: Case report and brief literature review. Bone 120: 482-486.
- Nakajima K, Tamai M, Okaniwa S, Nakamura Y, Kobayashi M, et al. (2013) Humoral hypercalcemia associated with gastric carcinoma secreting parathyroid hormone: a case report and review of the literature. Endocr J 60(5): 557-562.
- Eller-Vainicher C, Ossola MW, Beck-Peccoz P, Chiodini I (2012) PTHrP-associated hypercalcemia of pregnancy resolved after delivery: a case report. Eur J Endocrinol 166(4): 753-756.
- Newman NB, Jabbour SK, Hon JD, Berman JJ, Malik D, et al. (2015) Hepatocellular Carcinoma Without Cirrhosis Presenting With Hypercalcemia: Case Report and Literature Review. J Clin Exp Hepatol 5(2): 163-166.
- Stefanko NS, Drolet BA (2019) Subcutaneous fat necrosis of the newborn and associated hypercalcemia: A systematic review of the literature. Pediatr Dermatol 36(1): 24-30.
- Shimonodan H, Nagayama J, Nagatoshi Y, Hatanaka M, Takada A, et al. (2005) Acute lymphocytic leukemia in adolescence with multiple osteolytic lesions and hypercalcemia mediated by lymphoblast‐producing parathyroid hormone‐related peptide: A case report and review of the literature. Pediatr Blood Cancer 45(3): 333-339.
- Koldkjær Sølling AS, Harsløf T, Kaal A, Rejnmark L, Langdahl B (2016) Hypercalcemia after discontinuation of long-term denosumab treatment. Osteoporos Int 27(7): 2383-2386.
- Garbim BB, D Ávila L, Rigatto SZP, Quadros KRDS, Belangero VMS, et al. (2017) Hypercalcemia in children: three cases report with unusual clinical presentations. J Bras Nefrol 39(2): 213-216.
- Marx SJ (2017) Calcimimetic Use in Familial Hypocalciuric Hypercalcemia-A Perspective in Endocrinology. J Clin Endocrinol Metab 102(11): 3933-3936.
- Visnyei K, Samuel M, Heacock L, Cortes JA (2014) Hypercalcemia in a male-to-female transgender patient after body contouring injections: a case report. J Med Case Rep 8: 71.
- Ghaznavi S A, Saad N M, Donovan L E (2016) The Biochemical Profile of Familial Hypocalciuric Hypercalcemia and Primary Hyperparathyroidism during Pregnancy and Lactation: Two Case Reports and Review of the Literature. Case Rep Endocrinol 2016: 2725486.
- Cengiz C, Rodriguez-Davalos M, deBoccardo G, Fiel MI, Rodriguez-Laiz G, et al. (2005) Recurrent hepatic sarcoidosis post-liver transplantation manifesting with severe hypercalcemia: a case report and review of the literature. Liver Transpl 11(12): 1611-1614.
- Sternlicht H, Glezerman IG (2015) Hypercalcemia of malignancy and new treatment options. Ther Clin Risk Manag 11: 1779-1788.
- Doyle MA, Malcolm JC (2013) An unusual case of malignancy-related hypercalcemia. Int J Gen Med 7: 21-27.
- Mishra A, Newman D (2014) An Interesting Case of Life-Threatening Hypercalcemia Secondary to Atypical Parathyroid Adenoma versus Parathyroid Carcinoma. Case Rep Med 2014: 473814.
- Hygum K, Wulff CN, Harsløf T, Boysen AK, Rossen PB, et al. (2015) Hypercalcemia in metastatic GIST caused by systemic elevated calcitriol: a case report and review of the literature. BMC Cancer 15: 788.
- Nemr S, Alluri S, Sundaramurthy D, Landry D, Braden G (2017) Hypercalcemia in Lung Cancer due to Simultaneously Elevated PTHrP and Ectopic Calcitriol Production: First Case Report. Case Rep Oncol Med 2017: 2583217.
- Abdullayev T, Korkmaz M, Kul M, Koray N (2020) A rare cause of neonatal hypercalcemia: Neonatal severe primary hyperparathyroidism: A case report and review of the literature. Int J Surg Case Rep 66: 365-369.
- Camponovo C, Aubry-Rozier B, Lamy O, Rodriguez E (2020) Hypercalcemia upon denosumab withdrawal in primary hyperparathyroidism: a case report and literature review. Osteoporos Int 31(12): 2485-2491.
- Olivier Lamy, Elena Gonzalez-Rodriguez, Delphine Stoll, Didier Hans, Bérengère Aubry-Rozier (2017) Severe Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: 9 Clinical Cases Report. J Clin Endocrinol Metab 102(2): 354-358.
- Ma YB, Hu J, Duan YF (2019) Acute pancreatitis connected with hypercalcemia crisis in hyperparathyroidism: A case report. World J Clin Cases 7(16): 2367-2373.
- Marstrand SD, Tofteng CL, Jarløv A, Borgwardt L, Schwarz P (2021) Concomitant familial hypocalciuric hypercalcemia and single parathyroid adenoma: a case report. J Med Case Rep 15(1): 471.
- Królewicz K, Steć Z, Niemczyk S (2021) Hypercalcemia in the nephrology department patients - incidence, etiology and impact on renal function. Pol Merkur Lekarski 49(289): 9-12.
- Bentata Y, Benabdelhak M, Haddiya I, Oulali N, Housni B (2022) Severe hypercalcemia requiring acute hemodialysis: A retrospective cohort study with increased incidence during the Covid-19 pandemic. Am J Emerg Med 51: 374-377.
- Turner J O (2017) Hypercalcaemia – presentation and management. Clin Med (London) 17(3): 270-273.
- Walsh J, Gittoes N, Selby P, Society for Endocrinology Clinical Committee (2016) SOCIETY FOR ENDOCRINOLOGY ENDOCRINE EMERGENCY GUIDANCE: Emergency management of acute hypercalcaemia in adult patients. Endocr Connect 5(5): G9-G11.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...