Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Research Article(ISSN: 2641-1687) 
Case of External Genitalia Abnormalities in A Patient with Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency with 46, XX Karyotype and Male Gender Identification Volume 2 - Issue 3
Serebryakova Inna P1*, Galakhova Ravilya K1, Vorokhobina Natalia V2, Kuznetsova Alla V1, Bashnina Elena B2, Baranov Vitalii L2, Maksiutova Indira R3 and Guseva Polina S4
- 1Endocrinology Department, North West State Medical University, Russia
- 2Chief of Endocrinology Department, North West State Medical University, Russia
- 3Endocrinologist of Polyclinic Department, Bashkiria’s Republican Clinical Hospital, Russia
- 4Chief of Endocrinology Department, Bashkiria’s Republican Clinical Hospital, Russia
Received: August 19, 2019; Published: November 15, 2019
Corresponding author: Serebryakova IP, PhD, Associate of Professor, Endocrinology Department, North West state medical university named after I.I. Mechnikov, Russia
DOI: 10.32474/JUNS.2019.02.000137
Abstract
The article describes a clinical case of a patient, phenotypically male, with male gender identity and male type of external genitalia, but two-sided cryptorchidism, who first time seek medical attention due to urological complaints in adulthood. During the diagnostics, it was found that he has internal female reproductive organs, hypospadia of the urethra and karyotype 46, XX. Hormonal and molecular genetic analysis allowed to diagnose the virile form of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Late diagnostics and therapy initiation of 21-hydroxylase deficiency led to the formation of adrenal tumors, stunting, long-existing urological problems.
Keywords: Сongenital adrenal hyperplasia; 21-hydroxylase; external genitalia virilization; hypospadias of the urethra; cryptorchidism
Introduction
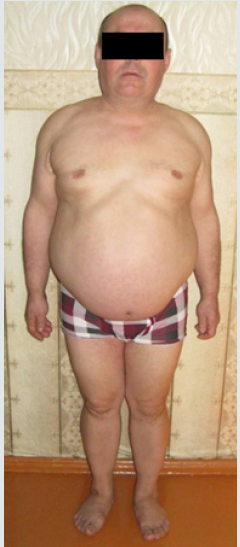
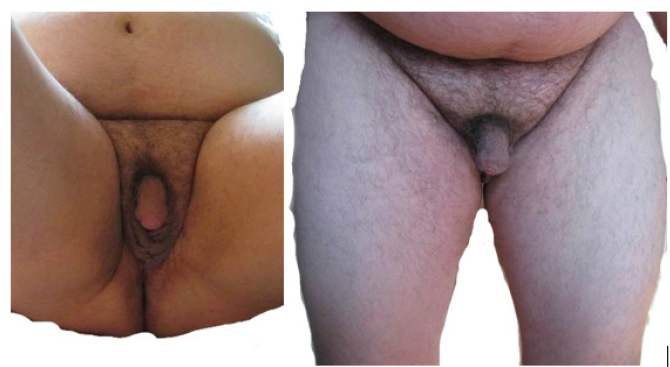
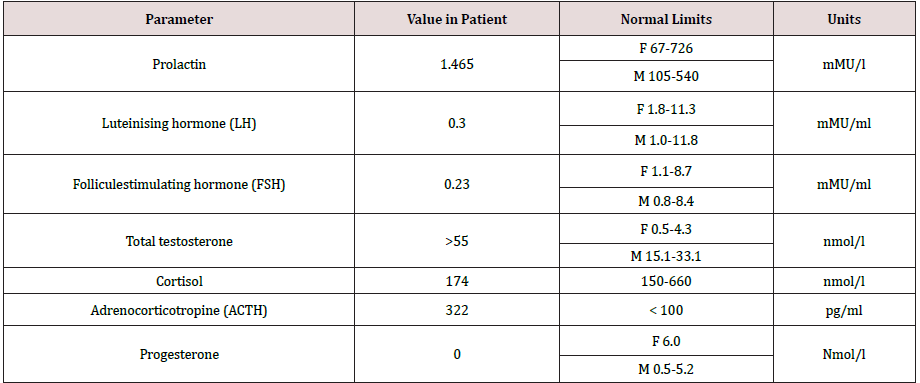
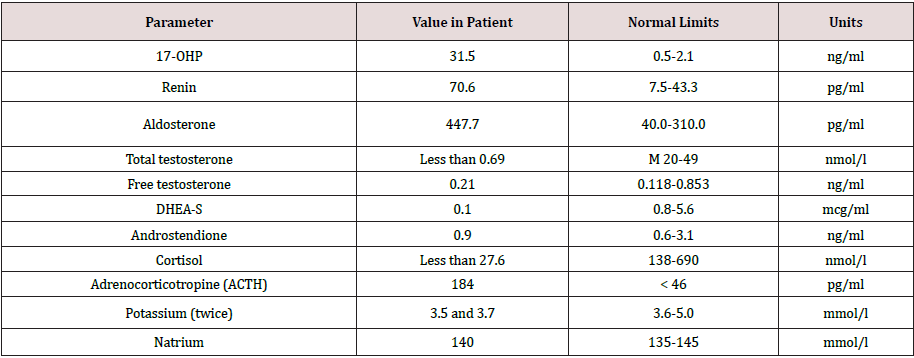
Congenital adrenal hyperplasia (CAH) is a group of disorders with an autosomal recessive type of inheritance, which is based on a defect of adrenal cortex enzymes involved in the cortisol biosynthesis. Deficiency of 21-hydroxylase (CYP21) lead to CAH in 95% cases. The prevalence of classical CAH due to CYP 21 deficiency varies in different populations from 1:5000 to 1:23000 newborns. Salt-wasting form of the disease is usually diagnosed in infancy, in connection with the development of adrenal insufficiency crisis which occures soon after birth and caused by severe deficiency or totally absence of cortisol and aldosterone [1,2]. The onset of simple virilising form of CAH occures, as a rule, at the age of 2-6 years and expressed by symptoms of premature puberty and premature growth of bone age. Chronic adrenal insufficiency symptoms, such as hyperpigmentation of the skin, muscle weakness, hypotension, poor exercise tolerance, fatigue, weakness, hypoglycemia, often masked by the anabolic action of androgen excess and not manifested in children. Moreover, due to intrauterine androgen hyperproduction, newborn girls have external genitalia virilisation. In some patient’s full phenotypical masculinization occures. Such patients often regarded as boys, and only premature puberty or the adrenal insufficiency crisis contributes to the further diagnostics of CAH. However, in medical articles describes a lot of clinical cases, when timely diagnostics of simple virile form was carried out in an adult person due to infertility, urogenital tract problems, occasionally detected tumors in the adrenal glands [3]. Biochemical marker of 21-hydroxylase deficiency is highly increased serum level of 17-hydroxyprogesteron. In routine practice mandatory screening of newborns for 21-hydroxylase deficiency by 17-OHP detection on 3-5-th day of life, allows to determine diagnosis of CAH in early period and prevent the development of many complications such as premature puberty, progressive virilisation, adrenal tumors and gender dysphoria [1]. But some patients remain undiagnosed for a long time. This clinical case was observed by our team. Materials and methods. Observation. Patient A, the male, 49 years. Was hospitalized for examination and therapy scheme modification in Adrenal Diseases Clinic of North-West State Medical University in January 2019. From anamnesis it is known that he was born the 7-th child in a large family, from which four children died in infant. At birth, two-sided cryptorchism was established, but further diagnostics was not performed. Until the age of 6-7 years development was according to age as patient informed us. At the age of 6-7 years during planned examination for testicles bringing down surgery the absence of testicles in the abdominal cavity was revealed, but the uterus was found. Up to 37 years, the patient’s history has not been documented, but according to the patient, he was not regularly observed by doctors. He graduated from school, then from institute, then worked as a teacher at school. He lives in a small village in Bashortostan. Never married. In 2006 (37 years old) he complained of difficulty urinating and was examined by urologist. On the basis of a physical examination, posterior urethritis was established. During ultrasound pelvic examination posterior to the urinary bladder liquid structure of irregular shape and thick wall 54*32*28 mm was revealed, filled with movable liquid content (Figures 1 & 2). Any additional pathological structures, including testicles, were not found in the pelvic cavity. To clarify the nature of unknown mass CT-tomography and MRI of the pelvis were conducted. According to CT examination on the right side and slightly below the urine bladder, at the level of the seminal vesicles, the volume formation of soft tissue density with clear contours, but an inhomogeneous nature due to the hypodens zone in the center is determined. Both adrenal glands are significantly enlarged due to the thickening of the adrenal body and both legs, the structure and shape are not changed. By MRI clarified the nature of formation, is was the uterus with an enlarged cavity filled with content and communicating with the prostatic part of the urethra. Laboratory examination from 2006 revealed some changes (Table 1), but 17- OHP level was not determined. Molecular genetic analysis of CYP21 was carried out: R356W and Int2spl mutations in heterozygous state were revealed. Karyotyping was tested – 46, XX. To exclude hypophysis adenoma MRI investigation was done, no pathologies were revealed. Thus, CAH due to 21-hydroxylase deficiency was diagnosed and dexamethasone intake to patient was recommended. Also rear optical urethrotomy was performed for correction of urethra stricture. We were unable to obtain documented evidence of the patient’s follow-up between 2006 and 2015. In 2015 he was re-operated by urologysts due to recurrence structure of bulbus part of the urethra. Plastic of glandular urethra by Blandy was performed. According to the patient, since 2018 he began dexamethasone intake in daily dose of 2mg. Physical examination. The patient is phenotypically male (body type male, hair growth in androgen-dependent areas, android allopecia) with male gender identity. He has short stature (145 sm) and overweight (79 kg) with the distribution of subcutaneous fat by Android type, BMI is 37,6 kg/m2 (Figure 3). According to the organs and systems by the physical study clinically significant pathological changes were not revealed. The external genitalias are formed according to the male type, the testicles in the scrotum are absent (Figure 4). Dates of hormonal evaluation presented in Table 2. Computer tomography of abdomen results: adrenal glands are enlarged, have uneven bumpy contours. On the border of the body and legs of the right adrenal gland is determined formation with clear smooth contours, 20 mm in diameter, +13+24 HU, accumulating contrast in the arterial phase to +90+100 HU, in parenchymal phase +80+90 HU, in delayed phase +36+40 HU (leaching coefficient – 65%). A similar formation 13*15 mm is defined in the body of the left adrenal gland. Pelvic MRI- views shown at Figure 5. Thus, the final clinical diagnosis was: Congenital adrenal hyperplasia due to 21-hydroxylase deficiency, virilising form, decompensation. Complications: Virilising external genitalia (stage V by Prader scale). Formations of both adrenals. Concomitant: Obesity (BMI-37.6 kg/m2). In conclusion, the scheme of medications intake was changed: dexamethasone changed on prednisolone 10 mg daily (5 mg twice a day), mineralocorticoid Cortineff was added in daily dose of 50 mcg at the morning. Also, hypocaloric diet and regular physical activity were recommended for weight loss. Upon reaching the compensation of hormonal disorders, the patient should be sent to a specialized urological hospital for surgical treatment, which should include removal of the uterus and ovaries, bladder plasty, implantation of testicular prostheses of the scrotum.
Figure 1: Ultrasound pelvic examination of patient A. (2006). Behind the bladder, a structure with thick walls and liquid contents is visualized.
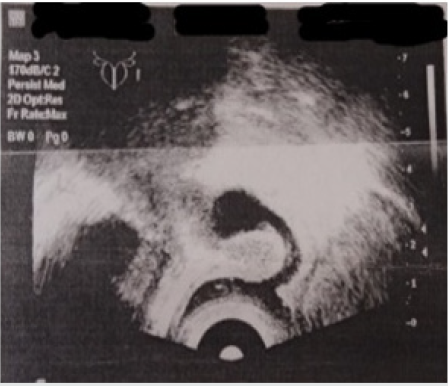
Figure 2: Ultrasound pelvic examination of patient A. (2006). Registration of the rhythm of liquid content movement of the formation.
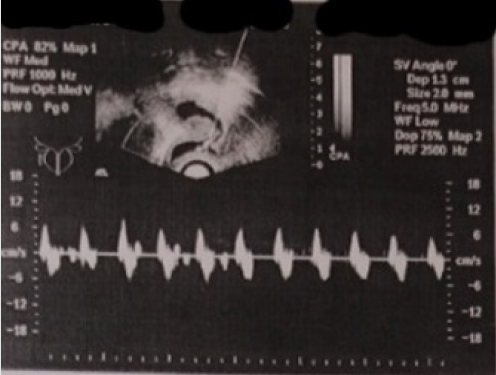
Table 2: Hormones and electrolytes evaluation of patient A. while taking dexamethasone 2 mg/daily (from 2019).
Discussion
The clinical case presented in this article shows that patients with late diagnosed CAH still occur in clinical practice. Most often, the reason for the diagnosis are randomly detected formations in the adrenal glands. The problem of late diagnosis of CAH is also relevant because irreversible changes are formed, such as stunting, severe forms of virilization, urological problems, adrenal tumors. Psychological problems associated with gender dysphoria are also a very serious issue, especially when the patient is faced with it in adult age [3,4]. In order to avoid such cases as much as possible, mandatory screening of newborns for early diagnosis of classical forms of 21-hydroxylase insufficiency was conducted. In the Russian Federation it has been carried out since 2006. The results of newborn screening in Russia showed significant variability in the frequency of classical forms of CAH, from 1:5.363 in the southern Federal district to 1:14.308 in the North-Western Federal district [5]. However, among people born before the introduction of total newborn screening, patients with late diagnosed cases of СAH will occur more often than in the future. In patient anamnesis was some highlights which suggests the disease, and to initiate a survey: the high mortality of children in infancy of the patient’s parents and bilateral cryptorchidism in newborn with phenotypic male genitalia. Especially the combination of cryptorchidism with hypospadias requires mandatory karyotyping and ultrasound of the pelvic organs [1,2]. In this case the age of primary diagnosis was at 6-7 years. However, for now the cause of hermaphroditism has not been definitively established by unknown various reasons. A very important aspect in the diagnostics of 21-hydroxylase deficiency is the molecular genetic analysis of the CYP21 gene [1,3]. Two frequently occurring mutations were identified in the patient’s genotype: R356W and Int2spl. It is known that both mutations are severe mutations leading to a significant loss of activity CYP21, presence of this mutation in homozygous position leads to the development of salt-losing forms of 21-hydroxylase insufficiency in 85-100% cases. Nevertheless, the patient did not have crises during his life, the history of the disease reflects the mismatch of “genotypephenotype”, although a marked increase in renin and aldosterone reflect a hidden mineralocorticoid insufficiency. These changes make prescribe not only suppressive therapy with glucocorticoids, but also replacement therapy with mineralocorticoids [2,4]. Achieving compensation of hormonal disorders will prepare the patient for a serious urological surgery in the near future.
References
- Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, et al. (2010) Congenital Adrenal Hyperplasia Due to Steroid 21-hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline.Journal of Clinical Endocrinology & Metabolism 95(9):4133-
- Grandfather II, Melnichenko GA (2016) Federal clinical recommendations (draft): Diagnostics and treatment and preventive measures for congenital dysfunction of the adrenal cortex in patients aged 28s.
- Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP, et al. (2000) Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, welldefined patients from Southern Germany. J Clin Endocrinol Metab85(3):1059-1065.
- Sazonova AI (2013) Somatic status and metabolic disorders in adult patients with various forms of VCD: dis. Cand. honey. sciences. M.
- Novikov VP (2012) The first results of expanded neonatal screening for hereditary metabolic diseases in the Russian Federation. Russian Bulletin Perinatol. Pediatrician5: 5-12.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...