
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1687
Case report(ISSN: 2641-1687) 
Case Report: Metastatic Ureteral Melanoma Volume 4 - Issue 4
Nguyen Myky1, EL Kaddouri Hamid1 and Chatzopoulos Charles2*
- 1Resident of Department of Urology CHIREC site Delta, Université Libre de Bruxelles, Belgium
- 2Chief of Department of Urology CHIREC site Delta, Université Libre de Bruxelles, Belgium
Received:October 27, 2023; Published:November 01, 2023
Corresponding author:Chatzopoulos Charles, Chief of department of Urology CHIREC site Delta, Université Libre de Bruxelles, Belgium
DOI: 10.32474/JUNS.2023.04.000198
Abstract
We are describing here a rare case of ureteral melanoma in a 70yo white male with previous history of cutaneous melanoma, which confirmed the secondary origin of the tumor. Ureterorenoscopy revealed a solid mass adherent to the distal ureteral wall. We performed a partial ureterectomy with ureteral reimplantation and psoas-hitch robot-assisted. There was no evidence of the disease at the surgical intervention. Even though metastatic melanoma of the urinary system is exceptional, early detection allows an appropriate management of the disease.
Keywords:Ureteral melanoma; melanoma; urinary tract obstruction symptoms; lymphadenectomy
Introduction
Melanoma is the most common and most serious form of skin cancer. Mucosal melanomas are a very rare entity and have different features compared to cutaneous melanomas. They carry a worse prognosis than cutaneous melanomas. The most common sites of primary mucosal melanomas are head and neck, anorectal, and vulvovaginal regions. The urinary tract (UT) is a rarer site of origin and accounts for less than 4% of all mucosal melanomas. The urethra is the most common site followed by the bladder and at last the ureter [1–3]. Whereas metastatic locations of melanomas are most frequently localized in the lungs, liver, brain, and bones. Even though metastatic spread of melanoma to the urinary system is rare. UT melanomas are most certainly secondary to metastatic spread from a primary lesion originating from the skin conventionally by lymphatic circulation [4,5].
The optimal management of UT melanomas is limited, because of its rarity and the challenging clinical semiology bound by its anatomical location. Patients are usually asymptomatic, have microor macroscopic hematuria or upper urinary tract obstruction symptoms. The risk factors, etiology and physiopathology of UT melanomas are not well-known. And it is difficult to distinguish from other poorly differentiated tumors. BRAF mutations are uncommon in mucosal melanomas. Treatment for UT melanomas are generally based on wide resection surgery and lymphadenectomy followed by adjuvant chemotherapy or immunotherapy [6,7]. We present a case of malignant melanoma in the ureter. We were not able to determine if it was.
Case report
Our patient (M.T.) is a white male of 70 years old presenting hematuria and renal colic for which he went to the emergency department. Lab results came back with lightly altered renal function and hematuria. An abdominal CT with contrast demonstrated a lesion of 2 cm long of the left ureter at the height of L5 (Figures 1-3) and ureterohydronephrosis. A double J ureteral stent was initially placed. An ureterorenoscopy was performed and demonstrated a non-pigmented and non-necrotic mass adherent to the ureteral wall like a polypoid mass of benign aspect. A retrograde ureteropyelography demonstrated a lesion of 2 cm long (Figure 3). There was no evidence of bladder lesion. The issue was favorable with a normalization of the serum level of creatinine and CRP. In front of the benign aspect of the mass, we decided to have a conservative approach and opted for a segmentary ureterectomy. Two weeks later, the patient underwent a robotic segmentary ureterectomy, ureteral reimplantation and Psoas-Hitch robot-assisted (Figure 4) due to the loss of the distal ureter and to perform the reimplantation, a psoas-Hitch was necessary. The histopathological anatomy revealed a ureteral melanoma with a thickness of 8 mm, R0 resection (Figure 5).
Figure 2: Arrow showing the obstruction of the ureteral due to the mass. The contrast doesn’t fill up the whole ureter.

Figure 3: Retrograde ureteropyelography demonstrating the absence of contrast where the lesion is nested.

Figure 4: Image from the Da Vinci Xi robot. visualization of the distal ureter. We can guess the localization of the lesion.
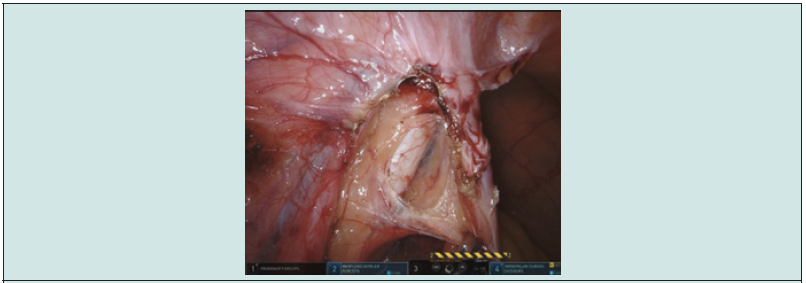
The pathology showed evidence of ulceration, a mitotic count of 8/mm2 and the subsequent gene testing demonstrated the tumor to be BRAF negative. No suspicious lymph nodes were found. The definitive diagnosis of melanoma was a surprise. Afterwards the patient mentioned an excision of melanoma of the back more than 10 years ago. A complementary PET-FDG and cerebral magnetic resonance indicated a suspicious hypermetabolic pulmonary nodule and a focal lesion of the small gastric curvature, but no cerebral lesion. A gastric biopsy was performed and confirmed a secondary invasion of the fundic mucosa.
The pulmonary nodule could not be sampled due to its location. A thoracal scanner the primitive (melanoma of the mucosa) or secondary origin (metastasis) of the tumor could not be confirmed on the pathological analysis. But due to his previous history of cutaneous melanoma on the back, a secondary origin is more probable. Our patient was initially diagnosed with a cutaneous melanoma of the back followed by excision 10 years ago. The initial histopathological analysis could not be retrieved. After discussion with the oncologists and dermatologists the patient received double immunotherapy: Nivolumab et Ipilimumab. Our patient is still alive and well.
Discussion
Melanomas of the upper urinary tract are extremely rare and are clinically very challenging. These are often discovered post-mortem [8]. The identification of melanomas by pathological analysis in the upper urinary tract is important for the clinician and the patient. The initial diagnosis includes CT imaging, endoscopic evaluation, and biopsy if possible. There are no established algorithms for the treatment of ureteral melanomas. Gakis and al. proposed an algorithm based on wide resection of the lesion if possible, with lymphadenectomy, chemotherapy, immunotherapy and radiation [6]. We couldn’t perform a biopsy during ureteroscopy, we therefore did not have an evidence-based approach. We had no evidence of the diagnostic at the time of the intervention. Still, there is reason to be optimistic concerning the long-term prognosis with the development and use of immunotherapy. That’s why the treatment of locoregional metastases should be performed with renal preservation if possible [7]. The segmentary ureterectomy with ureteral reimplantation and psoas-hitch was robot-assisted. This approach has advantages on oncological perspectives (wide margins), as to have less anastomotic leakage and ureteral stricture compared to an end-to-end anastomosis. Also, a segmentary ureterectomy allows the preservation of the kidney and quality of life of the patient. What’s more, the robotic laparoscopic approach permitted easier access to the lesion situated at the level of L5 vertebrae on the CT imaging. From an oncological perspective, at the time of the surgery, the malignant origin of the lesion was not known. Dispersion of metastatic lesions at other localizations was unknown at the time and the patient was clinically well enough to undergo the diagnostic and therapeutic surgery.
Conclusion
Malignant melanoma of the urinary tract is exceptionally a primary disease, it is mostly a metastasis of cutaneous melanoma localized in the urinary tract [8]. We believe that wide resection with organ preservation is a valid therapeutic option for similar cases.
References
- Richard D Carvajal, Omid Hamid, Charlotte Ariyan (2023) Locoregional mucosal melanoma Epidemiology, clinical diagnosis, and treatment.
- Acikalin A, Bagir E, Karim S, Bisgin A, Izol V, et al. (2020) Primary melanoma of the urinary tract; Clinicopathologic and molecular review of a case series. Pathol Res Pract 216(9): 153095.
- Cazzato G, Colagrande A, Cimmino A, Caporusso C, Candance PMV, et al. (2021) Urological Melanoma: A Comprehensive Review of a Rare Subclass of Mucosal Melanoma with Emphasis on Differential Diagnosis and Therapeutic Approaches. Cancers 13(17): 4424.
- Broderick Sutton, Robert Chan, Mark Sutton, Timothy Boone (2013) Primary Malignant Melanoma of the Genitourinary Tract with Upper and Lower Tracts Involvement. Case Rep Urol 2013: 217254.
- Lebacle, Géraldine Pignot, C. Mateus, Pierre Bigot, Laurence Rocher, et al. (2012) Mélanome métastatique aux voies excrétrices supérieures. À propos de trois cas et revue de la littérature. Progrès en Urologie 22(12): 736-739.
- Gakis G, Merseburger AS, Sotlar K, A Kuczyk M, Sievert KD, et al. (2009) Metastasis of malignant melanoma in the ureter: Possible algorithms for a therapeutic approach. Int J Urol 16(4): 407-409.
- MacNeil J, Hossack TA (2016) Case of Metastatic Melanoma in the Ureter. Case Rep Urol 2016: 1853015.
- Stein BS, Kendall AR (1984) Malignant Melanoma of the Genitourinary Tract. J Urol 132(5): 859-868.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...






