Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1373
Research ArticleOpen Access
Assessing The Risk of Transfer of a Transdermal Hormonal Drug Through Skin-To-Skin Contact by an In Vitro Permeation Testing Method Volume 3 - Issue 1
Nahid S Kamal1*, Rokon Uz Zaman1, Behnam Dasht Bozorg1, Sarah Ibrahim2, Ahmed S Zidan1 and Muhammad Ashraf1*
- 1Division of Product Quality Research, Office of Testing and Research, Office of Pharmaceutical Quality, Center for Drug Evaluation and Research, Food and Drug Administration, MD, USA
- 2Office of Generic Drug, Center for Drug Evaluation and Research, Food and Drug Administration, MD, USA
Received: August 24, 2022; Published:September 06, 2022
Corresponding author: Nahid S. Kamal and Muhammad Ashraf, DPQR/OTR/OPQ/CDER, Food and Drug Administration, 10903 New Hampshire Avenue, Silver Spring, MD, USA
DOI: 10.32474/LOJPCR.2022.03.000155
Abstract
Objective: Transdermal hormonal drugs without protective covering may pose a risk for skin-to-skin hormone transfer through contact from the dosed to undosed person. Currently, no in vitro methodologies are available to assess the potential risk of skinto- skin drug transfer. This study aimed to develop an in vitro permeation test (IVPT) to evaluate the impact of use conditions (e.g., dosing duration and skin-to-skin contact time), on the potential risk of skin-to skin transfer of hormonal drugs.
Method: The risk of hormone transfer from the dosed skin to undosed skin was evaluated by employing human cadaver dermatomed skin mounted on vertical diffusion cells under various use conditions. Testosterone and estradiol transdermal hormonal gels were used as model hormonal products.
Results: Extent of hormone permeation through dosed skin was found directly proportional to the dosing duration of the formulation. Following dosing duration of 0.5-2 h, drug transfer from the dosed to undosed skin approached equilibrium after 24 h of skin-to-skin contact time. Following dosing duration of 8 h, drug transfer from dosed to undosed skin increased proportionally and significantly (p<0.05) after skin-to-skin contact times of 0.5-8 h.
Conclusion: A validated IVPT method may be used as a predictive tool to evaluate how dosing duration and contact time may affect the potential for skin-to-skin transfer of a hormonal drug. This IVPT tool could ultimately be utilized to develop drug products with a lower risk of skin-to-skin transfer and assess or mitigate skin-to-skin transfer risk of existing hormonal transdermal drugs.
Keywords: In Vitro Permeation Test (IVPT); Skin-to-skin transfer; Dosing duration; Topically applied drug products; Hormonal replacement therapy
Introduction
Hormonal drugs for replacement therapy are commonly administered via a transdermal route of drug administration. Hormone administration via a transdermal route has the advantage of by-passing the first-pass metabolism and minimizing the side effects that may be associated with peak plasma drug concentrations as is the case in oral administration [1]. Various topical drug products such as gels, creams, emulsions, sprays, or patches are employed for transdermal hormonal drug delivery. Although gels, creams, emulsions, or sprays are generally preferred over patches due to their lower skin irritability [2], a disadvantage of these topical products is their lack of protective covering without which residual hormonal products could pose a potential risk of drug transfer to others who may inadvertently come in direct skin-to-skin contact with the dosed skin. For instance, a small fraction of testosterone has been demonstrated to be systemically absorbed through the stratum corneum while most of the hormone remained unabsorbed on the skin surface of a patient for a longer period of time [3,4]. This unabsorbed hormone on the skin surface of a patient poses a risk of drug transfer via skin-to-skin contact to a non-patient. While transdermal testosterone and estradiol may provide substantial clinical benefit to a patient, these hormones may also lead to serious side effects to non-patients if transferred via inadvertent skin-to-skin contact such as hormonal imbalance. This risk has been described via several case reports in which children suffered pronounced virilization, early puberty, and premature epiphyseal closure of the bones due to secondary exposure to testosterone gel through skinto- skin contacts with male patients [5-9]. Additionally, children also experienced nipple swelling or breast enlargement due to transdermal estradiol exposure through contacts with female patients [10]. Skin-to-skin transfer of testosterone gel to women through male contacts has been reported to result in hirsutism, acne, coarsening of the voice, clitoral hypertrophy and male pattern alopecia [11,12]. Importantly, clothing has been shown to decrease the transfer of testosterone by preventing direct skin-to-skin contact [13,14] and washing of testosterone gel from the skin has been shown to significantly reduce the risk of skin-to-skin drug transfer [15].
The potential of drug transfer via skin contact with non-patients has been investigated in several pharmacokinetic clinical studies. In these studies, volunteers made physical contact of their dosed skin with the undosed skin of non-patients for a certain duration [4,15-17]. These studies were performed under a variety of occlusive or non-occlusive conditions [15,18]. However, these studies do not necessarily replicate the natural states of patients in which skin contact may be prolonged due to, for instance, intimate contact. Duplicating such studies with prolonged contact to a high degree of fidelity would be challenging. Moreover, conducting studies that expose non-patients to potentially toxic doses of drugs with their deleterious side effects for extended periods of time is not ethically appropriate. This is rendered even more challenging as in vivo animal studies are not considered suitable to extrapolate to human subjects because the animal skin (or fur) is structurally not similar to human skin. This leaves only in vitro approach for assessing the potential risk of drug transfer through skin contact, which to our knowledge has not yet been investigated.
The objective of the present study was to develop an in vitro permeation test (IVPT) to assess the potential of skin-to-skin drug transfer by exposing human cadaver dermatomed skin to various hormonal drug products. This study evaluated the effect of various experimental conditions, such as different formulations, formulation residence or wear time, and duration of skin-to-skin contact, on drug transfer. The formulation residence or wear time has been termed as dosing duration in this paper. Two model hormonal drug products were selected: AndroGel® (Testosterone 1.62%) and EstroGel® (Estradiol 0.06%). These two drug products were considered suitable for this study because there is a body of evidence establishing the clinically meaningful transfer of testosterone or estradiol from dosed to un-dosed subjects [3,4,19].
AndroGel® and EstroGel® are hydroalcoholic gels. Androgel® contains 1.62% of testosterone. Other excipients include carbopol 980, isopropyl myristate, sodium hydroxide, ethyl alcohol, and purified water. AndroGel® is indicated for the treatment of adult males with testosterone deficiency. One actuation of the pump delivers 1.25 g of gel which is equivalent to 20.25 mg of testosterone. Andro- Gel® is applied to the maximum surface area of the skin of the shoulders and upper arms once daily [20]. EstroGel® contains 0.06% of estradiol (17β-estradiol). Other excipients include carbomer 934P, triethanolamine, alcohol, and purified water. EstroGel® is indicated for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause in women. One actuation of the pump delivers 1.25 g of gel which is the single approved dose and is equivalent to 0.75 mg of estradiol. EstroGel® is applied over a large area (750 cm2) of arm skin, from the wrist to shoulder in a thin layer [21].
Materials and Methods
Materials
AndroGel® (Testosterone gel 1.62%), lot # D90024A, exp. date 03/2021 and EstroGel® (Estradiol gel 0.06%), lot # NHCR, exp. date 08/20, and lot # PDBT, exp. date 03/2021 were purchased from Brookville Pharmacy (Rockville, MD). Testosterone and estradiol USP reference standards were purchased from Sigma Aldrich Chemicals (St. Louis, MO). Acetonitrile and Methanol were purchased from VWR International (Radnor, PA). All other solvents and materials used in the study were of analytical grade.
Skin Samples
The human cadaver dermatomed skin of 600 ± 200 μm thickness obtained from shoulder and or thigh of donors of 40-60 years of age and free of any dermal diseases was purchased from Science Care (Phoenix, AZ USA) and stored at -80 °C.
Instrumentation
The HPLC system consisted of an Agilent-1260 series (Agilent technologies, Santa Clara, CA, USA) equipped with a diode array detector (DAD). Separation was achieved on a Phenomenex Luna C18 column (4.6 mm × 150 mm, 5 μm packing) fitted with a Phenomenex Luna C18 (2) guard column. In vitro permeation experiments were performed by using Vision Microette 18-cell system (Teledyne Hanson Research, Chatsworth, CA).
Methods
IVPT method development
An IVPT method was developed employing vertical diffusion cells to study the potential of drug transfer from a dosed skin to undosed skin through skin-to-skin contact. The diffusion cells, methodologies and study conditions utilized in the IVPT study were validated as per draft guidance for acyclovir topical cream 5% [22]. Briefly, vertical diffusion cells, consisting of a donor cell with cap (occlusive condition) or an open top (non-occlusive condition), and receiver cells of 12 mL capacity were used. The skin was thawed at room temperature and immersed in deionized water for hydration. The hydration medium was replaced with fresh medium three times every 10 minutes. The hydrated skin was dried with Kim wipes, cut into pieces of about 2.9 cm² to cover the exposure area of the receiver cell and examined visually for any surface damage with magnifying glass. The cut skin pieces were mounted onto the receiver cell orifices (1.72 cm2 diameter) with the stratum corneum side of the skin facing air and the dermal side in contact with the receiver medium. The receiver medium consisted of phosphate buffer saline of pH 7.4 for testosterone and phosphate buffer saline with 30% propylene glycol of pH 7.4 for estradiol. The receiver chambers were examined to ensure the absence of air bubbles below the dermal side of the skin. The receiver medium was maintained at 32 ± 1ºC by circulating water to mimic physiological temperature of the human skin surface and stirred at 600 ± 50 rpm. The skin integrity was tested by measuring trans-epidermal water loss (TEWL) with the help of a Vapometer. TEWL reading of 10 g/m2/h or lower ensured adequate hydration of skin.
Approximately 280 ± 20 mg of testosterone gel equivalent to 4.54 ± 0.324 mg of testosterone; or estradiol gel equivalent to 0.168 ± 0.012 mg of estradiol was applied on 1.766 cm2 of skin mounted on diffusion cell respectively. The gel was applied on the skin using a 1 mL positive displacement pipette and spread evenly over the entire skin surface with the help of a round-ended glass and then permeation experiment was started. The samples were withdrawn from the receiver medium at predetermined intervals throughout the course of permeation test for experiments A-C, described in the next subsection. The volume of receiver medium withdrawn was replaced by the fresh medium for maintaining the sink conditions. The drug permeated into the receiver medium, and drug recovered from dosed and undosed skins were quantified using validated HPLC methods.
Investigations on the effect of use conditions on skin-toskin transfer
Three separate experiments (A, B, C) were carried out to study the effects of various use conditions to determine the potential for skin-to-skin drug transfer, as described in Table 1. Each experiment was replicated either four or six times based on the availability of skin.
Table 1: Experimental design to investigate the effect of dosing duration and skin-to-skin contact time on the in vitro drug transfer.
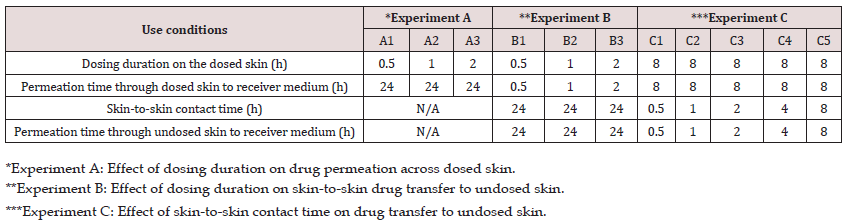
In the first experiment (A), the effect of dosing duration on the rate and extent of drug permeation from the dosed skin to the receiver medium in diffusion cell was investigated. For this purpose, the dosing duration for the dosed skin was varied from 0.5 to 2 h. while permeation was continued up to 24 h. in all cases. Briefly, the formulation was applied on the skin and after the specified dosing duration, the residual formulation was carefully removed from the dosed skin with the help of cotton swabs without interrupting the permeation experiment. For this purpose, three dry and three wet (water) cotton swabs were used. All experiments were performed under non-occlusive conditions (n=6) by leaving the donor cell exposed to air. A control in vitro permeation test (n=6) under occlusive condition was also performed where the donor cell was covered by a cap after applying the formulation and no formulation was removed from the skin surface.
In the second experiment (B), the effect of dosing duration on drug transfer from the dosed to the undosed skin and subsequent permeation into the receiver medium was investigated. For this purpose, skin was dosed with the formulation exactly in the same fashion as in experiment A, where skin mounted on a diffusion cell was dosed for a specified dosing duration of either 0.5, 1 or 2 h and permeation allowed into receiver medium under non-occlusive condition. At the end of dosing duration, the experiment was stopped, and residual formulation was removed and cleaned from the dosed skin as described previously. Then the cleaned dosed skin was placed on an undosed skin that was already mounted on a diffusion cell containing fresh receiver medium. The dosed skins were placed on each other in such a way that the stratum corneum of both skins were in close contact with each other. A glass disc was carefully placed on the dosed skin and the cell was closed with a clamp to ensure intimate contact between the dosed and undosed skin. The permeation test was initiated to allow the skin-to-skin drug transfer for 24 h. The 24 h of skin-to-skin contact time is not likely to occur in real life. A longer contact time of 24 h of skin-toskin contact time was selected as a worst-case scenario to understand the fate of drug retained by the dosed skin and its transfer to undosed skin which, in turn, helped us to design experiment C.
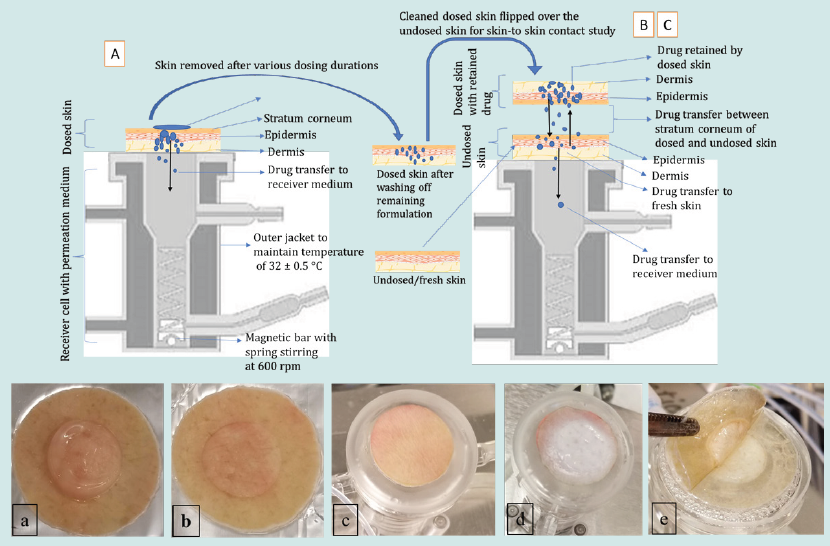
In the third experiment (C), the effect of various skin-to-skin contact time on drug transfer from the dosed to the undosed skin was studied. In this experiment the dosing duration of the dosed skin was kept constant for 8 h prior to bringing it in contact with the undosed skin. The cleaned dosed skin was then flipped over the undosed skin and secured to ensure skin-to-skin contact in the same way as previously described in experiment B. The dosed and undosed skins were then removed after a specified skin-to-skin contact time of either 0.5,1, 2, 4 or 8 h and permeation also stopped at the same time. The schematic presentation of the experiments A-C is shown in (Figure 1).
Figure 1: Upper panel: schematic presentation of experiments A-C employing vertical diffusion cells. Lower panel: a) image of residual gel on the dosed skin, b) clean dosed skin after washing off residual gel, c) undosed/fresh skin mounted on the diffusion cell with fresh medium underneath, d) dosed skin flipped over the undosed skin to enable skin-to skin contact, and e) removing skin at the end of the contact time for quantifying drug transfer.
Skin drug recovery
The dosed and undosed skins after removal from the diffusion cells were grounded into small pieces and then immersed in 5 mL of mobile phase. The skin in mobile phase was sonicated for 99 mins at 32 ± 0.5°C using a bath sonicator. The above skin samples were then centrifugated at 5000 rpm for 30 mins at 32 ± 0.5°C, followed by filtration. The filtrate was then analyzed by HPLC.
Analytical method
A reversed-phase HPLC method, adopted from literature, was used for the analysis of testosterone [23] and estradiol [24] contents in skin and receiver medium samples. Both methods were validated as per ICH Q2(R1) guidelines for specificity, linearity, accuracy, precision, range, limit of quantitation (LOQ), limit of detection (LOD) and system suitability [25]. Chromatographic separation of testosterone and estradiol were achieved on a Phenomenex Luna column using a methanol-water (70:30 v/v) mobile phase for testosterone and an acetonitrile-methanol-water (45:05:50 v/v) mobile phase for estradiol. Both compounds were eluted isocratically at a flow rate of 1 mL/min. Testosterone was analyzed with UV detection at 254 nm. Estradiol was analyzed with UV detection at 280 nm. The injection volume was 10 μL. The calibration range for testosterone was from 5-100 μg/mL with limit of detection (LOD) of 0.1 μg/mL with an acceptable precision (< 10%). The estradiol peak was detected by using DAD UV detector at 280 nm. The calibration range for estradiol was from 0.5-100 μg/mL with limit of detection (LOD) of 0.1 μg/mL with an acceptable precision (< 10%).
Data and Statistical Analysis
The permeation parameters such as cumulative drug permeated (Eq. 1), steady state flux (Eq. 2) and percent drug permeated (Eq. 3) were calculated per square centimeter (cm2) of the skin from the obtained AUC data using Eq. 1, 2 or 3, respectively [23,26,27]. The drug retention in the skin was normalized to a constant skin thickness of 373.33 μm for testosterone and 283.33 μm for estradiol based on the original skin sample thickness. The drug concentration in each receiver medium sample was calculated by correcting the sampling effect according to the equation described by Hayton and Chen [26].

where Q is the cumulative amount of drug permeating through the skin, A is the surface area of permeation at a given time t, Cd is the amount of drug in donor chamber and P is the permeability coefficient obtained from the slope of a plot of cumulative permeation of drug across skin samples against square root of time.
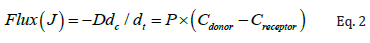
where J is the flux of drug from donor to receiver chambers in μg/cm2/h, dc/dt is the concentration gradient, D is diffusion coefficient, P is the permeability coefficient of the skin tissue to the drug, Cdonor is the amount of drug in the donor chamber, and Creceptor is amount of the drug in the receiver chamber at the end of the study.

In case of experiment B and C, the amount of drug retained by the dosed skin following various dosing duration were back calculated using Eq. 4 below. The obtained total amount retained by the dosed skin was then considered as 100% loading amount of drug on the undosed skin during skin-to-skin drug transfer studies. All data were expressed as mean ± standard deviations, with either four or six experimental replicates. Statistical significance for each experiment was determined using one-way analysis of variance (ANOVA). A p-value of less than 0.05 was considered statistically significant.

Figure 2: Effect of dosing duration (0.5, 1 and 2 h) on testosterone permeation (mean ± SD of n=6). A) cumulative amount of drug permeated as a function of permeation time, B) percent testosterone permeation after 24 h, C) Flux of testosterone.

The results in Figure 2A show the effect of dosing duration 0.5, 1, or 2 h on the cumulative amount of testosterone permeated through the dosed skin into the receiver medium. At dosing duration of 0.5, 1 and 2 h, the cumulative amount of testosterone that permeated through the unit surface area of skin following 24 h of permeation was found to be 3.63 ± 1.81, 5.0 ±3.52 and 5.6 ± 2.27 μg/mL/cm2, respectively, under non-occlusive condition. Figure 2B shows the percent testosterone permeated through the dosed skin into the receiver medium was 2.3 ± 1.19, 2.7 ± 1.8 and 2.9 ± 1.17 % after 24 h of permeation when dosing duration was set at 0.5, 1 or 2 h, respectively. Figure 2C shows the steady state flux of testosterone was 2.3 ± 0.16, 2.75 ±1.30 and 3.1±1.12 μg/mL/cm2/h for dosing duration of 0.5, 1, and 2 h, respectively. These permeation results clearly indicate that increase in the dosing duration from 0.5 to 2 h exhibited an increase in testosterone permeation across the skin. However, the cumulative permeation or percent permeation was not statistically significant (p>0.05) at various dosing durations which may be attributed to close proximity of dosing durations to each other (0.5, 1 and 2 h). Notably, for the dosing durations of 0.5, 1, or 2 h studied, the retention of testosterone in the skin after allowing permeation for 24 h was found below the LOD.
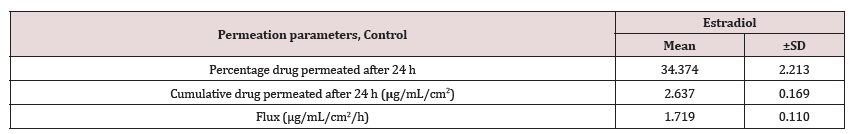
Contrary to the extent of testosterone permeation, the estradiol permeation in receiver medium was found below the LOD even after 24 h of permeation for all dosing durations (0.5, 1 and 2 h). This observation may be explained by assuming very slow permeation of estradiol. Therefore, estradiol could not permeate in detectable amounts into the receiver medium during the shorter dosing duration of 0.5 to 2 h. On the other hand, in case of the control run, where the dosing duration was long enough (24 h) at occlusive condition, estradiol permeated into receiver medium in measurable quantities, as shown in Table 2. The results in Table 2 for control show the percent estradiol permeated, cumulative estradiol permeated, and estradiol flux were 34.37 ± 2.21, 2.63 ± 0.17 and 1.72 ± 0.11, respectively.
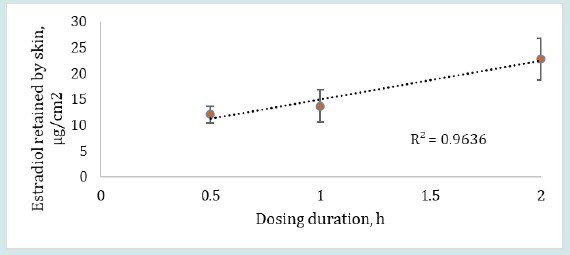
The results in Figure 3 show that the retention of estradiol by the dosed skin was found to be 12.07 ± 1.63, 13.70 ± 3.16 and 22.78 ± 4.06 μg/cm2 when dosing duration was 0.5, 1 or 2 h, respectively, after continuous permeation for 24 h. The retention of estradiol by skin clearly exhibited an increase in drug absorption when the dosing duration was increased from 0.5 to 2 h. However, the skin retention of estradiol was not statistically significant (p>0.05) for different dosing durations which may be attributed to the selected dosing durations (0.5, 1 and 2 h) being close in time to each other. The low permeation results of estradiol may be due to hormone accumulation in the subcutaneous region or due to estradiol’s relatively high affinity for the lipophilic and hydrophilic interface of the stratum corneum and epidermis from where the drug may permeate slowly into the circulation [27,28].
Figure 3: Estradiol retained by the skin (mean ± SD) after 24 h of permeation when dosing duration was varied from 0.5 – 2 h, (n=6).
Effect of dosing duration on skin-to-skin drug transfer
The results in Table 3 show the effect of dosing duration on the testosterone transfer through skin-to-skin contact to undosed skin and into the receiver medium in contact with undosed skin. The testosterone recovered from the dosed skins following skin-to-skin contact of 24 h with undosed skins was 463.60 ± 60.28, 509.39 ± 22.44 and 565.99 ± 100.09 μg/cm2 for dosing duration of 0.5, 1 or 2 h, respectively. Testosterone absorbed and retained by the undosed skin following the same skin-to-skin contact time and dosing durations was 426.35 ±83.69, 449.41 ± 66.32 and 455.31 ± 42.15 μg/cm2, respectively. The cumulative amount of testosterone that finally reached into the receiver medium from the dosed through undosed skins was observed to be 5.19, 4.51 and 7.82 μg/cm2 after 0.5, 1, or 2 h of dosing duration, respectively. These results indicated that testosterone recovered from dosed skin, undosed skin or cumulative amount in the receiver medium all exhibited an increase in drug transfer with increase in dosing duration.
Table 3: Testosterone amount recovered from dosed skin, undosed skin and receiver medium after 24 h of skin-to-skin contact time (n=6).

After 24 h of skin-to-skin drug transfer, most of the retained drug was assumed to be transferred from the dosed to undosed skin after this duration of skin-to-skin contact time. Surprisingly, we did not observe such results. Instead, the amount of testosterone retained by both dosed and undosed skins were approaching close to each other for all dosing durations evaluated.
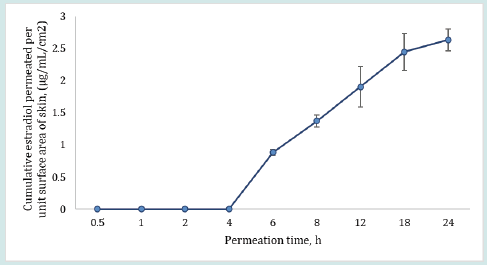
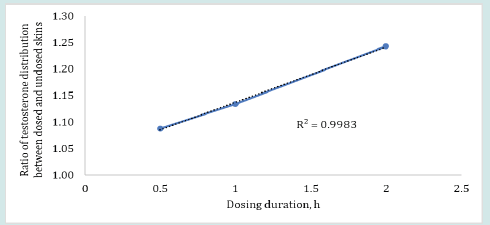
The plot in Figure 4 shows that the ratio of testosterone distribution between the dosed and undosed skin after 24 h of skin-toskin contact increased with increasing dosing duration. The testosterone distribution ratios between dosed and undosed skin were relatively close but the difference was statistically significant (p = 0.0262, regression (R2) = 0.9983). The smaller ratio of testosterone distribution after 24 h of permeation may be attributed to the drug content in dosed or undosed skin which may be slowly approaching equilibrium. On the other hand, the amount of estradiol recovered from the dosed skin or undosed skin or from the receiver medium in contact with undosed skin following the same skin-to-skin contact time after specified dosing duration were found below LOD for most of the cells (n=6). The possible reason for observing undetectable lower estradiol retention by both dosed and undosed skins could be attributed to the equilibrium distribution of the estradiol in both dosed and undosed skins which further lowered the sensitivity of the estradiol detection. Notably, the percent drug recovered from the skin wash after each dosing period (0.5 to 2 h) were 95- 98%. The cumulative estradiol permeation results for control formulation revealed that estradiol starts to appear in the receiver medium after 4 h during 24 h of permeation test study even though the estradiol formulation was kept on the skin intact for 24 h at occlusive conditions (Figure 5).
Figure 4: Ratios of testosterone distribution or transfer between the dosed and undosed skin following 0.5, 1 or 2 h of dosing duration and 24 h of skin-to-skin contact (n=4).
Effect of contact time on skin-to-skin drug transfer
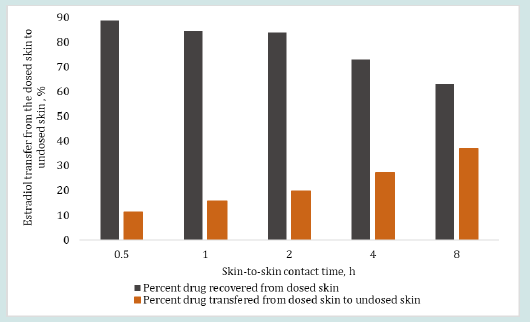
The results in Figure 6 and 7 show the effect of changing skinto- skin contact time on drug transfer from the dosed skin to the undosed skin and into the receiver medium. The dosing duration was kept constant at 8 h for all dosed skins while the skin-to-skin contact time of dosed skin with undosed skin was varied from 0.5 to 8 h. The percent testosterone recovered from the undosed skin after the specified skin-to-skin contact times, as shown in Figure 6, were 15.50, 16.03, 21.81, 19.12 and 23.86%. The testosterone recovered from the receiver mediums in contact with the undosed skin were below LOD after up to 2 h of skin-to skin contact time and 6.92 and 6.70% after 4 and 8 h of skin-to skin contact time, respectively. The overall testosterone transfer from the dosed to undosed skin showed that testosterone transfer increased linearly and significantly (p=0.000785, R2 = 0.985) with increasing skin-to-skin contact time. The testosterone content in the receiver medium after 0.5 to 2 h of skin-to skin contact was found below the LOD because of shorter permeation time. Importantly, most of the results of the testosterone drug transfer did not exceed 50% of % relative standard deviation (RSD). The variability in these experiments was at tributed to the inherent variability from differences in the donors’ race, age, sex, skin thickness and site of the skin source on the body and the pairing of two stratum corneum together. The results in Figure 7 show the effect of 0.5 to 8 h of skin-to-skin contact time on the estradiol transfer from the dosed to undosed skin and into the receiver medium following same dosing duration of 8 h. The percent estradiol recovered from the undosed skins were 11.31, 15.62, 19.58, 27.25 or 37.06%, as shown in Figure 7 for the respective contact times. In all cases, the permeated amount in the receiver medium via the undosed skins were found below LOD. Overall, the results clearly show a linear transfer of estradiol that significantly increased when the duration of skin-to-skin contact was increased (p= 0.00223, R2 of 0.9699).
Figure 6: Effect of skin-to-skin contact time on the percent testosterone transfer from the dosed skin (after 8 h of dosing) to the undosed skin and into the receiver medium in contact with undosed skin after 0.5, 1, 2, 4 and 8 h of skin-to-skin contact time. The total drug retained by the dosed skin after 8 hr of dosing duration was back calculated by employing Eq. 4.
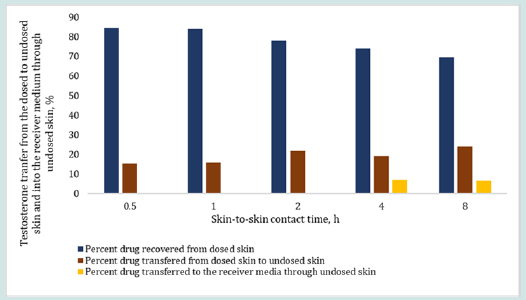
Figure 7: Effect of skin-to-skin contact time on the percent estradiol transfer from the dosed skin (after 8 h of dosing duration) to the undosed skin (including receiver medium containing undosed skin) after 0.5, 1, 2, 4 and 8 h of skin-to-skin contact time (obtained from Eq. 1). The total drug retention by the dosed skin after 8 hr of dosing duration was considered as 100% loading dose on the undosed skin for skin-to-skin contact study.
Conclusions
The data provided by this work demonstrated that the potential risk of transdermal hormonal drug transfer to undosed persons through skin-to-skin contact with a dosed skin may be assessed by employing an in vitro permeation testing method. The extent of drug transfer to an undosed skin through skin-to-skin contact with a dosed skin may depend on the dosing duration of dosed skin and its duration of contact with undosed skin. An adequately developed and validated IVPT method may serve as a tool to quantify the extent of drug transfer from the dosed skin to undosed skin for product development of transdermal hormonal drugs or other transdermal drugs.
Acknowledgment
“This project was supported in part by appointing ORISEs (Behnam Dasht Bozorg and Rokon Uz Zaman) to the Research Participation Program at the U.S. Food and Drug Administration administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.”
Disclaimer
This scientific publication reflects the views of the authors and should not be construed to represent FDA’s views or policies.
References
- Busse KL, HI Maibach (2011) Transdermal Estradiol and Testosterone Transfer in Man: Existence, Models, and Strategies for Prevention. Skin Pharmacology and Physiology 24(2): 57-66.
- Samsioe G (2004) Transdermal hormone therapy: Gels and patches. Climacteric 7(4): 347-356.
- Miller MG, AD Rogol and TL ZumBrunnen (2012) Secondary exposure to testosterone from patients receiving replacement therapy with transdermal testosterone gels. Current Medical Research and Opinion 28(2): 267-269.
- Wester RC, X Hui and HI Maibach (2006) In Vivo Human Transfer of Topical Bioactive Drug between Individuals: Estradiol. Journal of Investigative Dermatology 126(10): 2190-2193.
- Bhowmick SK, T Ricke and KR Rettig (2007) Sexual precocity in a 16-month-old boy induced by indirect topical exposure to testosterone. Clinical pediatrics 46(6): 540-543.
- Yu YM, N Punyasavatsu, D Elder, AJD Erocle (1999) Sexual development in a two-year-old boy induced by topical exposure to testosterone. Pediatrics 104(2): 23-23.
- Franklin SL, ME Geffner (2003) Precocious puberty secondary to topical testosterone exposure. Journal of Pediatric Endocrinology and Metabolism 16(1): 107-110.
- Stephen MD, Caycet Jehaimi, Patrick G Brosnan, Michael Yafi (2008) Sexual precocity in a 2-year-old boy caused by indirect exposure to testosterone cream. Endocr Pract 14(8):1027–30.
- Kunz GJ, Karen O Kelein, Robert D Clemons, Michael E Gottschalk, Kenneth Lee Jones (2004) Virilization of Young Children After Topical Androgen Use by Their Parents. 114(1): 282-284.
- Voelker R (2010) Estrogen spray poses risks to children, pets through contact with treated skin. JAMA 304(9): 953-953.
- ZO M and S N (2007) Postmenopausal virilization after spousal use of topical androgens. Fertil Steril 87: 975–6.
- Rolf C and N E (1998) Potential adverse effects of long-term testosterone therapy. Baillieres Clin Endocrinol Metab 12(3): 521–34.
- Stahlman J, Margaret Britto, Sherahe Fitzpatrick, Cecilia Mcwhirter, Samuel A Testion et al (2012) Effect of application site, clothing barrier, and application site washing on testosterone transfer with a 1.62% testosterone gel. Current Medical Research and Opinion 28(2): 281-290.
- Stahlman J, Margaret Britto, Sherahe Fitzpatrick, Cecilia Mcwhirter, Samuel A Testion et al (2012) Serum testosterone levels in non-dosed females after secondary exposure to 1.62% testosterone gel: effects of clothing barrier on testosterone absorption. Current medical research and opinion 28(2): 291-30.
- W d R (2009) Hyperandrogenism after transfer of topical testosterone gel: case report and review of published and unpublished studies. Human Reproduction 24 (2):425–428.
- ZumBrunnen TL, Ingrid Meuwsen, Michiel devries, John J Bronnan (2006) The effect of washing and the absence of interindividual transfer of estradiol gel. Am J Drug Deliv 4: 89–95.
- Taylor MB, MJ Gutierrez (2008) Absorption, Bioavailability, and Partner Transfer of Estradiol from a Topical Emulsion 28(6): 712-718.
- Stahlman J, Margaret Britto, Sherahe Fitzpatrick, Cecilia Mcwhirter, Samuel A Testion et al (2012) Serum testosterone levels in non-dosed females after secondary exposure to 1.62% testosterone gel: effects of clothing barrier on testosterone absorption. Current Medical Research and Opinion28(2): 291-301.
- Rolf C, U kine, G Lemmnitz, E Nieschlag (2002) Interpersonal testosterone transfer after topical application of a newly developed testosterone gel preparation. Clinical Endocrinology 56(5): 637-641.
- FDA (2020) Medication Guide: ANDROGEL® CIII, (testosterone gel) 1.62% 2015 05/2015 [cited 2020 10/19/2020]
- WebMD EstroGel. 2018 7/20/2018
- FDA Draft Guidance on Acyclovir Topical Cream 5%. Guidance for Industry 2016 December 1/16/2020]
- Zidan AS, Kamal N, Alayoubi A, Seggel M and Ibrahim S et al (2017) Effect of Isopropyl Myristate on Transdermal Permeation of Testosterone from Carbopol Gel. Journal of pharmaceutical sciences 106(7): 1805-1813.
- YS K, Naresh Pavurala, Yang yang, Rakhi shah, Yong Sheng yang et al (2017) In vitro drug transfer due to drug retention in human epidermis pretreated with application of marketed estradiol transdermal systems. AAPS PharmSciTech 18(6): 2131-40.
- ICH, Q2(R1) (1995) Validation of Analytical Procedures: Text and Methodology.
- Hayton WL, T Chen (1982) Correction of perfusate concentration for sample removal. Journal of pharmaceutical sciences 71(7): 820-821.
- (2008) emc. Sandrena 0.5 mg gel.
- Hadgraft J (1983) Percutaneous absorption: Possibilities and problems. International journal of pharmaceutics 16(3): 255-270.