Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1373
Research ArticleOpen Access
A Hmsh2 c.2332 T>A Mutation and Membrane-hMSH2/TCRγδ/NKG2D-Mediated Cytotoxicity of Human Vγ9vδ2 T Cells in Ovarian Serous Cystadenocarcinoma Volume 2 - Issue 4
Yumei Dai1* and Hui Chen2
- 1Department of Clinical Laboratory Medicine, Guangzhou Women & Children’s Medical Centre, Guangzhou Institute of Pediatrics, China
- 2Department of Immunology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and School of Peking Union Medical College, the National Key Laboratory of Molecular Biology, China
Received: April 01, 2021 Published: April 08, 2021
Corresponding author: Yumei Dai, Department of Clinical Laboratory Medicine, Guangzhou Women & Children’s Medical Centre, Guangzhou Medical University, Guangzhou Institute of Pediatrics, Guangzhou 510623, China
DOI: 10.32474/LOJPCR.2021.02.000142
Abstract
Membrane human MutS homologue 2 (mhMSH2) is a well-characterized endogenous ligand for human Vγ9Vδ2 T cells, but its germline gene expression in mhMSH2-overexpressing ovarian serous cystadenocarcinoma (OSC) cells and tissues is unclear. Herein, we discovered a silent hmsh2 c.2332 T>A mutation in the OSC HO8910 cell line (GenBank accession no.MG674653) and identified serval novel soluble forms of hMSH2 protein from its whole cell protein extracts and culture supernatant. The mhMSH2/ TCRγδ/NKG2D-mediated recognition and cytotoxicity of Vγ9Vδ2 T cells were validated in mhMSH2/6-overexpressing SKOV3 cells. Positive expression of cytoplasmic and/or membrane hMSH2/6, cytoplasmic and/or nuclear hMSH3 and complete loss of nuclear expression of hMSH2 was observed in all examined ovarian cancer tissues. The clinical significance of the novel hmsh2 c.2332 T>A mutation, the soluble full-length and truncated forms of hMSH2 proteins, and the complete loss of nuclear hMSH2 protein expression in OSC development and human Vγ9Vδ2 T cell-mediated anti-OSC immunity remain to be further elucidated.
Keywords: Hmsh2; Mutation; Membrane hMSH2; Vγ9Vδ2 T cells; Ovarian serous cystadenocarcinoma.
Abbreviations: hMSH2: Human MutS Homologue 2; MMR: Mismatch Repair; M: membrane; EBV: Epstein-Barr virus; OSC: Ovarian Serous Cystadenocarcinoma; ATCC: American Type Culture Collection; IHC: Immunochemistry; mAb: monoclonal Antibody; FCM: Flow Cytometry; PFA: Paraformaldehyde; FITC: Fluorescein Isothiocyanate; DAPI: 4’,6-diamidino-2-phenylindole; LS: Lynch Syndrome.
Introduction
Human MutS homologue 2 (hMSH2), a critical element of
the DNA mismatch repair (MMR) system, is normally localized
in the nucleus of host cells and dimerizes with hMSH3 or hMSH6
(hMSH3/6) to form complexes that participate in DNA damage and
cell apoptotic signaling. Inherited or acquired defects in the hmsh2
gene or protein lead to dysfunctional correction of errors in DNA
synthesis and duplication, the cell cycle and Ig isotype switching
of B cells and thus have a close association with the genesis of
many types of tumors [1,2]. In our published study, we reported
a broad overexpression of membrane hMSH2 (mhMSH2) in a
series of epithelial tumor cell lines and Epstein-Barr virus (EBV)-
transformed B lymphoid cells [3]. mhMSH2 overexpressed on
malignant cells was subsequent characterized as a stress-inducible
endogenous protein ligand for human Vγ9Vδ2 T cells, a subset of
innate immune cells that play a crucial role in antitumor/antiviral
immunity and autoimmunity [3-5]. The induction of ectopic
mhMSH2 expression on renal carcinoma cells by oxidative stress
via the p38 mitogen-activated protein kinase and c-Jun N-terminal
kinase pathways with interleukin-18 promotion was subsequently
revealed, but the systemic expression of hmsh2 gene in mhMSH2-
overexpressing carcinomas is still unknown [6].
There are 238,700 estimated new cases of ovarian cancer and
151,900 deaths per year worldwide, and thus, this disease is the
fifth most common cause of female cancer-related death in the
United States in 2018 [7,8]. Among the 4 subtypes of ovarian cancer
(high-grade serous, endometrioid, clear cell ovarian carcinomas
and non-epithelial subtypes), Ovarian Serous Cystadenocarcinoma
(OSC) is the most common and lethal type in women [9]. Previously, we reported the unusual ectopic mhMSH2 expression on the
human OSC cell lines HO8910 and SKOV3 and the specific binding
of mutated mhMSH2 on SKOV3 cells to synthesized OT3 peptides
of human Vδ2 TCR [3,10]. In this work, we further elucidated the
hmsh2 gene mutation and ectopic subcellular protein expression in
HO8910 cells and the mhMSH2-mediated recognition and cytolytic
effects of Vγ9Vδ2 T cells towards SKOV3 cells. The abnormal
subcellular distribution of hMSH2/3/6 in different subtypes of
ovarian cancer tissues was demonstrated by immunochemistry
(IHC).
Materials and Methods
Cell lines, culture medium and tumor tissues
HO8910 cells (OSC) were maintained in RPMI-1640 complete medium (Invitrogen, Shanghai, China). SKOV3 (OSC) and HK-2 (proximal tubular cell line derived from normal kidney) cells were cultured in 10% FBS DMEM/F12 medium (Niuyin, Beijing, China). All cell lines were purchased from the Cell Culture Center of the Institute of Basic Medicine, Chinese Academy of Medical Sciences and confirmed to have no mycoplasma contamination before use. Expansion and subset separation of effector human Vγ9Vδ2 T cells were carried out as we previously described [3]. Ovarian cancer tissues belonging to different histotype (OV-1, serous adenocarcinoma; OV-2, embryo sinus carcinoma; OV-3, cyst-myxomatous adenoma) were freshly obtained from Peking Union Hospital with informed consent provided by the patients. The use of human tissues for research was also approved by the ethics committee of the Guangzhou Women and Children’s Medical Centre, Guangzhou Medical University, Guangzhou Institute of Pediatrics (2015020917).
Gene sequencing of hmsh2 in HO8910 cells
Appropriate numbers of HO8910 cells in the logarithmic growth phase were collected for total mRNA extraction and cDNA reverse transcription. The target hmsh2 gene was amplified using fragment PCR as we described in Subcellular hMSH2 expression is aberrant in membrane-hMSH2-overexpressing cervical, lung and gastric cancer cell lines and tissues (article in progression). Three PCR products with predicted lengths (990, 1250 and 1549bp) were purified and introduced into the pGEM-T easy vector (Promega, USA). Recombinant plasmids were positively selected and characterized by Not I digestion before gene sequencing. Human msh2 variants in HO8910 cells were screened by alignment with the full-length hmsh2 sequence (NM_000251) using Laser gene 7 software.
Soluble full-length and truncated forms of hMSH2 in the whole cell extracts and culture supernatant of HO8910 cells
Soluble forms of hMSH2 protein in the complete protein extracts and the cell culture supernatant of HO8910 cells were simultaneously separated by SDS-PAGE and blotted with antihMSH2 McAb (65021-1 Ig, 1:500, Proteintech Group, USA) and with goat anti-mouse IgG/HRP secondary antibody (1:5000, Zhongshan Jinqiao Company, China). 𝛽-actin was blotted as endogenous reference control (MW 43 kDa).
Growth curves of HO8910 cells
For growth curves of both OSC cell lines, appropriate numbers of trypsin-digested HO8910, SKOV3 and HK-2 cells were planted in 24-well plates in triplicate and cultured for 8 consecutive days in a 5% CO2 atmosphere at 37°C. Growth curves of the examined cell lines were obtained by using the cell counting method. The expansion rates of target OSC cells were compared with that of HK-2 control cells.
Quantitative real-time PCR for hmsh2 mRNA expression in HO8910 cells
The human msh2 gene transcription levels in HO8910, SKOV3 and HK-2 cells were comparatively analyzed by qRT-PCR. The results were processed with Sequence Detector Version 1.2 (Applied Biosystems, USA) and Sigma Plot 11.0 as we previously described [3].
Ectopic membrane, cytoplasmic and nuclear expression of hMSH2 in HO8910 cells
The membrane, cytoplasmic and nuclear hMSH2 expression in HO8910, SKOV3 and HK-2 cells were further investigated by laser confocal microscopy with different fixation reagents. The target cells (2-3×105) were cultured overnight on pre-autoclaved glass cover slips in a 24-well format, properly fixed with 4% paraformaldehyde (PFA) or ice-cold methanol for 10-15 min and blocked with 0.5% BSA for 30 min at 4°C before labeling with specific anti-hMSH2 (N- 20, Santa Cruz Biotechnology, USA) polyclonal antibody or rabbit IgG and fluorescein isothiocyanate (FITC)-conjugated goat antirabbit secondary antibody (Zhongshan Jinqiao Company, Beijing, China). The nuclei of the examined tumor cells were stained with 4’,6-diamidino-2-phenylindole (DAPI) (1:1000, Sigma, USA) before being mounted on a Leica DMIRE2 inverted microscope (objective, 40×; numerical aperture, 1.25).
Participation of mhMSH2/TCRγδ/NKG2D in Vγ9Vδ2 T cell-mediated anti-OSC immunity
For further analysis of the role of mhMSH2 in human Vγ9Vδ2 T cell-mediated anti-OSC immunity, effector Vγ9Vδ2 T cells were incubated with SKOV3 cells at different ratios (effector: target [E: T] 20:1, 10:1, 5:1 and 2.5:1). Cytotoxicity was measured with the LDH method, as we previously described [3]. For cytotoxicity blockade assays, target OSC cells were pretreated with anti-hMSH2 (N20, Santa Cruz Biotechnology), anti-NKG2D (149810, R&D Systems) and anti-TCRγδ (B1.1, Immunotech, France) antibodies before incubation with effector Vγ9Vδ2 T cells at different E:T ratios (20:1, 10:1, 5:1 and 2.5:1). The blockade cytotoxicity was measured and compared to that of the rabbit IgG blockade control group. Target SKOV3 cells were stained with anti-hMSH2, anti-hMSH3 and anti-hMSH6 antibodies to confirm the cell surface expression of hMSH2/3/6 antigens before cytotoxicity and antibody-blocking cytotoxicity assays.
Subcellular hMSH2/3/6 Distribution In OSC Tissues
For IHC analyses, freshly collected ovarian cancer tissues were classically made into paraffin sections (3-4 μm in thickness) after proper neutral formalin fixation. The biopsies were heated at 60°C overnight and gradient dehydrated with methanol before antigen retrieval in boiled citrate buffer, followed by overnight incubation with purified mouse anti-hMSH2 monoclonal antibody (mAb) (clone G219-1129,1:200), mouse anti-hMSH3 mAb (clone 52, 1:20), mouse anti-hMSH6 mAb (clone 44, 1:30) (BD Pharmingen, USA) or isotype-matched mIgG1 at 4°C in a moist environment after 3% H2O2 treatment. The coloration was developed with PV9000 reagents (Zhongshan Jinqiao Company, Beijing, China) and captured with a Leica DM3000 imaging system.
Statistical Analysis
GraphPad Prism 7.2 (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis and data plotting. Data are presented as the mean ± SD. Differences between/among groups were compared with Student’s t-test (two-tailed) or one-way ANOVA, P<0.05 was considered statistically significant.
Results
Hmsh2 gene sequencing and soluble full-length and truncated hMSH2 protein blotting
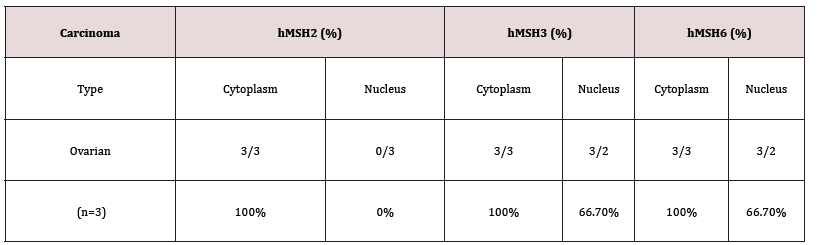
Figure 1:hmsh2 gene mutation and soluble truncated hMSH2 protein in HO8910 cell extract and culture supernatant.< A, Fragment PCR amplification of the hmsh2 gene in the OSC cell line HO8910. The predicted lengths of the PCR products were 990 bp (fragment 1, lane 1), 1250 bp (fragment 2, lane 2) and 1549 bp (fragment 3, lane 3) in length. Lane 4, β-actin. B, A c.2332 T>A point mutation occurred in the full gene sequence of hmsh2 in HO8910 cells. STD, standard hmsh2 gene (NM_000251). C, Truncated hMSH2 variants in HO8910 whole cell extracts and culture supernatant were blotted with an anti-hMSH2 antibody.
The PCR products of hmsh2 in HO8910 cells were 990, 1250 and 1549 bp in length (Figure 1A). By aligning the spliced full gene sequence with hmsh2 (NM_000251), we observed a point mutation (hmsh2 c.2332 T>A) in the HO8910 cell line (Figure 1B). It was a silent mutation that did not alter the primary structure of hMSH2 protein. The gene sequencing data of mutated hmsh2 in HO8910 cells were submitted to GenBank (Accession number: MG674653) for release. No additional gene mutation of hmsh2 was found in other mhMSH2-overexpressing epithelial tumor cell lines (data not shown here). By SDS-PAGE separation and western blots, several soluble forms of hMSH2 proteins were identified from the whole HO8910 cell protein extract [including the full-length form (MW ~105 kDa) and four truncated variants (MW~70, ~62, ~55 and ~40 kDa)] and the cell culture supernatant (MW~88, ~68 kDa) (Figure 1C).
Hmsh2 gene transcription and ectopic cytoplasm/ membrane expression
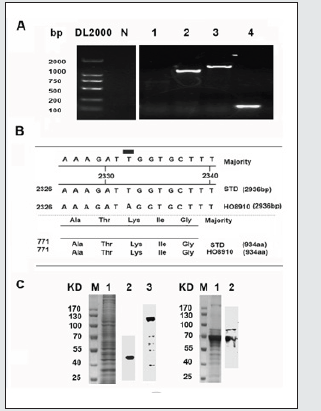
The growth and proliferation of both OSC cell lines were depicted with growth curves determined by cell counting assays. Both HO8910 cells and SKOV3 cells achieved rapid growth on day 5 and expanded much faster than the normal control HK-2 cells (Figure 2A). The mRNA expression of hmsh2 was slightly decreased in SKOV3 cells but substantially elevated in HO8910 cells compared with HK-2 cells (Figure 2B). Further detection of the ectopic membrane overexpression of hMSH2 on target OSC cells with laser confocal microscopy showed that both HO8910 and SKOV3 displayed different degrees of green fluorescence on the cell surface after labelling with a specific anti-hMSH2 antibody. The cell surface green fluorescence was much stronger on SKOV3 cells than on HO8910 cells. No green fluorescence was observed on normal control HK-2 cells (Figure 2C, the upper panels of pictures). The subcellular distribution of hMSH2 in HO8910 and SKOV3 cells was further determined by confocal microscopy with ice-cold methanol fixation. The membranes of both cells displayed different degrees of green fluorescence. The density of green fluorescence in the cytoplasm and the nuclei of SKOV3 cells was much stronger than that of HO8910 cells. There was no green fluorescence observed in HK-2 cells (Figure 2C, the lower panels of pictures).
Figure 2: Hmsh2 gene transcription and ectopic subcellular location in HO8910 cells. A, Growth curves of the mhMSH2-overexpressing OSC cell lines HO8910 and SKOV3. HK-2 cells were used as a normal control. B, Relative hMSH2 mRNA expression ratio of HO8910 and SKOV3 cells to normal control HK-2 cells. C, Ectopic subcellular expression of hMSH2 in HO8910, SKOV3 and HK-2 cells. Both HO8910 and SKOV3 displayed strong fluorescence on their cell surface after staining with a specific anti-hMSH2 antibody. Strong green fluorescence was also observed in the cytoplasm and nucleus of SKOV3 cells. Scale bar, HO8910 and SKOV3, 75 μm; HK-2, 50 μm.
Membrane hMSH2-mediated recognition and cytotoxicity of OSC cells by Vγ9Vδ2 T cells
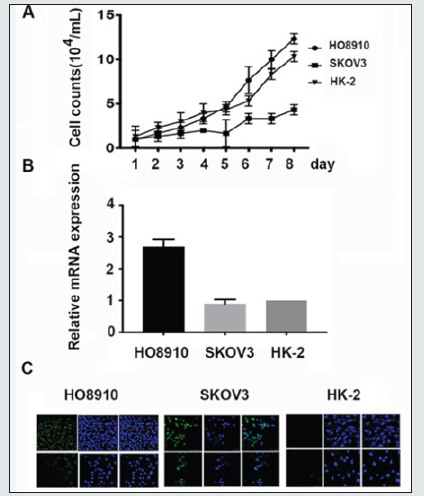
Previously, we identified the membrane-overexpressed hMSH2 on several epithelial tumor cell lines as a stress-inducible endogenous protein ligand for human Vγ9Vδ2 T cells [3,6]. Herein, mhMSH2-mediated recognition and cytotoxicity of Vγ9Vδ2 T cells towards target OSC cells were confirmed by cytotoxicity and independent specific antibody blockade cytotoxicity assays. mhMSH2 overexpression on OSC targets was validated with flow cytometry (FCM) before cytotoxicity and cytotoxicity blockade assays (Figure 3A). At E:T ratios of 20:1, 10:1, 5:1 and 2.5:1, the cytotoxic efficiency of effector human Vγ9Vδ2 T cells against target SKOV3 cells was 53%, 26%, 23% and 12%, respectively (Figure 3B). The ectopic mhMSH2-mediated recognition and cytotoxicity could be strongly blocked by specific anti-hMSH2, anti-NKG2D or anti-TCRγδ antibodies at different E:T ratios, indicating the participation of mhMSH2 in Vγ9Vδ2 T cell-mediated anti-OSC immunity by NKG2D/γδ TCR recognition (Figure 3C).
Figure 3: Membrane hMSH2-mediated recognition and cytolytic effects via TCRγδ and NKG2D on OSC cells. A, FCM analysis of mhMSH2/3/6 expression on SKOV3 cells. B, Cytotoxicity assay of human V𝛾9V𝛿2T cells against SKOV3 cells at E:T ratios of 20:1, 10:1, 5:1 and 2.5:1. C, Cytotoxicity blockade assay of membrane-overexpressed hMSH2, NKG2D and γδ TCR in the V𝛾9V𝛿2T cell-mediated cytolysis of SKOV3 cells. *, P<0.05; **, P<0.01; ***, P<0.001.
Subcellular distribution of hMSH2/3/6 in OSC tissues
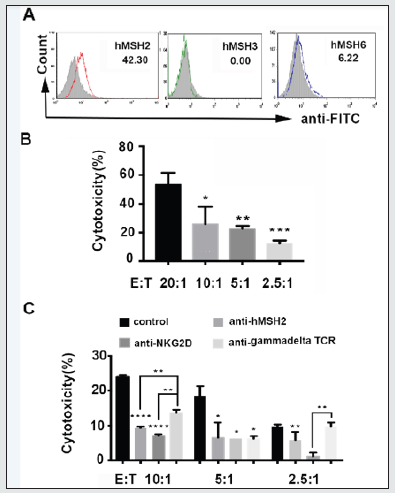
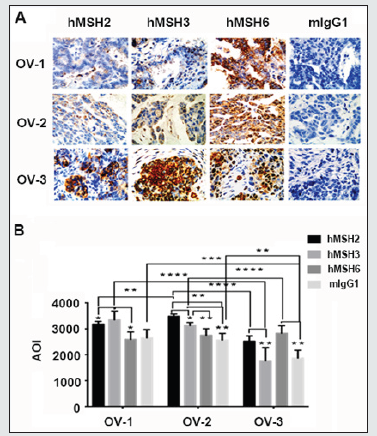
As shown in Table 1, all ovarian cancer cells displayed positive ectopic cytoplasmic and/or membrane hMSH2/6 expression and cytoplasmic and/or nuclear hMSH3 expression, while loss of nuclear expression of hMSH2 was observed in all 3 categories of ovarian cancer tissues. Strong and clear cytoplasmic and/or membrane hMSH2/3/6 expression was strikingly observed in ovary cyst-myxomatous adenoma nests (Figure 4A). Total loss of nuclear expression of hMSH6 was found in 66.67% (2/3) of the examined ovarian cancer tissues (Figure 4A). hMSH2/3/6 expression showed substantial heterogeneity among individual ovarian cancer patients (Figure 4B).
Figure 4: Subcellular distribution of hMSH2/3/6 in ovarian cancer tissues. A, IHC analyses of hMSH2, hMSH3 and hMSH6 distribution in 3 subtypes of ovarian cancer tissues (OV-1-3). Columns 1-4, ovarian cancer tissues stained with specific anti-hMSH2 mAb (clone G219), anti-hMSH3 mAb (clone 52), anti-hMSH6 mAb (clone 44) or mouse IgG1. Original magnification, 400×. B, Area of intensity (AOI) of hMSH2/3/6 expression in ovarian cancer tissues was analyzed with ImageJ software and is presented as the mean ±SD (N=3, n=5). *, P<0.05; **, P<0.01; ***, P<0.001.
Discussion
Human msh2 is one of the most crucial genes involved in the
DNA MMR pathway and was strongly associated with increased
tumor mutational burden in a multivariate analysis [11]. Recent
studies have shown that high hMSH2 expression is significantly
associated with smoking, while low hMSH2 expression is an
indicator of MMR deficiency in lung adenocarcinoma [11]. The high
expression of hMSH2 accompanied by increased PD-L1 expression
and CD8+ T cell infiltration therefore leads to the development of a
prominent immunotherapy-responsive microenvironment for lung
adenocarcinoma and acts as a potential surrogate biomarker of
tumor mutational burden to identify immune checkpoint blockade
responders in this disease [11]. Moreover, recent studies revealed
that MSH2-MSH6 played a crucial role in activation-induced
deaminase-initiated antibody diversity by recognizing uracil(s) in
the Ig gene loci to generate DNA breaks [12]. Many studies have
identified mutations in hmsh2 as diagnostic and/or prognostic
factors in carcinomas not only in the US and Canada but also in
the Middle East and China [13-15]. Human msh gene mutations
also have strong potential as novel candidate triple-negative
breast cancer predisposition genes and are closely associated
with acute adverse events and survival in rectal cancer patients
receiving postoperative chemoradiotherapy [16-19]. Pathogenic
or likely pathogenic germline mutations in pms2, msh2 or msh6
were detected in 0.5% (6/1,179) of lung cancer patients [18]. In
this study, we screened a novel mutation in the gene sequence of
hmsh2 at c.2332 T>A in the OSC cell line HO8910. This is a silent
mutation that theoretically does not result in a change to the amino
acid sequence of hMSH2 protein or to the phenotype of HO8910
cells. However, a rapid expansion and a strong increase in hmsh2
mRNA expression were observed in this cell line compared with the
control cell line.
Moreover, several soluble full-length or truncated variants of
hMSH2 proteins were observed in the whole cell protein extract
and the culture supernatant of HO8910. We deduce that these
altered biological characteristics of HO8910 cells and the genesis
of OSC might be linked to the hmsh2 c.2332 T>A variation, as
evidence showed that germline mutations of hmsh2 and its family
members were closely associated with the pathogenesis of Lynch
syndrome (LS). For example, hmsh2 c.2152 C>T alteration has
been recently reported as a founder mutation in Portugal; its high
proportion implies combined screening for this mutation and some
other previously reported founder mutations will be helpful in
the genetic testing of Portuguese families with suspected LS [19].
The occurrence and clinical significance of hmsh2 c.2332 T>A
and soluble full-length and truncated forms of hMSH2 proteins in
clinical OSC patients remain to be clarified in the future.
Compared with HO8910 cells, SKOV3 cells displayed stronger ectopic membrane and nuclear expression of hMSH2 in laser confocal microscopy and FCM analyses, suggesting better membrane anchoring and a more efficient nuclear importing system in SKOV3 cells. The ability of the notably overexpressed mhMSH2 on SKOV3 cells to promote human Vγ9Vδ2 T cell-mediated recognition and cytotoxicity via TCRγδ/NKG2D receptors towards target OSC cells was later validated by independent cytotoxicity and specific antibody blockade cytotoxicity assays, providing further evidence for mhMSH2 functioning as an OSC-associated self-antigen (ligand) for human Vγ9Vδ2 T cells in anti-ovarian cancer immunity [10]. In the absence of information/data demonstrating that the observed mutation impacts function, one cannot determine if the mutation is physiologically relevant. In further investigations on the subcellular distribution of hMSH2 and its companion proteins in ovarian cancer tissues, we found that all examined ovarian cancer specimens (serous adenocarcinoma, embryo sinus carcinoma, cystmyxomatous adenoma) displayed positive ectopic cytoplasmic and/or membrane hMSH2/6 expression and cytoplasmic and/or nuclear hMSH3 expression, and total loss of nuclear expression of hMSH2/6 commonly occurred in almost all of the ovarian cancer tissues. Considering the high hmsh2 gene transcription and the loss of nuclear distribution of hMSH2 protein in HO8910 cells, we hypothesized that the nuclear importing system of hMSH2 and/ or hMSH6 protein was dysfunctional. By contrast, recently, it has been reported that a high overall rate (16.2%) of MMR deficiency was surprisingly observed in ovarian endometrioid carcinoma and was significantly associated with increased IFOG (International Federation of Obstetrics and Gynecology) grade and CD8+ intraepithelial lymphocyte infiltration but not with cancer-specific death [19]. These findings suggest a promising future in which loss of nuclear expression of hMSH2 and/or hMSH6 protein may function as effective screening/diagnostic markers or therapeutic targets for different subtypes of ovarian cancers and as endogenous immune ligands not only for Vγ9Vδ2 T cells [20]. We expected that a human cell-based assay system for functional testing of hMSH2 and its family members will facilitate the identification of highrisk ovarian carcinoma patients and the generation of individual autoantigen-targeted immunotherapies for OSC.
Conclusion
In this study, we identified a silent hmsh2 c.2332 T>A mutation (GenBank accession no. MG674653) along with serval novel soluble truncated forms of hMSH2 variants in HO8910 cells and validated the mhMSH2/TCRγδ/NKG2D-mediated recognition and cytotoxicity of Vγ9Vδ2 T cells against mhMSH2/6-overexpressing SKOV3 cells. We also demonstrated the abnormal subcellular distribution of hMSH2/3/6 in the examined ovarian cancer tissues. The clinical significance of the critical findings in OSC genesis and human Vγ9Vδ2 T cell-mediated anti-OSC immunity remain to be further investigated.
Conflict of Interest
The authors have no financial conflicts of interest regarding the publication of this paper.
Acknowledgment
We sincerely thank Professor Xu Liu, Yandong Zhang and Yunzhou Gao from Basic Medical Sciences, Chinese Academy of Medical Sciences and School of Peking Union Medical College, for their detailed technical instructions on laser confocal microscopy and IHC analyses. Special thanks are extended to various peer reviewers for their enlightening suggestions for improving the manuscript. This work was funded by grants from the National Natural Science Foundation (81572804, 81971541) and by the Guangzhou Institute of Pediatrics/Guangzhou Women and Children’s Medical Center Fund (IP-2018-021).
References
- Fishel R, Ewel A, Lee S, Lescoe MK, Griffith J (1994) Binding of mismatched microsatellite DNA sequences by the human MSH2 protein. Science 266(5189): 1403-1405.
- Nepal RM, Tong L, Kolaj B, Edelmann W, Martin A (2009) Msh2-dependent DNA repair mitigates a unique susceptibility of B cell progenitors to c-Myc-induced lymphomas. Proc Natl Acad Sci U S A 106(44): 18698-18703.
- Dai Y, Chen H, Mo C, Cui L, He W(2012) Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human gammadelta T cells to induce innate anti-tumor/virus immunity. J Biol Chem 287(20): 16812-16819.
- Dai YM, Liu HY, Liu YF, Zhang Y, He W(2018) EBV transformation induces overexpression of hMSH2/3/6 on B lymphocytes and enhances gammadelta T-cell-mediated cytotoxicity via TCR and NKG2D. Immunology154(7): 673-682.
- Comeau K, Paradis P, Schiffrin EL (2020) Human and murine memory gammadelta T cells: Evidence for acquired immune memory in bacterial and viral infections and autoimmunity. Cell Immunol 357: 104217.
- Mo C, Dai Y, Kang N, Cui L, He W (2012) Ectopic expression of human MutS homologue 2 on renal carcinoma cells is induced by oxidative stress with interleukin-18 promotion via p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) signaling pathways. J Biol Chem 287(23): 19242-19254.
- Parte SC, Batra SK, Kakar SS (2018) Characterization of stem cell and cancer stem cell populations in ovary and ovarian tumors. J Ovarian Res 11(1): 60-69.
- Ji JX, Cochrane DR, Tessier Cloutier B, Chen SY, Ho G, et al ( 2020) Arginine Depletion Therapy with ADI-PEG20 Limits Tumor Growth in Argininosuccinate Synthase-Deficient Ovarian Cancer, Including Small-Cell Carcinoma of the Ovary, Hypercalcemic Type. Clin Cancer Res 26(16): 4402-4413.
- Huang W, Zhang Y, Xu Y, Yang S, Li B, et al (2020) Comprehensive analysis of the expression of sodium/potassium-ATPase alpha subunits and prognosis of ovarian serous cystadenocarcinoma. Cancer Cell Int 20: 300-309.
- Chen H, He X, Wang Z, Wu D, Zhang H, et al (2008) Identification of human T cell receptor gammadelta-recognized epitopes/proteins via CDR3delta peptide-based immunobiochemical strategy. J Biol Chem 283(18): 12528-12537.
- Jia M, Yao L, Yang Q, Chi T (2020) Association of MSH2 Expression With Tumor Mutational Burden and the Immune Microenvironment in Lung Adenocarcinoma. Front Oncol 10: 160-168.
- Zanotti KJ, Maul RW, Yang W, Gearhart PJ (2019) DNA Breaks in Ig V Regions Are Predominantly Single Stranded and Are Generated by UNG and MSH6 DNA Repair Pathways. J Immunol 202(5): 1573-1581.
- Alkhalidi H, Kfoury H (2012) Status of mismatch repair genes hMSH2 and hMSH6 in colorectal cancer in Saudi patients: an immunohistochemical analysis. East Mediterr Health J 18(11): 1114-1117.
- Win AK, Lindor NM, Young JP, Macrae FA, Young GP, et al (2012) Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst 104(18): 1363-1372.
- Hu F, Li D, Wang Y, Yao X, Zhang W, et al (2013) Novel DNA variants and mutation frequencies of hMLH1 and hMSH2 genes in colorectal cancer in the Northeast China population. PLoS One 8(4): e60233.
- Yi D, Xu L, Luo J, You X, Huang T, et al (2019) Germline TP53 and MSH6 mutations implicated in sporadic triple-negative breast cancer (TNBC): a preliminary study. Hum Genomics 13(1): 1-4.
- Yang J, Huang Y, Feng Y, Li H, Feng T, et al (2019) Associations of Genetic Variations in Mismatch Repair Genes MSH3 and PMS1 with Acute Adverse Events and Survival in Patients with Rectal Cancer Receiving Postoperative Chemoradiotherapy. Cancer Res Treat 51(3): 1198-1206.
- Sun S, Liu Y, Eisfeld AK, Zhen F, Jin S, et al (2019) Identification of Germline Mismatch Repair Gene Mutations in Lung Cancer Patients With Paired Tumor-Normal Next Generation Sequencing: A Retrospective Study. Front Oncol 9: 500-550.
- Pinheiro M, Francisco I, Pinto C, Peixoto A, Veiga I, et al (2019) The nonsense mutation MSH2 c.2152C>T shows a founder effect in Portuguese Lynch syndrome families. Genes Chromosomes Cancer 58(9): 657-664.
- Rambau PF, Duggan MA, Ghatage P, Warfa K, Steed H, et al (2016) Significant frequency of MSH2/MSH6 abnormality in ovarian endometrioid carcinoma supports histotype-specific Lynch syndrome screening in ovarian carcinomas. Histopathology 269(2): 288-297.