Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1373
Review ArticleOpen Access
Glioblastoma Multiforme: a miPEP and miRNA Approach Volume 2 - Issue 2
Sepehr Saberian1, Sharif Morsalin1, Jinbo Fang1, Christian M Mustroph2, Seema Yousuf3, David A Gimbel2, Veena N Rao1 and E Shyam P Reddy1*
- 1Cancer Biology Program, Department of Ob/Gyn, Morehouse School of Medicine, Atlanta, GA,
- 2Department of Neurosurgery, Emory University, Atlanta, GA
- 3Department of Emergency Medicine, Emory University, Atlanta, GA
Received:March 04, 2022; Published:March 28, 2022
Corresponding author: E Shyam P Reddy, Ph.D, Professor and Director, GCC Distinguished Cancer Scholar, Cancer Biology Program, Department of OB/GYN, Morehouse School of Medicine, Atlanta, GA, USA
DOI: 10.32474/LOJPCR.2022.03.000153
Abstract
Glioblastoma multiforme (GBM) is perhaps the most devastating tumor of the central nervous system (CNS), with an approximately 7% five-year survival rate. The micro-RNAs (miRNA) miRNA-200a and are downregulated in GBM. We hypothesize that miPEP-200a and miPEP-200b, discovered in our lab, has potential to restore levels of miRNA-200a and miRNA-200b and function as inhibitors of migration of glioma cells. For this work, a literature search was conducted, identifying various upregulated and downregulated members of the miRNA family in GBM. A search for the miPEP-200 family was also performed. An in-depth analysis of the blood-brain barrier (BBB) components was conducted to determine feasible candidates for therapeutic treatment of GBM. There has recently been a substantial increase in studies examining the role of miRNA in GBM. A thorough review of existing literature showed that in GBM, 73% (256) of miRNAs involved are upregulated and 27% (95) are downregulated. The latter group contains miRNA-200a and miRNA-200b. It’s been shown that miRNA has excellent stability in the blood stream. Although miRNA can cross the BBB from the cerebrospinal fluid (CSF) to the blood, the reverse movement has not yet been shown. Considering the characteristics of the BBB combined with experimental data from various studies showing the ability of miRNA to cross the BBB, miRNA has potential for use as a therapeutic agent in GBM. Specifically, miRNA-200a and miRNA-200b are known to be downregulated in GBM. Interestingly, our laboratory recently discovered the novel proteins miPEP-200a and miPEP-200b, both products of pri-miRNA translation. These proteins may demonstrate great potential for upregulating miRNA-200a and miRNA- 200b, given data suggesting this positive feedback mechanism found in one study. Since miPEP-200a and 200b were shown to inhibit migration of cancer cells and Epithelial to Mesenchymal Transition (EMT) of cancer cells, we propose that these miPEP-200a and miPEP-200b may function as therapeutic agents for targeting the migration of glioma cells.
Keywords:Gene Expression; Glioblastoma; glioblastoma multiforme; tumor; miRNA
Introduction
Background
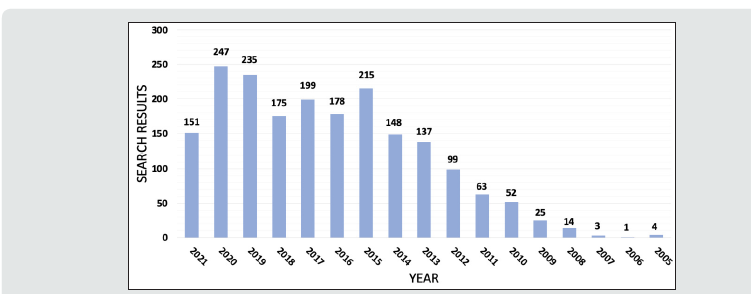
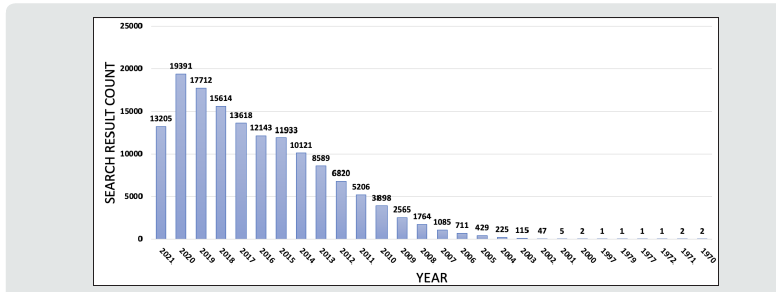
Glioblastoma multiforme (GBM) remains the most aggressive primary tumor of the central nervous system (CNS), with a median survival of 12-16 months with the current standard of resection, radiation, and chemotherapy [1,2]. Despite being the most common malignant primary brain tumor and accounting for 3-4% of all tumor-related deaths, treatment advances have been limited with continued overall poor prognosis [3,4]. High cellular heterogeneity coupled with its infiltrative nature have made advances in systematic management difficult while making curative complete resection impossible [5]. There has been increasing interest in studying the role of micro-RNAs (miRNA) in GBM due to their role in treatment resistance, checkpoint avoidance, and tumorigenesis [6]. A PubMed query for the search phrase “glioblastoma AND miRNA” in October 2021 demonstrates a remarkable increase from 2005, which mirrors interest in miRNA Figures 1 & 2.
Micro-RNAs are a subset of a larger category of RNA molecules called noncoding-RNAs (ncRNA) [7]. These RNAs have traditionally been thought of as having a function other than the production of proteins. The general mechanism to produce proteins intracellularly is the following: DNA is first transcribed to messenger RNA (mRNA) via transcription factors. This “copy” is then transported outside the nucleus with various modifications made to the mRNA in the process. Once in the cytoplasm, the mRNA is recognized by ribosomes, which then translate its code, codon by codon, into proteins, which then perform their functions [8]. However, miRNA does not participate in protein synthesis. Instead, it can bind with high specificity to mRNA strands and inhibit their eventual translation to proteins via different mechanisms [9]. Interestingly, miRNA has been found to be highly involved in many neoplastic processes in recent years. It has been postulated that miRNA dysregulation is involved in most, if not all, cancer types. More specifically, the miRNA family has been shown to be involved in the epithelial-to-mesenchymal (ETM) transition process in various types of cancer. Previously, we have published results from our study demonstrating miRNA’s involvement in ETM in cells of prostatic adenocarcinoma [10].
Clinical Presentation
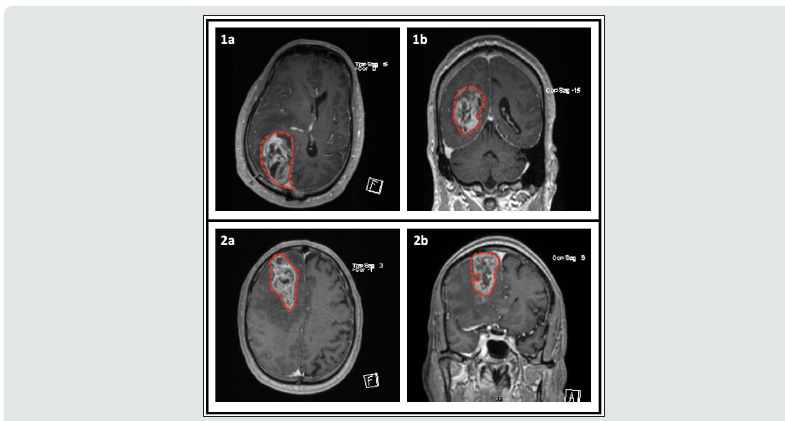
The clinical presentation of GBM remains varied and can include seizures, headaches, changes in mental status, and neurological deficits. Clinical presentation depends on the location of the tumor and its effect on structural areas of the brain. Changes in personality and disinhibition can be attributed to frontal cortex damage, auditory and visual dysfunction can be attributed to temporal lobe damage, and tumors near motor areas can cause weakness. Imaging, while varied, typically demonstrates a heterogeneously enhancing hypercellular lesion with possible areas of necrosis with surrounding vasogenic edema [11]. Figure 3 shows contrasted MRIs of two patients with GBM.
Figure 3:Contrasted brain MRI of two patients with Glioblastoma Multiforme. Tumor is outlined with dashed red line. Patient 1 (Images 1a and 1b): This patient is status post right parietal craniotomy and right parieto-occipital intraparenchymal mass resection. Mild linear diffusion restriction along the margins of the resection cavity compatible with devitalized soft tissue. Patient 2 (Images 2a and 2b): Intraparenchymal mass in the right frontal lobe measuring 6.7 x 3.3 x 5.7 cm, with findings most consistent with high grade glial neoplasm. Moderate microvascular ischemic changes including chronic infarcts in the bilateral basal ganglia, thalami, and pons/midbrain.
miRNA and Glioblastoma Multiforme
As a result of recent interest in miRNA and its role in different cancers, many studies have examined its involvement in GBM. Although the vast majority of miRNA dysregulation in GBM has been shown to be in the form of overactivity, specific miRNAs, such as miR-593, have been found to be underactive in this setting [12,13]. An experiment involving circMELK in GBM showed that it could sponge miR-593, leading to neoplastic processes such as hyperproliferation and tumor invasion, among others. This is due to suppression of the regular activity of miR-592; it serves to inhibit translation of the oncogene Eph receptor B2 [14]. On the other hand, miR-21 suppression has been shown to induce apoptosis in vitro. This indicates that overexpression of miR-21 in GBM leads to inhibition of apoptosis, causing unregulated cellular proliferation [15]. A review by Shea et al. reports a comprehensive list of the most current miRNA subtypes found in GBM and their roles in the pathogenesis of the disease [16]. Of the 351 miRNAs involved, approximately 73% (256) are upregulated, and 27% (95) are downregulated [12]. Given that GBM is a hyperproliferative process, these findings imply that most miRNA subtypes involved in GBM target tumor suppressors since miRNA is an inhibitory regulator of translation.
An interesting property of miRNAs in the setting of GBM is their ability to “transmit” resistance to adjacent cells. Buruiană et al. showed that extracellular vesicles (EV) derived from GBM contain miR-21 and miR-451, which can fuse with microglia and monocytes, leading to their proliferation. Additionally, by way of miR-21, both GBM and monocytes begin to show signs of proliferation, more aggressive invasion, as well as increased overall angiogenesis [17]. These properties may render GBM extremely resistant to treatment compared to other neoplastic processes. In one study, miR-1238 was shown to be overexpressed not only in GBM patients compared to healthy individuals but also in temozolomide (TMZ)-resistant GBM [18]. Interestingly, in another experiment, when TMZ was administered to TMZ-sensitive GBM cells in a sustained manner, TMZ-resistance developed with concurrent overexpression of miR- 21. Following this process, silencing of miR-21 led to increased apoptosis along with decreased tumor invasion, indicating that miR-21 acted as an oncogene [19]. These studies demonstrate not only a clear role that miRNAs play in GBM development but, far more importantly, evidence that modification of their levels can lead to decreases in rates of tumor progression.
As multiple studies have shown, STAT3 activation appears to be a common culprit in GBM development and growth in the setting o miRNA dysregulation [20-22]. STAT3 is a regulator of transcription involved in the immune system’s proper functioning. It is tightly regulated, and any deviation from its physiologic activity levels may lead to autoimmune diseases, lowered immune function, or neoplastic processes, including cancer [23]. miRNAs affect STAT3 either directly or in an upstream manner. Specific miRNAs have been shown to decrease the production of STAT3 stimulators, which in turn reduce STAT3 activity. This mechanism can be thought of as “inhibition of inhibitors.” Other downregulated miRNAs in GBM, such as miR-519, have been shown to bind with the untranslated region of the STAT3 mRNA itself, thereby decreasing its production [24]. This mechanism can be thought of as a “failure of inhibition.” Decreasing STAT3 activity by reducing miR-21 levels has been demonstrated to reduce neoplastic cell survival and further proliferation significantly [25-26]. These findings make a strong case for miRNA’s role in glioma genesis and provide evidence that innovating new methods targeted at regulating miRNA levels could show remarkable improvements in GBM management and treatment. Furthermore, miRNAs may not only be used for GBM management but could be used as biomarkers. Several studies have examined various miRNA families as potential serum biomarkers for GBM diagnosis and prognosis. Although a significant number of miRNAs are dysregulated in GBM, not all can be relied on as useful serum markers. To identify promising biomarkers, these studies utilized quantitative reverse transcription-polymerase chain reaction to analyze patient serum levels of various miRNAs [27-35].
Potential Role of miPEP
Our laboratory very recent discovery of miPEPs may play a pivotal role in GMB pathogenesis, progression, and perhaps even treatment. As previously discussed, miRNA is a “noncoding-RNA” family member. Despite this misleading name, we have found that miPEP is, in fact, a protein product of ncRNA (pri-miRNA) translation [36]. We showed that pri-miRNA, a precursor of mature miRNA, possesses an open reading frame that can interact with ribosomes to produce miPEPs [36]. Mammalian prostate and breast cells were found to both express protein products of pri-miRNA translation [10]. The specific functionality of miPEPs is still quite unclear, given their recent discovery in mammalian cells. However, in one study, a positive feedback mechanism was described. In this study, miPEP was shown to stimulate the synthesis of mature miRNA in plant cells [37]. Although yet to be proven in mammalian cells, this relationship likely does exist to a certain extent. By studying the cellular pathways involved in diseases where miRNA is known to be a culprit, we can infer that a miRNA/miPEP interaction likely takes place [10].
In the context of GBM, the existence of this miRNA/miPEP interaction could lead to an entirely new understanding of the disease; it would add another dimension to the complexity of this already-intricate tumor. Given that this interaction exists (although at this time, this remains a hypothesis), we can examine different scenarios in which it has implications. It is also essential to mention recent developments in miRNA functionality. As previously discussed, miRNA’s primary mechanism of action is in the cytoplasm via interaction with its target mRNA strands. However, in 2018, one study demonstrated the existence of mature miRNA within the nucleus [38]. Furthermore, other experiments have demonstrated the ability of miRNA to interact with the nuclear DNA and to downregulate specific genes [39]. This may initially seem trivial; however, it provides incredible insight for the development of therapeutic agents.
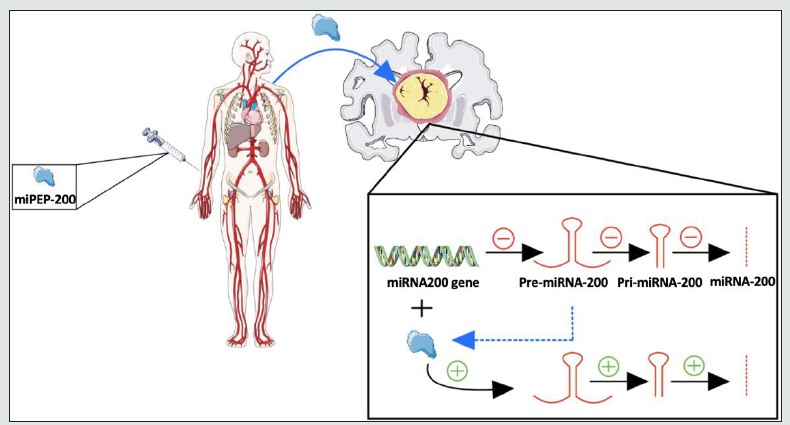
We have thus far established that in GBM, most miRNA subtypes involved are upregulated, and only 27% are downregulated. We have also discussed that upregulated miRNAs serve to inhibit the production of tumor suppressors, while downregulated miRNAs serve to inhibit oncogenic proteins. This logic is based on the main functionality of miRNA, which is to target mRNA strands, leading to halted translation. With our previous assumption of the positive feedback miRNA/miPEP interaction, we expect the addition of miPEPs produced from the upregulated miRNA population to worsen disease progression. Similarly, miPEPs made from the downregulated miRNA population may slow disease progression. However, we must also consider the more significant proportion of upregulated miRNAs (73%) than downregulated miRNAs (27%). Based on population size alone, if the highest possible concentration of miPEPs from the latter population is added, the maximal rate of downregulated miRNA production would likely still not be able to outweigh the upregulated population. There may, however, be a way to circumvent this intracellular roadblock. As mentioned previously, one of the newest developments in miRNA functionality is its activity in the nucleus and, even further, its ability to regulate gene expression via direct interaction with DNA [38-39]. By identifying the specific intranuclear miRNAs that interact with the genes producing the upregulated miRNA population in GBM; it allows us to silence those genes using a minimal amount of miRNA. As such, we simply silence the individual genes that lead to multiple copies of mature miRNA. In addition, this would concurrently increase the efficacy of miPEP. This is a classic example of a synergistic effect, in which the efficacy of compounds A and B together is far greater than the additive efficacy of compounds A and B individually. Figure 4 provides a visual representation of this proposed mechanism.
Figure 4:Mechanism for potential rescue of miRNA-200a and miRNA-200b levels in GBM by administration of miPEP-200a and miPEP-200b (Dashed blue line - organic synthesis of miPEP from translation of pre-miRNA)
Blood-Brain Barrier
One of the most significant impediments to developing therapeutic agents against GBM with high efficacy is the notoriously selective BBB. The cerebral vessels are comprised of an endothelium, connected by tight junctions, without any fenestrations. Various types of cells are also involved in the BBB. In addition to endothelial cells, the BBB includes mural cells, astrocytes, and immune cells [40]. The smooth muscle cells surrounding the endothelium and pericytes are mural cells. Pericytes are located within the basement membrane. Pericytes possess proteins that aid contraction, and by extending their process along the outer surface of the endothelium, these cells can differentially regular capillary diameter. Although these cells are an essential component of the functioning BBB, they also play a crucial role in angiogenesis and during BBB development. Although not a cellular component, the basement membrane (BM) is another part of the BBB. It contains various molecules such as heparin sulfate, collagen, and laminin.
The BM serves as one of the main barriers to entry into the cerebral parenchyma. It can be further divided into the vascular glia limitans perivascularis and the inner vascular BM, the former of which is produced by secretions from astrocytes. The inner vascular BM, on the other hand, is produced by secretions from pericytes and endothelial cells. Astrocytes are a cellular component of the BBB that can surround segments of the cerebral microvasculature. They also extend foot processes that contact neurons. These cells are unique in that they provide communication between neurons and the vasculature. This communication is crucial, as it relays neural activity to the vasculature, allowing for very tight regulation of blood flow based on neuronal activity and metabolic requirements [41].
There have been several studies focused on the role of astrocytes in GBM, many of which have demonstrated that these cells are involved in one capacity or another. [42-46] Interestingly, astrocytes have not been shown to play a role in the initial development of the BBB, unlike pericytes. Instead, they serve to maintain the function of the BBB following its development. Considering all the discussed components of the BBB, this elaborate make-up renders penetration to and from the brain parenchyma very selective and challenging in nature. Despite the BBB’s highly selective nature, there are mechanisms in place for the entry of specific molecules. Highly lipid-soluble substances can cross the BBB via simple diffusion [47]. Additionally, substances with low lipid solubility can cross the BBB, not by simple diffusion but rather carrier-mediate transport. Specific proteins can also enter the brain parenchyma through receptor-mediated transcytosis or absorptive mediate transcytosis [48]. These exceptions to the BBB’s highly selective nature present various opportunities for the delivery of therapeutic agents to restore normal miRNA levels.
miRNA-200 and miPEP-200: Implications for Pharmacotherapy
The overexpression of various miRNAs has been elucidated in GBM. In essence, the goal of therapy concerning miRNA is to decrease the production or inhibit the activity of the upregulated miRNAs and vice versa for the downregulated miRNA. The miRNA-200 family consists of miRNA-200a, miRNA-200b, miRNA- 200c, miRNA-141, and miRNA-429, all of which have been shown by many studies to be downregulated in GBM tissue samples. Although this is generally the case, some members of the miRNA-200 family are differentially upregulated in specific grades of gliomas. For instance, miRNA-429 and miRNA-141-3p have been shown to be upregulated in high-grade gliomas compared to low-grade gliomas and healthy brain tissue.
Additionally, an association between miRNA-200 family dysregulation and epigenetic changes such as methylation of DNA and other histone modifications have been demonstrated. These changes were shown to lead to GBM disease progression. miRNA- 200b has specifically been shown to target multiple genes involved in GBM metastasis through BBB modification, rendering it more permeable. Studies examining the correlation between levels of the miRNA-200 family and their respective functions have revealed several important roles of its members. miR-200a has been shown to be downregulated in human gliomas in general as well as GBM, with evidence showing that by interfering with the SIM2-s gene, miR-200a behaves as a tumor suppressor. Hence low levels indicate an inability to prevent tumorigenesis. miR-200b was also found to have a similar expression profile, but instead, it targets the RAB family and ZEB2, both of which have oncogenic properties. Again, miR-200b acts as a tumor suppressor by inhibiting oncogenic products. Interestingly, miR-200b has been found to correlate with glioma histopathologic grading, making it a suitable candidate for use as a prognostic biomarker in gliomas. miR200c is also under expressed in various gliomas and has been shown to target moesin. Lastly, miR-141 was found to target TGF-β2, a key upregulated growth factor presents in many neoplastic processes, acting as a tumor suppressor. Interestingly, miRNAs of this family can regulate other miRNAs and be regulated by multiple other miRNAs. As a result, the intricate regulatory web that forms is extremely promising for identifying which miRNAs or combinations of miRNA can have the most positive impact in GBM [48].
Due to our relatively recent discovery of pri-miRNA-200a and pri-miRNA-200b-encoded proteins, termed miPEP-200a and miPEP-200b [36], there currently are no publications on the involvement of these proteins in GBM. However, we have shown in a previous study that both miPEP-200a and miPEP-200b markedly reduce not only prostate cancer migration, but also the process of epithelial-to-mesenchymal transition [36]. To achieve further granularity, the expression profiles of various proteins were obtained and, interestingly, vimentin was found to be considerably downregulated because of the increased expression of miPEP-200a and miPEP-200b. Furthermore, miRNA-200a and miRNA-200b are known to have similar effects on vimentin expression. Our findings were congruent with those of other studies, as vimentin is known to be involved in both cancer progression and the epithelialto- mesenchymal transition [36]. Interestingly, in a 2019 study by Nowicki et al., it was shown that vimentin plays a key role in increasing migration of GBM cells [49]. Thus, if miPEP-200a and miPEP-200b display a similar behavior in GBM as they did in prostate cancer, they may prove useful in developing a new class of GBM therapy which we call it as miPEP-therapy.
As discussed earlier, there is convincing evidence that miRNA can act as a gene expression regulator via direct interaction with DNA. Therefore, further studies identifying the precise gene that leads to each upregulated miRNA are the first step in developing an efficacious pharmaceutical agent for GBM. The next step would be to identify the regulatory miRNA capable of interacting with that gene. Finally, and perhaps the most difficult of the processes, we must locally increase the concentration of the isolated regulatory miRNA in the vicinity of the tumor. There are two main methods of accomplishing this: via infusion of miRNA into the bloodstream and the second via direct injection of the compound by way of intracerebral delivery. Each method has its advantages and disadvantages. Surprisingly, unlike other molecules containing nucleic acids, intravenous injection of miRNA does not lead to nearimmediate denaturation [50]; it has exceptional stability in the circulation, making it a good candidate for systemic distribution. On the other hand, one obstacle that remains is the BBB. It has been shown that miRNA can enter the bloodstream from the cerebrospinal fluid (CSF); however, the reverse direction of miRNA transport from the blood to the CSF has not yet been shown [51].
Further experiments to analyze the nature of this unidirectional flow of miRNA would allow for a better understanding of its mechanism. With the intracerebral method of drug delivery, the BBB is no longer an obstacle, as the drug is delivered directly to the ventricles, where the BBB has already been encountered [52]. Despite this relatively effective method of delivery, direct access to the parenchyma is required via either craniotomy or an injection tube. This procedure is far more invasive than an intravenous injection; furthermore, physical access to the brain parenchyma is associated with an elevated risk of serious infections, most commonly with pathogens such as Cutibacterium acnes, Staphylococcus epidermidis, and Enterobacter cloacae [53]. Of the two methods, intravenous injection of miRNA appears more ideal when considering patient comfort and potential for therapeutic efficacy.
We propose an additional area of study for developing pharmaceutical agents aimed at GBM. With the discovery of miPEPs in mammalian cells, novel approaches to treating various diseases, including GBM, are now possible. If miPEP behaves similarly in plant cells as in mammalian cells, that is, positive feedback regulation of its respective miRNA, then targeting diseases with under-expression of miRNA with miPEP appears to be a reasonable approach [11]. In GBM, however, overexpression of miRNA is generally the problem at hand. Under an earlier subsection, we have described the proposed mechanism for using miPEP in GBM as a therapeutic agent (mi-PEP therapy).
Discussion
With the recent increase in experiments involving miRNAs and the discovery of miPEPs, new opportunities may present in creating solutions to managing or even treating aggressive diseases. The vast amount of knowledge that has been gathered has tremendously aided in classifying various families of miRNAs and their respective roles in diseases spanning multiple organ systems ranging from simple organ dysfunction to the most aggressive forms of cancer. This paper has examined the relationship between miRNAs and GBM to propose potential targets for treating this disease. A clear correlation has been shown with many miRNAs and GBM where either under expression or overexpression has been observed compared to healthy individuals. These molecules can act as either tumor suppressors or oncogenes in different settings, and their respective expression status provides insight into their potential role. In the setting of miRNA under expression, it is likely acting as a tumor suppressor, and in the setting of overexpression, it is likely acting as an oncogene [48].
This simple framework provides a robust starting ground for identifying intracellular and extracellular targets for therapeutic intervention. Furthermore, with our recent discovery of miPEPs, we now know that we are not limited to developing miRNAbased agents for combatting this disease. miPEPs present another regulatory step for modifying miRNA levels indirectly yet innovatively. Taking advantage of this knowledge could lead to more direct medication delivery, smaller doses of medication needed, and even better outcomes due to the functional synergy between miPEP and miRNA. Although theoretically reasonable, this proposal requires validation by extensive experiments to identify the biochemical stability of miPEP in the microenvironment of the bloodstream, its relative size and charge, lipid solubility, and ability to cross the BBB. Aside from determining these essential molecular characteristics of miPEP, its functional ability must also be exhaustively studied. Given its recent discovery, miPEP presents a set of challenges in the context of identifying not only its tissue specificity but its functional specificity. If it is found that miPEP demonstrates a high degree of molecular promiscuity, it will render it an inferior candidate for use as targeted therapy. If molecular promiscuity is demonstrated, the specific effects of miPEP on these alternate organs, cellular components, and gene products must first be fully elucidated prior to consideration for treating specific pathologies, such as GBM.
Conclusions
If our proposal for the synergistic relationship between miPEP and miRNA holds, entirely new treatment methods can be developed for preventing, managing, or even treating GBM. Just as this aggressive tumor tends to evolve to resist therapeutic interventions, we must also fundamentally change how we approach it from a pharmacologic standpoint. This new miRNA revolution in medicine can prove extremely helpful in battling GBM. This, paired with the discovery of miPEPs, provides valid and exciting new avenues for researchers to begin a search for GBM treatment. With further research on the molecular characteristics of the various classes of miRNAs and identifying properties of their corresponding miPEPs, we can better understand how to circumvent the obstacles presented by the BBB and precisely and safely deliver these agents to GBM cells.
Acknowledgements
We thank all the members of Reddy and Rao laboratories. This study was funded in part by the U.S. Army Medical Research and Materiel Command under W81XWH-08-1-0628, W81XWH-09-1-0236, W81XWH-10-1-0418 (Reddy, ESP) and the Georgia Cancer Coalition Distinguished Cancer Scholar award (Reddy, ESP and Rao, VN), U54/56 Morehouse School of Medicine/ University of Alabama at Birmingham/Tuskegee University Partnership Grant (NIH 2U54CA118948, 3U54CA118638-05S1 to Dr Reddy), RCMI, U54 RR026137 and U54 MD007588.
References
- Fishel R, Ewel A, Lee S, Lescoe MK, Griffith J (1994) Binding of mismatched microsatellite DNA sequences by the human MSH2 protein. Science 266(5189): 1403-1405.
- Nepal RM, Tong L, Kolaj B, Edelmann W, Martin A (2009) Msh2-dependent DNA repair mitigates a unique susceptibility of B cell progenitors to c-Myc-induced lymphomas. Proc Natl Acad Sci U S A 106(44): 18698-18703.
- Dai Y, Chen H, Mo C, Cui L, He W(2012) Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human gammadelta T cells to induce innate anti-tumor/virus immunity. J Biol Chem 287(20): 16812-16819.
- Dai YM, Liu HY, Liu YF, Zhang Y, He W(2018) EBV transformation induces overexpression of hMSH2/3/6 on B lymphocytes and enhances gammadelta T-cell-mediated cytotoxicity via TCR and NKG2D. Immunology154(7): 673-682.
- Comeau K, Paradis P, Schiffrin EL (2020) Human and murine memory gammadelta T cells: Evidence for acquired immune memory in bacterial and viral infections and autoimmunity. Cell Immunol 357: 104217.
- Mo C, Dai Y, Kang N, Cui L, He W (2012) Ectopic expression of human MutS homologue 2 on renal carcinoma cells is induced by oxidative stress with interleukin-18 promotion via p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) signaling pathways. J Biol Chem 287(23): 19242-19254.
- Parte SC, Batra SK, Kakar SS (2018) Characterization of stem cell and cancer stem cell populations in ovary and ovarian tumors. J Ovarian Res 11(1): 60-69.
- Ji JX, Cochrane DR, Tessier Cloutier B, Chen SY, Ho G, et al ( 2020) Arginine Depletion Therapy with ADI-PEG20 Limits Tumor Growth in Argininosuccinate Synthase-Deficient Ovarian Cancer, Including Small-Cell Carcinoma of the Ovary, Hypercalcemic Type. Clin Cancer Res 26(16): 4402-4413.
- Huang W, Zhang Y, Xu Y, Yang S, Li B, et al (2020) Comprehensive analysis of the expression of sodium/potassium-ATPase alpha subunits and prognosis of ovarian serous cystadenocarcinoma. Cancer Cell Int 20: 300-309.
- Chen H, He X, Wang Z, Wu D, Zhang H, et al (2008) Identification of human T cell receptor gammadelta-recognized epitopes/proteins via CDR3delta peptide-based immunobiochemical strategy. J Biol Chem 283(18): 12528-12537.
- Jia M, Yao L, Yang Q, Chi T (2020) Association of MSH2 Expression With Tumor Mutational Burden and the Immune Microenvironment in Lung Adenocarcinoma. Front Oncol 10: 160-168.
- Zanotti KJ, Maul RW, Yang W, Gearhart PJ (2019) DNA Breaks in Ig V Regions Are Predominantly Single Stranded and Are Generated by UNG and MSH6 DNA Repair Pathways. J Immunol 202(5): 1573-1581.
- Alkhalidi H, Kfoury H (2012) Status of mismatch repair genes hMSH2 and hMSH6 in colorectal cancer in Saudi patients: an immunohistochemical analysis. East Mediterr Health J 18(11): 1114-1117.
- Win AK, Lindor NM, Young JP, Macrae FA, Young GP, et al (2012) Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst 104(18): 1363-1372.
- Hu F, Li D, Wang Y, Yao X, Zhang W, et al (2013) Novel DNA variants and mutation frequencies of hMLH1 and hMSH2 genes in colorectal cancer in the Northeast China population. PLoS One 8(4): e60233.
- Yi D, Xu L, Luo J, You X, Huang T, et al (2019) Germline TP53 and MSH6 mutations implicated in sporadic triple-negative breast cancer (TNBC): a preliminary study. Hum Genomics 13(1): 1-4.
- Yang J, Huang Y, Feng Y, Li H, Feng T, et al (2019) Associations of Genetic Variations in Mismatch Repair Genes MSH3 and PMS1 with Acute Adverse Events and Survival in Patients with Rectal Cancer Receiving Postoperative Chemoradiotherapy. Cancer Res Treat 51(3): 1198-1206.
- Sun S, Liu Y, Eisfeld AK, Zhen F, Jin S, et al (2019) Identification of Germline Mismatch Repair Gene Mutations in Lung Cancer Patients With Paired Tumor-Normal Next Generation Sequencing: A Retrospective Study. Front Oncol 9: 500-550.
- Pinheiro M, Francisco I, Pinto C, Peixoto A, Veiga I, et al (2019) The nonsense mutation MSH2 c.2152C>T shows a founder effect in Portuguese Lynch syndrome families. Genes Chromosomes Cancer 58(9): 657-664.
- Rambau PF, Duggan MA, Ghatage P, Warfa K, Steed H, et al (2016) Significant frequency of MSH2/MSH6 abnormality in ovarian endometrioid carcinoma supports histotype-specific Lynch syndrome screening in ovarian carcinomas. Histopathology 269(2): 288-297.