
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1373
Research ArticleOpen Access
An Analysis of Risk Associated to Concomitant Use of Potential Inhibitors of Statins Metabolism Volume 3 - Issue 2
Carlos Treceño Lobato1,2*, María-Isabel Jiménez-Serranía2, Javier Herradón Muñoz1, Elena Valles Martín2 and José Luis Nájera García1
- 1Council of Professional Colleges of Pharmacists of Castilla y León, Castile and Leon, Spain
- 2ADViSE Research Group. Department of Health Sciences, Faculty of Health Sciences, Miguel de Cervantes European University (UEMC), Spai
Received:June 27, 2023; Published: July 03, 2023
Corresponding author: Carlos Treceño Lobato, Council of Professional Colleges of Pharmacists of Castilla y León. Castile and Leon,Spain
DOI: 10.32474/LOJPCR.2023.03.000159
Abstract
Background: Some statins are metabolised by CYP3A4 pathway and concomitant treatments with potential inhibitors of this
isoenzyme could influence in the occurrence of adverse drug reactions (ADR) related to an increase in dose.
Objective: To evaluate the ADR incidence rate of statins combined with CYP3A4 inhibitors and to detect new signals of ADR in
real-life ambulatory settings.
Methods: The authors performed an observational cross-sectional study of surveyed patients treated with statins (atorvastatin,
lovastatin, simvastatin –CYP3A4 metabolized-, fluvastatin, pitavastatin, pravastatin, rosuvastatin –other metabolization pathways-)
and CYP3A4 inhibitors (amiodarone, cyclosporine, cilostazol, diltiazem, dronedarone, fluoxetine, verapamil). Descriptive, clinical,
and ADRs incidence data were reported and analyzed through a bivariate and applied an adaptation of Bayesian methodology
(BCPNN) to detect new signals.
Results: A total of 112 patients were surveyed, the mean concomitant number of treatments per patient was 7,5±3,7 and
56,3% were on atorvastatin. The authors obtained higher risk of musculoskeletal or limb pain (0.58; 95% confidence interval
0.17-1.92) and paresthesias-myasthenia (0.67; 0.22–2.32) with CYP3A4 metabolised statins and higher risk of myalgia (OR,1.41;
0.42–4.74) with non-CYP3A4 metabolised statins. These results are confirmed with detection of positive signals of musculoskeletal
or limb pain linked to atorvastatin (FDR=0.016). A signal of osteoporosis linked to [atorvastatin+fluoxetine] (FDR=0.011) was also
detected.
Conclusion: The risk profile of statins metabolised by CYP3A4 did not largely differ with concomitant isoenzyme inhibitors,
being the most reported ADR the musculoskeletal or limb pain. Caution is recommended with calcium antagonists and cyclosporine.
Atorvastatin-fluoxetine combination enhance the risk of osteoporosis.
Keywords:Statin; CYP3A4 inhibitor; musculoskeletal pain; osteoporosis; interaction
Introduction
Statins, also known as hydroxymetil-glutaril coenzyme A reductase inhibitors (HMG CoA reductase), a key enzyme for liver cholesterol synthesis, use several metabolization pathways. So, atorvastatin, simvastatin and lovastatin are metabolised by the CYP3A4 isoenzyme of the P450 cytochrome [1,2] while other statins use different pathways, such as fluvastatin, which fundamentally utilises the CYP2C9 isoform, although, to a lesser extent, is also metabolised by CYP2C8 and CYP3A4 [3]. Lastly, pitavastatin, rosuvastatin and pravastatin do not employ the P450 cytochrome as a metabolization pathway [4]. It is a issue of concern the potential drug-drug interaction of CYP3A4-metabolised statins with other medications co-prescribed for chronic treatment that inhibit this isoenzyme, such as calcium antagonists, like diltiazem and verapamil, antiarrhythmics, like group-III agents amiodarone and dronedarone, and cyclosporine or fluoxetine. Consequently, these medications should not be combined with each other in the treatment of patients on atorvastatin, simvastatin or lovastatin [5- 7].
Therefore, it seems to be reasonable to determine the frequency of this potential drug-drug interaction, as well as its potential clinical impact on patients’ health. Thus, it is estimated that up to 5.5% of patients are concomitantly treated with a statin and a CYP3A4 inhibitor [8]. Among CYP3A4 inhibitors, a difference should be made between those that are used for acute treatment, such as erythromycin, clarithromycin, fluconazol, itraconazol and ketoconazol, and those employed for chronic treatment such as antihypertensive agents or antidepressants. When taking into account only chronic therapy, we find that 2.75 % of patients are treated concomitantly with a statin and a CYP3A4 inhibitor. However, it should be borne in mind that this percentage may be higher, depending on the specific medication under investigation.
Another point to be considered is the percentage of patients concurrently using a interacting susceptible statin -simvastatin, atorvastatin or lovastatin- and a CYP3A4 as chronic co-medications This drug combination is likely to give rise to a potential drugdrug interaction that may, in turn, result in an increased risk of muscle toxicity and other harmful effects, like increased creatine kinase levels, renal damage or hyperkalemia [9,10]. Currently, there is strong evidence supporting that such an interaction results in increased plasma levels of the statin in question [11,12]. Nevertheless, because of the individual differences across the patients in increased statin levels, it is hard to estimate the likelihood for this kind of interaction to occur in the individual patient. It is difficult as well to determine the potential clinical impact attributable to such an interaction. Studies on drug metabolism show that simvastatin and lovastatin are particularly sensitive to the inhibitory effects on CYP3A4 isoenzyme induced by other medications, while atorvastatin metabolism is affected to a lesser extent [13].
Because of this variability, it is not easy to state whether this potential interaction affects patients’ clinical outcome. This holds particularly true in the cases in which the medication is prescribed on an outpatient basis. In addition, concerning the reporting of mild adverse events, statins have the potential to frequently cause similar reactions independently of the above-described interaction. Also, the poor compliance of these patients or the occurrence of significant interactions with other medications may result in problems with statin safety. The community pharmacy may be a suitable setting to collect a significant sample of patients taking their medication on a real-life basis. This is why a community pharmacy network was established in 2015 in the Spanish region of Castilla y León aimed to increment the number of notifications of adverse drug reactions (ADRs), the final aim of the network being to promote the research on drug safety in the area.
Objective
The aim of this study was to evaluate the ADR incidence rate of statins combined with CYP3A4 inhibitors and to detect new signals of ADR in real-life ambulatory settings.
Method and Materials
We conducted a cross-sectional, observational, multicentre study at the 100 community pharmacies forming the Sentinel Network of Castilla y León (Spain). The Spanish Drug Agency and the Ethics Committee of Valladolid University Hospital (CON-EST-2017-01; EPA 18-255) approved the study. The 100 participating community pharmacies were classified according to sociodemographic strata, which allowed data extrapolation by the usual methodology [14]. Of these 100 community pharmacies, 45 were located in urban, 12 in semi-urban, and 43 in rural areas.
Patients on statins (i. e. atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin) concurrently treated with a chronic CYP3A4 isoenzyme inhibitor (i. e. amiodarone, cyclosporine, cilostazol, diltiazem, dronedarone and fluoxetine) [15] participated in the study. The above CYP3A4 isoenzyme inhibitors were selected because patients had been taking them uninterruptedly within the previous six months. Protease inhibitors were excluded because they are not dispensed at Spanish community pharmacies. Likewise, we excluded those CYP3A4 isoenzyme inhibitors that are prescribed only for acute treatment, such as macrolide antibiotics or azole antifungals. Amlodipine was not included because it is a weaker inhibitor. Lastly, fibrates were excluded because they are used for the treatment of dyslipidemias when statins are contraindicated, or no tolerated and concomitant use of statins and fibrates is contraindicated. Each of the community pharmacies integrated in the Sentinel Network randomly selected patients who had undergone chronic therapy with both a statin and a CYP34A isoenzyme inhibitor for longer than 6 months. The protocol for patient recruitment and data collection was compliant with the Spanish Organic Law on Personal Data Protection (LOPD, according with its Spanish acronym) and the Organic Law 15/1999 of December 13th on patients’ privacy. The patients were divided into two cohorts. One of the cohorts was made up of patients on statins using the CYP3A4 isoenzyme as metabolization pathway (i. e. atorvastatin, lovastatin, and simvastatin), while the other cohort included the patients on statins using a metabolization pathway other than CYP3A4 (i. e. pitavastatin, rosuvastatin, fluvastatin, and pravastatin).
The definitive sample was made up of those patients who gave their informed consent to participate in the study. Data collection was carried out by means of a structured questionnaire, including the following information: patients’ descriptive demographic data (age, sex); clinical data (diagnostic criteria for which statins were prescribed, active pharmacological treatments, dosage and); and data on diverse adverse drugs reactions (ADRs) experienced by the patients within the previous six months (see Appendix 1). Registered adverse events were related to the risk of neuromuscular toxicity. In addition, ADRs and associated symptoms and signs that the pharmacist gathered during the interview with the patients were entered in the questionnaire as complementary information, we conducted a descriptive analysis of the sample with the following variables: age, sex, pharmacological treatments, and INR values. Additionally, we determined the incidence rate of ADRs in the two patient cohorts, and conducted a bivariate analysis based on the ADR incidence rates in both cohorts by taking into consideration the pharmacological treatment and the remaining cohort variables by estimating the ORs (95% CI).
In order to adjust the estimator according to age, gender, and number of active treatments, linear regression standardization was used. Here, a conversion factor was obtained for each discrete variable category, taking as a reference the stratification of the cohort on CYP3A4 metabolised statins, and applying it to cohort on non-metabolized CYP3A4 statins using the following weighting factor.
Weighting factor = gender conversion factor (a) * age conversion factor (b) * number of active treatments conversion factor (c); so that a=1, b=1, and c=1 for all categories of the variable in the metabolised CYP3A4 statins.
*if a variable did not present cases in some category of the CYP3A4-metabolised statins cohort, an identical treatment (adding 100 units) would be applied in each category of the variable in both cohorts in order to obtain real values for the conversion factor.
The data from the cross-sectional study performed at the community pharmacies were supplemented with the data from spontaneous reporting of ADRs submitted to the Spanish Pharmacovigilance System (SPS). The SPS has operated since nearly 30 years and allows all healthcare professionals to notify any suspected ADRs, particularly in the cases in which the adverse reaction is serious or the suspected drug is a medication subject to special surveillance or recently commercialised in Spain. ADRs were coded by standardised terms (MedDRA) [16] The search of notifications submitted to the Pharmacovigilance Centre of Castilla y León during the year 2018 was performed on 6-March-2019. The data for the present study were obtained from the Spanish Pharmacovigilance database (Spanish acronym: FEDRA) of the Spanish Human Use Drug Pharmacovigilance System (SEFV-H, according to its Spanish acronym), run by the Spanish Agency for Drugs and Medical Devices (Spanish acronym: AEMPS).
In order to detect new ADR signals we applied the Bayesian Confidence Propagation Neural Network (BCPNN) with expansion to the multiple comparison framework [17]. This method relies on the posterior likelihood of the null hypothesis (post.H0) and allows to indirectly obtain the false positive Bayesian estimator (FDR), which represents a measure for the detected signals. For data analysis, the following arguments were considered into the model: relative risk value higher than 1 (RR>1); minimum number of cases by pair [drug-adverse reaction] to be considered as a potential signal (N=1); decision rule for signal generation: false positive rate (false discovery rate, FDR); limit or threshold for the decision rule: FDR<0.05; statistic used for sorting out the pairs drug-adverse reaction: posterior likelihood of the null hypothesis (post. H0); and estimation of the distribution of the statistic of interest by approaching the normal distribution [18,19] and by means of empirical estimation based on Monte Carlo simulations (NB.MC=10000) [20].
Results
During the study period (i. e. 1-June-2018 / 31-July-2019), 112 community pharmacy surveys were fulfilled. Approximately 80% of patients used atorvastatin or simvastatin (none with lovastatin), 46% a calcium-antagonist agent (verapamil or diltiazem), and 36% a group-III antiarrhythmic (amiodarone or dronedarone). Patients’ mean age was 71.1 years, and 57.1 % were males. The mean number of concomitant treatments was over 7. Table 1 displays the main characteristics of the study sample.
SD: standard deviation; y: years.
In the cohort of patients on atorvastatin or simvastatin, 180 ADR was reported (2.0 ADR per patient), while this figure was 35 in the case of patients taking a statin other than atorvastatin or simvastatin (1.6 ADR per patient). All ADRs presented a higher notification rate in the cohort of patients on a CYP3A4-metabolised statin except in the case of myalgia. In addition, the number of patients who did not have any ADRs notified was the highest one in the group of patients on statins other than CYP3A4-metabolised agents. The most frequently ADR in the group of patients treated with atorvastatin or simvastatin were those involving muscular pain (38% of patients) (Table 2).
Table 2: Frequency of ADR and crude and standarized odds ratio for the main reported adverse reactions for CYP3A4 metabolised statins vs rest of statins.

Some of the values in the contingency table are 0 (OR cannot be calculated) Abbreviations: CI, confidence interval; OR: odds ration N/A: not applicable
There was a relationship between the co-administration of verapamil or diltiazem and a statin, on the one hand, and notifying musculoskeletal pain, on the other. In addition, and non-significant relationship between the use of these calcium antagonists and the occurrence of paresthesia or myasthenia was noted, while the relationship between the aforementioned concomitant treatment and the absence of neuromuscular symptoms was negative (Table 3). As shown in Table 3, there was a stadistically significant positive relationship between the use of fluoxetine and the higher prevalence of osteoporosis, as well as between the therapy with cyclosporine and the presence of increased liver enzyme levels. When evaluating the number of ADRs, we found that calcium antagonists caused 1.78 ADRs per patient (diltiazem 1.74, verapamil 1.83), and the group-III antiarrhythmics caused 1.83 ADRs per patient (amiodarone: 1.85, dronedarone: 1.67), whereas cyclosporine showed a higher ADR rate per patient of 1.85 and, particularly, fluoxetine (2.5) (Table 3).
Table 3: ADRs per patient and standarized odds ratio for the main reported adverse reactions for statins and CYP3A4 inhibitors.
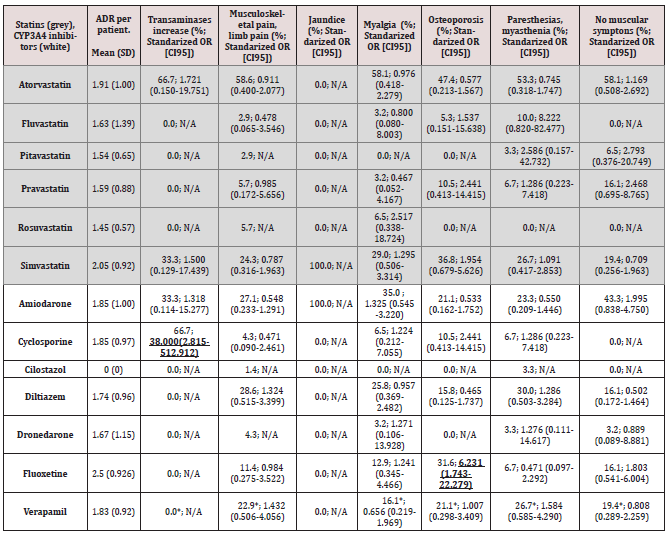
Some of the values in the contingency table are 0 (OR cannot be calculated). Abbreviations: CI, confidence interval; OR, odds ratio; SD, Standard deviation N/A, not applicable. Note: Bold and underlined values indicate statistically significant differences
To detect new signals for ADRs, we applied the method called Bayesian Confidence Propagation Neural Network (BCPNN) with expansion to the multiple comparison framework. When normal distribution model was performed (Table 4), we found an osteoporosis signal linked to the use of atorvastatin (FDR<0.001) as a single drug -but no significant signal co-administered with fluoxetine (FDR=0.079)-, and osteoporosis linked to fluoxetine (FDR=0.030). When empirical estimation based on Monte Carlo simulations (NB.MC=10000) was considered (Table 5), we found the same significant osteoporosis signals linked to atorvastatin (FDR<0.001) and fluoxetine (FDR=0.010) and now a positive signal for co-administration of both (FDR=0.011). We also observed an musculoskeletal pain signal linked to atorvastatin (FDR=0.016) and transaminases increase linked to ciclosporin (FDR=0.048). These signals are also consistent with the above findings. Moreover, we observed positive signals of musculoskeletal and limb pain linked to tiotropium, transdermal nitroglycerin and topical calcipotriol. Only in the SPC of tiotropium is registered an musculoskeletal pain ADR (joint swelling).
Table 4: Detection of positive signals (normal distribution model) for statins and CYP3A4 inhibitors. False discovery rate (FDR estimator) <0.05.

Abreviations: ADR, adverse drug reaction FDR, false discovery rate Post.H0, posterior probability of the null hypothesis
Table 5: Detection of positive signals (MonteCarlo simulations model) for statins and CYP3A4 inhibitors. False discovery rate (FDR estimator) <0.05.
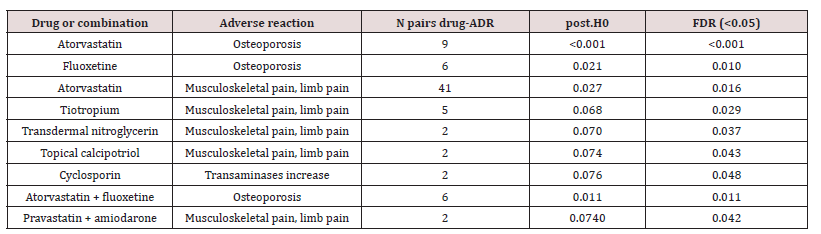
Abbreviations: ADR, adverse drug reaction; FDR, false discovery rate; Post.H0, posterior probability of the null hypothesis
When evaluating ADRs related to muscular toxicity found in the cross-sectional study, we encountered 180 ADRs in 112 patients, with musculoskeletal and limb pain being the most frequent ADR (51.7% of patients complained of this condition) (Table 6). Of note, 14 ADRs were reported in Spain in 2018: 9 rhabdomyolysis, 4 myopathies, and 1 necrotising myositis, the latter being considered a very rare adverse reaction in atorvastatin SPC (Table 5).
Table 6: Adverse events recorded in the cross-sectional study comparing with those coming from spontaneous reporting.

*Rare adverse reactions present in the Summary of Product Characteristics (SPC) (simvastatin and atorvastatin) + Very rare adverse reactions present in the Summary of Product Characteristics (SPC) (simvastatin and atorvastatin)
Discussion
The most outstanding finding of our study is the fact that an elevated percentage of patients used concomitantly a chronic CYP3A4 isoenzyme inhibitor agent and a statin (i. e, atorvastatin or simvastatin) that uses CYP3A4 isoenzyme as metabolization pathway. When looking at statin consumption in Spain (defined daily doses [DDD] per 1,000 inhabitants per day) in the year 2012 [21] (atorvastatin: 46.6%; simvastatin: 33.6%; rosuvastatin: 8.5%; pravastatin: 4.9%; pitavastatin: 1.8%; lovastatin: 0.9%), we observed that the distribution was similar in our study: atorvastatin 56.3%; simvastatin 24.1%; pravastatin 8.9%; fluvastatin, rosuvastatin and pitavastatin 3.6%.
We can conclude, therefore, that the consumption pattern does not greatly differ in our sample of patients who use a statin co-prescribed with a CYP3A4-isoenzyme inhibitor agent. This suggests that statin prescription pattern in these patients does not differ largely from the habitual use pattern for these medications, since the use percentage of the statins that are metabolised by the CYP3A4 isoenzyme (i. e. atorvastatin, simvastatin and lovastatin) approximated to 80%, which can be considered an inappropriate prescription pattern. When comparing the cohort of patients on a CYP3A4-metabolised statin with the cohort of patients treated with the other statins, we found that the number of ADRs notified for the former cohort was 20% higher (i.e. 2.0 vs. 1.6), with more ADRs being notified and a lower number of patients who did not have any ADRs notified. Some earlier studies also found this risk pattern associated with the co-administration of statins and CYP3A4 inhibitors.[1, 5] In addition, some of these studies have warned about the potential clinical risk of such a co-administration.6 However, current evidence for this risk is limited.
In our study, the consumption of atorvastatin seemed to be somewhat higher as compared to simvastatin among the patients taking CYP3A4 inhibitors than in the rest of patients on a statin. On the other hand, there were no relevant differences between simvastatin and atorvastatin in relation to the risk of muscular toxicity, and the differences, if any, were dependant on the dose [22,23]. So, when looking at the recommendations included in atorvastatin and simvastatin SPCs about the co-administration of these medications with CYP34A inhibitors [24,25], one can see that such recommendations vary with the statin dose and the CYP34Ainhibitor strength, and this holds true for both atorvastatin and simvastatin. In the case of the patients on simvastatin, the coadministration of this statin with cyclosporine (3 patients in our study; that is, 50% of patients treated with cyclosporine) is contraindicated, and it is recommended monitoring of the treatment and not to exceed 10 mg in those treated with atorvastatin. Therefore, 66% of patients in our study who were being treated concomitantly with a statin and cyclosporine were taking a contraindicated statin.
The drug-drug interaction of simvastatin and atorvastatin with cyclosporine has been reported to be associated with a higher risk of myopathies and muscular toxicity, this risk being higher than that associated with other drug combinations [26]. Another group of patients that presented a higher notification rate in our study were those taking the calcium antagonists diltiazem and verapamil. In this case, the SPCs of atorvastatin and simvastatin recommend not to exceed the dose of 20 mg (simvastatin) or monitoring the treatment (atorvastatin). In addition, the SPCs of simvastatin and atorvastatin state that there may be an increment in statin plasma levels, being lower than those found with cyclosporine. In our study, there was a statistically significant relationship between notifying musculoskeletal or limb pain and co-administering a calcium-antagonist agent with a statin as compared with the rest of participating patients. Previous studies have reported an increased risk of ADR among these patients, specifically, a higher risk of renal damage and hypokalemia [27].
Concerning the group III-antiarrhythmics, we found a lower risk compared to that associated with the other inhibitors, save the risk of notifying myalgia, which presented a slightly higher risk. On the other hand, 80.4% of patients in our study used atorvastatin or simvastatin. This finding is in keeping with earlier studies, in which the authors stressed both the importance of the dose and the risk of muscular toxicity [28,29]. In our study, 53% of patients used atorvastatin or simvastatin at a dose exceeding 20 mg.
Our findings indicated that there was a statistically significant relationship between the use of fluoxetine and a higher risk of notifying osteoporosis. In this case, the ADR cannot be ascribed to the effect of a potential interaction with statins caused by CYP3A4 isoenzyme inhibition, because osteoporosis is not an ADR associated with increased plasma levels of the hypolipemiant, but an effect attributable to the antidepressant agent itself [30]. It is a controversial issue, in general and, in particular with respect to fluoxetine, whether or not selective serotonin reuptake inhibitors (SSRIs) have the potential to cause or promote osteopenia. Some studies have found evidence for a significant risk of osteopenia with SSRIs [30-33], while others have failed to demonstrate such a risk [34-36]. At any rate, few studies have addressed the clinical effects derived from the potential risk of osteoporosis on an outpatient basis. SSRIs are likely to affect the bone metabolism, since the increased circulating serotonin can promote osteoclast growth, and, consequently, affects bone loss. However, additional factors, such as the depressed mood itself, should not be discarded [37].
Undoubtedly, this signal, as in the case of the increment in transaminase levels linked to cysclosporin administration, cannot be ascribed to the interaction with statins, and should be attributable instead to the drug safety profile itself, which confirms the quality of the data collected for our study. We observed positive signals of musculoskeletal and limb pain linked to tiotropium, transdermal nitroglycerin and topical calcipotriol. In the SPC of tiotropium is registered a musculoskeletal pain ADR, topical calcipotriol is indicated in the treatment of psoriasis and psoriasis is also related to limb pain.
When contrasting the results from the cross-sectional study with the data coming from Spanish spontaneous reporting database submitted during the year 2018, we found a different pattern. So, the most prevalent and mildest ADRs were found in the crosssectional study, whilst the least frequent and most serious ADRs were reported to the notification system. This finding coincides with previously published studies [38]. It should be underlined that all ADRs involving the muscular system included in the SPCs of atorvastatin and simvastatin were seen when combining the results from the cross-sectional study with the data from the spontaneous reporting. On the other hand, the prevalence of mild ADRs in our study (more than 70% involved musculoskeletal pain, and approximately 30% involved myalgia or paraesthesia) is higher than the prevalence of mild ADRs stated in the drug technical documents. This suggests that the results from pharmacovigilance studies or clinical trials might be underestimating the true prevalence of mild reactions.
Study Limitations
Our study presents several drawbacks and limitations, including the potential risk of biases due to the lack of blinding, which may have overestimated the best-known adverse reactions or the risk of ADRs associated with age, sex or additional therapies. Another limitation of our study is the small sample size, which might have hampered the detection of rare ADRs in evaluating the potential drug interactions with CYP3A4 inhibitors or detection of signals for active ingredients not prescribed in the sample such as lovastatin. Due of that we applied Monte Carlo simulations to clarify the results. Furthermore, the sample size prevented us to conduct an analysis by individual drug..
Disclaimer
The sections Discussion and Conclusions in this study contain only the opinion of the authors thereof, and, in no way, are intended to represent the stance of the SEFV-H or AEMPS on the issue under investigation.
Conclusions
In summary, data collection based on a community pharmacy network enables one to gather information on the risk of potential drug interactions of statins with other medicins (i. e. CYP3A4 inhibitors) when these medications are used on an outpatient basis. The findings of this study show that the statin consumption pattern did not largely differ from the general pattern when these drugs are co-administered with CYP3A4 inhibitors, despite the established risk of ADRs, particularly muscular toxicity. Patients taking a statin that uses this metabolization pathway (i. e. atorvastatin and simvastatin) report more neuromuscular reactions, above all musculoskeletal pain, and fewer patients do not report any ADRs of this kind.
Patients on cyclosporine and the calcium antagonist’s diltiazem and verapamil are at a higher risk of complaining of a neuromuscular ADR. Therefore, it is of particular importance to avoid administering the above drugs in combination with atorvastatin or simvastatin, especially at high doses. Patients on atorvastin and/or fluoxetine bear a higher risk of reporting osteoporosis as an ADR, and those taking cyclosporine are at a greater risk of developing increased transaminase levels. When combining the results from the crosssectional study with the data coming from spontaneous reporting, we found all the ADRs included in the Summary of Product Characteristics of the medications under investigation.
Funding Support
The Sentinel Network in Castilla y León received funding support from Esteve Pharmaceutical S.A. to assume the administrative cost of the project.
Conflicts of Interest
The authors report no other conflict of interest related to the subject of this research.
Acknowledgments
The authors would like to acknowledge the contributions of all pharmacists of Sentinel Network in Castilla y León (Spain) for their commitment and effort. We would like to thank the following Institutions for their assistance and support with the collection of data: Consejería de Sanidad de la Junta de Castilla y León, Dirección General de Salud Pública (DGSP), Centro Regional de Farmacovigilancia de Castilla y León, Centro de Estudios Sobre la Seguridad de los Medicamentos (CESME), Facultad De Medicina, Universidad de Valladolid, Valladolid (Spain).
References
- Molden E, Skovlund E, Braathen P (2008) Risk management of simvastatin or atorvastatin interactions with CYP3A4 inhibitors. Drug Saf 31(7): 587-596.
- Bakhai A, Rigney U, Hollis S, Emmas C (2012) Co-administration of statins with cytochrome P450 3A4 inhibitors in a UK primary care population. Pharmacoepidemiol Drug Saf 21(5): 485-493.
- Mukai Y, Narita M, Akiyama E, Ohashi K, Horiuchi Y, et al. (2017) Co-administration of Fluvastatin and CYP3A4 and CYP2C8 Inhibitors May Increase the Exposure to Fluvastatin in Carriers of CYP2C9 Genetic Variants. Biol Pharm Bull 40 (7): 1078-1085.
- Neuvonen PJ (2015) Drug interactions with HMG-CoA reductase inhibitors (statins): the importance of CYP enzymes, transporters and pharmacogenetics. Expert Opin Drug Metab Toxicol 11(9): 1435-1447.
- Wang YC, Hsieh TC, Chou CL, Wu JL, Fang TC (2016) Risks of Adverse Events Following Coprescription of Statins and Calcium Channel Blockers: A Nationwide Population-Based Study Medicine (Baltimore) 95(2): e2487.
- Morival C, Westerlynck R, Bouzillé G, Cuggia M, Le Corre P (2018) Prevalence and nature of statin drug-drug interactions in a university hospital by electronic health record mining. Eur J Clin Pharmacol 74(4): 525-534.
- Bakhai A, Rigney U, Hollis S, Emmas C (2012) Co-administration of statins with cytochrome P450 3A4 inhibitors in a UK primary care population. Pharmacoepidemiol Drug Saf. 21(5): 485-493.
- Devold HM, Molden E, Skurtveit S, Furu K (2009) Co-medication of statins and CYP3A4 inhibitors before and after introduction of new reimbursement policy Br J Clin Pharmacol 67(2): 234-241.
- Akimoto H, Negishi A, Oshima S, Okita M, Numajiri S (2018) Onset timing of statin-induced musculoskeletal adverse events and concomitant drug-associated shift in onset timing of MAEs. Pharmacol Res Perspect 6(6): e00439.
- Silva M, Matthews ML, Jarvis C, Nolan NM, Belliveau P, et al. (2007) Meta-analysis of drug-induced adverse events associated with intensive-dose statin therapy. Clin Ther 29(2): 253-260.
- Hirota T, Ieiri I (2015) Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol 11(9): 1435-1447.
- Neuvonen PJ, Backman JT, Niemi M (2008) Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin Clin Pharmacokinet 47(7): 463-474.
- Bottorff MB (2006) Statin safety and drug interactions: clinical implications. Am J Cardiol 97(8A): 27C-31C.
- Vega Alonso AT, Zurriaga Llorens O, Galmés Truyols A, Lozano Alonso JE, Paisán Maestro L, et al. (2006) Group of Research for the RECENT Project Health sentinel networks in Spain. Consensus for a guide of principles and methodsGac Sanit 20(6): 496-502.
- Zhou SF, Xue CC, Yu XQ, Li C, Wang G, et al. (2007) Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Ther Drug Monit 29(6): 687-710.
- Brown EG, Wood L, Wood S (1999) The medical dictionary for regulatory activities (MedDRA). Drug Saf 20: 109-117.
- Ahmed I, Haramburu F, Fourrier-Reglat A, Frantz T, Carmen KJ al. (2009) Bayesian pharmacovigilance signal detection methods revisited in a multiple comparison setting. Stat Med 28(13): 1774-1792.
- Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, et al. (1998) A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 54(4): 315-321.
- Gould AL (2003) Practical pharmacovigilance analysis strategies. Pharmacoepidemiol Drug Saf 12(7): 559-574.
- Nóren N, editores (2002) A Monte Carlo Method for Bayesian Dependency Derivation. Gothenburg: Chalmers University of Technology.
- De Abajo F, García del Pozo J. Utilización de medicamentos hipolipemiantes en España durante el periodo 2000-2012. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS. 2006.
- Rönnqvist J, Hallberg P, Yue QY, Wadelius M(2018) Fusidic Acid: A Neglected Risk Factor for Statin-Associated Myopathy. Clin Med Insights Cardiol 12: 1179546818815162.
- Silva M, Matthews ML, Jarvis C, Nolan NM, Belliveau P, et al. (2007) Meta-analysis of drug-induced adverse events associated with intensive-dose statin therapy. Clin Ther 29(2):253-260.
- (2017) Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Centro de Información online de Medicamentos de la AEMPS-CIMA. Ministerio de Sanidad, Servicios Sociales e Igualdad. Ficha Técnica simvastatina 10 mg cinfa ®.
- (2017) Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Centro de Información online de Medicamentos de la AEMPS-CIMA. Ministerio de Sanidad, Servicios Sociales e Igualdad. Ficha Técnica atorvastatina 10 mg cinfa ®.
- Evans M, Rees A (2002) Effects of HMG-CoA reductase inhibitors on skeletal muscle: are all statins the same? Drug Saf 25(9): 649-663.
- Zhou YT, Yu LS, Zeng S, Huang YW, Xu HM, et al. (2014) Pharmacokinetic drug-drug interactions between 1,4-dihydropyridine calcium channel blockers and statins: factors determining interaction strength and relevant clinical risk management Ther Clin Risk Manag 10: 17-26.
- Borders-Hemphill V (2009) Concurrent use of statins and amiodarone. Consult Pharm 24(5): 372-379.
- Gavronski M, Volmer D, Hartikainen S, Zharkovsky A (2015) Potential drug interactions with statins: Estonian register-based study. Open Med (Wars) 10(1): 254-260.
- Rauma PH, Honkanen RJ, Williams LJ, Tuppurainen MT, Kröger HP, et al. (2016) Effects of antidepressants on postmenopausal bone loss - A 5-year longitudinal study from the OSTPRE cohort. Bone 89: 25-31.
- Tsapakis EM, Gamie Z, Tran GT, Adshead S, Lampard A, et al. (2012) The adverse skeletal effects of selective serotonin reuptake inhibitors. Eur Psychiatry 27(3):156-169.
- Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, et al. (2007) Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med 167(12): 1240-1245.
- Bab I, Yirmiya R (2010) Depression, selective serotonin reuptake inhibitors, and osteoporosis. Curr Osteoporos Rep 8(4): 185-191.
- Diem SJ, Ruppert K, Cauley JA, Lian Y, Bromberger JT (2013) Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab 98(11): 4355-4363.
- Schwan S, Hallberg P (2009) SSRIs, bone mineral density, and risk of fractures--a review. Eur Neuropsychopharmacol. 19(10): 683-692.
- Schweiger JU, Schweiger U, Hüppe M, Kahl KG, Greggersen W, et al. (2018) The Use of Antidepressive Agents and Bone Mineral Density in Women: A Meta-Analysis. Int J Environ Res Public Health 15(7): 1373.
- Kerbage H, Bahadori S, Léger J, Carel JC, Purper Ouakil D (2014) Effect of SSRIs on bone metabolism. Encephale 40(1): 56-61.
- Carvajal A, Sáinz M, Velasco V, García Ortega P, Treceño C, et al. (2015) Emergency contraceptive pill safety profile. Comparison of the results of a follow-up study to those coming from spontaneous reporting. Pharmacoepidemiol Drug Saf 24(1): 93-97.





