Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Research Article(ISSN: 2637-6660) 
Technology for Hydrogen as Future Energy Carrier Volume 2 - Issue 1
Piyush M Maurya1* and Kalpana S Deokar2
- 1Lecturer, Department of Chemistry, Zeal Education Society’s Zeal Polytechnic, Maharashtra, India
- 2H.O.D., Department of Chemistry, Zeal Education Society’s, Zeal Polytechnic, Maharashtra, India
Received: July 05, 2019; Published:July 24, 2019
*Corresponding author:Piyush M Maurya,Lecturer, Department of Chemistry, Zeal Education Society’s Zeal Polytechnic, Maharashtra, India
DOI: 10.32474/ANOAJ.2019.02.000127
Abstract
The energy requirement of the world has been increasing due to increase in world population, technological development and increased living standards. A large fraction (~85 %) of the power consumed in the world is generated by burning of fossil fuels. The fossil fuels such as petroleum and natural gas are non-renewable and are expected to last for another 40 to 60 years based on current energy consumption. The other critical issue is the global warming, which is mainly caused by CO2 emissions generated from the combustion of fossil fuels. As a consequence, investigations of alternate energy strategies have recently become important. The most important requirement of alternative energy sources is their environmental compatibility. The fact that burning of hydrogen produces only harmless water vapors, hydrogen is likely to become one of the most attractive energy carriers in the future. It is important to develop technologies for economical production of hydrogen and for manufacturing compact containers for storage of hydrogen. There are several different methods for storage of hydrogen, the most common of them are: A. Compressed gas storage in tanks, B. Liquid hydrogen storage at low temperatures and C. Storage of gas in solid materials. It is seen that the methods and materials developed till today, are not satisfactory (especially not economical) and thus the development of hydrogen storage materials is an active field of research. Several different pharmacosiderites have been prepared and characterized using advanced techniques such as X-ray diffraction, SEM etc. It is seen that these compounds have porous structure and hopefully hydrogen can be stored in these pores. Arrangements are made to study the hydrogen uptake in the synthesized samples at IIT, Mumbai. Results of our studies as regards preparation and characterization of above compounds will be presented.
Keywords: Hydrogen Storage; Pharmacosiderites; Porous Materials
Introduction
Hydrogen is an excellent energy carrier for both transportation and stationary applications. It is one of the best alternative fuels due to its abundance, easy synthesis, and non-polluting nature when used in fuel cells. The global energy requirement can be easily satisfied by the hydrogen economy. Hydrogen can be easily produced directly from sunlight and water by biological organisms. It can also be produced indirectly by thermal processing of biomass or fossil fuels, and by advanced technology combined with CO2 sequestration, unlimited quantities of hydrogen can be produced [1]. The advantage of using hydrogen as fuel is that the burning of hydrogen results in energy in the form of heat and water is produced. Use of hydrogen eliminates carbon monoxide and CO2 emissions and thus reduces the greenhouse warming. In a fuel cell, hydrogen is directly converted into electricity. Pure hydrogen enters the anode channel in a fuel cell and diffuses through a porous anode towards the catalyst. The hydrogen molecules lose the electrons and become positively charged ions (2H+ + 2e-). The electrons flow through the external circuit to the cathode resulting in electric current. The protons pass through the Proton Exchange membrane and recombines with electrons and oxygen molecules on the cathode side catalyst layer to form water and heat (2H+ + 2e- n³ Heat + H2O). The heat released gives the fuel cell an operating temperature of 60 - 80oC.
Storage of Hydrogen
To use hydrogen as a fuel, the hydrogen has to be stored in a safe manner. The various ways in which the hydrogen can be stored are as follows:
a) High Pressure Gas Cylinders: The gas cylinder used for storing hydrogen should be able to withstand high pressures. The new light weight composite cylinders can withstand high pressures up to 80 MPa. The storage capacity can reach the volumetric density of 36 Kg/m3
b) Liquid hydrogen in Cryogenic Tanks: Liquid hydrogen is stored in cryogenic tanks at 21.2 K and ambient temperature. The volumetric density of liquid hydrogen is 70.8 Kg/m3 that are approximately twice achieved in high pressure gas cylinders.
c) Adsorbed Hydrogen on Materials with Large Specific Area: The gas is adsorbed on the adsorbent material by weak vanderwal forces. The amount of adsorption depends on the surface area of the adsorbent material.
d) Absorbed on the Interstitial Sites in a Host Metal: In absorption, hydrogen is dissociated into hydrogen atoms and then gets incorporated into the solid lattice framework [2,3].
e) Chemically bonded into covalent and ionic compounds: Many metals and alloys are capable of reversibly absorbing large amounts of hydrogen. Charging can be done using molecular hydrogen gas or hydrogen atoms from an electrolyte. Group I, II and III light metals like Li, Mg, B and Al can combine with hydrogen to form a large variety of metal hydrogen complexes. These are especially interesting because of their light weight and number of hydrogen atom per metal atom that is generally 2. Through oxidation of reactive metals: Hydrogen can be stored in reactive metals like Li, Na, Mg, Al and Zn. These metals easily react with water to form corresponding hydroxide and liberate the hydrogen from the water. Finally, the metal hydroxides can be reduced to metals in solar furnace..
Hydrogen Storage in Solids
The high-pressure compression or liquefaction of hydrogen is useful for the stationary applications of hydrogen. But when it comes to application related to transportation these two technologies have limitations. The hydrogen gas has to be compressed at a dangerously high pressure in excess of 1000 bar. Liquefaction requires extreme cooling to about 21K and then insulation is also required. Compressed hydrogen gas and cryogenically stored liquid hydrogen are the most developed technologies. Recent studies show that they meet the technical aspects of the DOE 2010 targets (6.0 wt% and 45 kg H2 per m3). They are unlikely to achieve the DOE 2015 target (9.0 wt% and 81 kg H2 per m3). The hydrogen storage in solids is a vast area of study. It includes study of hydrogen storage in hydride form. In this method alloys are used that are capable of holding large amounts of hydrogen by bonding and forming hydrides [4]. One of the drawbacks encountered in this method is degradatio
Material and Methods
Among the possible solid-state materials investigated, hydrogen adsorption on the porous materials is one of the best alternatives. Zeolites, Metal Organic Frameworks (MOF), porous carbons are some of the adsorbents studied for hydrogen storage. Literature studies on hydrogen storage show that, Metal-organic frameworks (MOFs) and related inorganic-organic (hybrid) materials have been developed and synthesized. In the study of porous materials like zeolites and activated carbons, it was proposed that high gas adsorption can be achieved on materials with large volume of micropores with an appropriate diameter. Various studies report that smaller the pore size higher is the hydrogen uptake. The ideal pore size seems to be approximately 2.8 to 3.3 Ang. Since this size pore is comparable to the kinetic diameter of H2. Pores of this size allow the dihydrogen molecules to interact with multiple portions of the framework. Pharmacosiderites are also microporous solids that can be a potential candidate for hydrogen storage because of tunnel like pores. The synthetically manufactured sodium pharmacosiderites has a pore size of 3.5 Ang. Also, the pore size can be altered if the Na ions can be exchanged with other ions. Pharmacosiderites already find useful applications in removal of 137Cs from nuclear wastewater and in humid sensing properties [5].
Pharmacosiderites
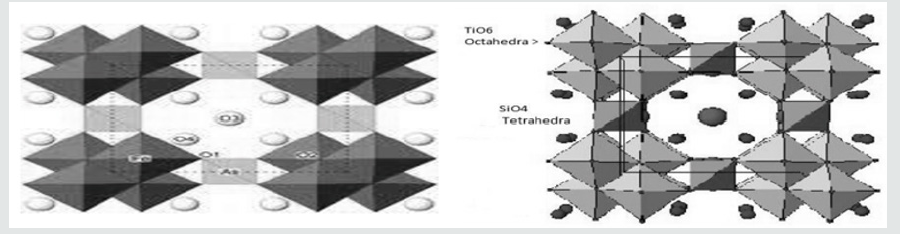
The naturally occurring pharmacosiderite is a nonaluminosilicate molecular sieve with a framework composition KFe4(OH)4(AsO4)3. This compound crystallizes in the cubic space group, P-43m, with a ~ 7.98Å. The structure consists of FeO6 octahedral and AsO4 tetrahedral connected to each other to form a three-dimensional network of channels, as seen in the Figure 1A. The pore has 8-membered ring openings with alternating arsenic tetrahedral and iron octahedral. Each pore is occupied by charge neutralizing cations and water molecules.
Result and Discussion
Synthesis of Pharmacosiderite
Synthetic analogues of pharmacosiderites are prepared in the laboratory by hydrothermal synthesis. We have synthesized pharmacosiderites in our laboratory, where Fe on octahedral site and as on tetrahedral site has been replaced by other elements. It may be mentioned that the above changes are not expected to destroy the porous structure though there may be a change in shape and size of the pore. For example, Chapman and Roe showed that pharmacosiderite consisting of alternating TiO6 octahedra and SiO4 tetrahedra in three orthogonal directions, has a cube like three-dimensional tunnel structure whose pore opening are approximately 3.5Å (Figure 1B). The hydrothermal synthesis is based on the reactants to be dissolved in an aqueous solution.
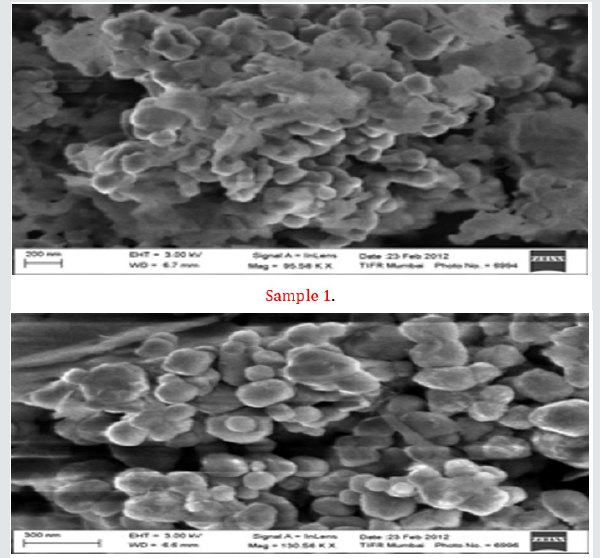
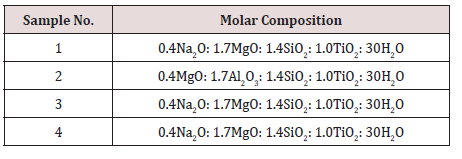
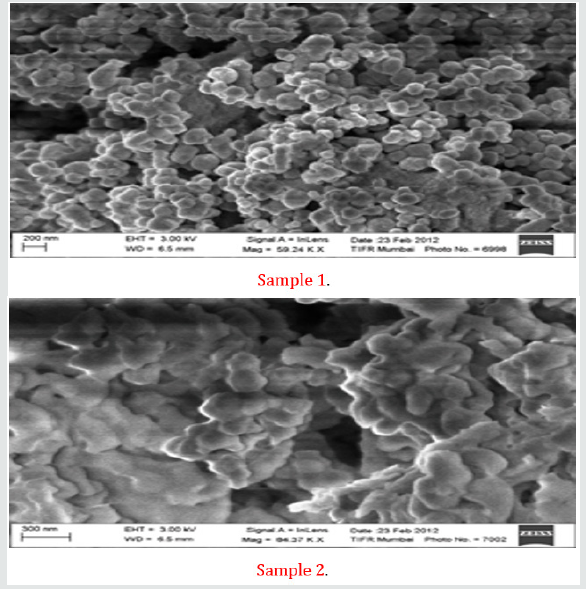
Adding titanium and silicon-sources to the synthesis together with sodium hydroxide and once all reactants are added and the solution is homogenous, the solution is transferred to a Teflon lined autoclave and kept at autogenous pressure at a certain temperature (ranging from 160°C to 500°C) for some time (ranging from a few days to a month, but normally a week). The product is recovered by filtering the sample and washed with did water. The sample is dried in a furnace at 50- 60°C for 24 hours. Even though this looks like a simple synthesis route there are a lot of different parameters to take into account, such as solvent, use of organic molecules, ratio between reactants, temperature, time and others. Each parameter is important in them, but they also affect each other. The Na-pharmacosiderite was synthesized in the laboratory by hydrothermal synthesis. The gel having a molar composition as depicted in Table 1, was kept for stirring overnight and then transferred to Teflon beaker and kept in specially manufactured stainless-steel vessel and treated at 200oC for 72 hours in a preheated oven. The product obtained was filtered and washed with dil. water and dried in room temperature for 24 hours. All the samples were characterized using the X-ray diffraction and SEM techniques. The critical result data of XRD and SEM are displayed in Figures 2 & 3. The preliminary analyses of critical data shows that the materials synthesized are porous and have nanostructures. The detailed analysis is awaited.
Further Work
After detailed analysis of sample few more samples of pharmacosiderites will be synthesized. These porous samples will be studied for their hydrogen storage capacities.
References
- David J Collins, Hong-Cai Zhou (2007) Hydrogen storage in metal-organic frameworks. Journals of Material Chemistry.
- K Mark Thomas (2007) Hydrogen adsorption and storage on porous materials. Catalysis Today 120(3-4): 389-398.
- RJ Westerwaal, WG Haige (2008) Evaluation solid state hydrogen system current status.
- RA Varin, T Czujko, ZS Wronski (2009) Nanomaterials for Solid State Hydrogen Storage.
- Seung-Hoon Jhi, Young-Kyun Kwon, Keith Bradley, Jean-Christophe P Gabriel (2004) Hydrogen storage by physisorption: beyond carbon. Solid State Communications 129(12): 769-773.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...