Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Research Article(ISSN: 2637-6660) 
Studie on Malachite Green Dye Degradation by Biogenic Metal Nano Cuo And Cuo/Zno Nano Composites Volume 1 - Issue 4
Madiha Batool1*, Zahid Qureshi1, Nida Mehboob2 and Abdul Salam Shah3
- 1Department of Chemistry, Government College University, Pakistan
- 2Post Graduate Islamic Collage (PGICC), Pakistan
- 3University of Kuala Lumpur (UniKl-MIIT), Kuala Lumpur, Malaysia
Received: July 17, 2018; Published: July 26, 2018
*Corresponding author: Madiha Batool, Department of Chemistry, Government College University, Pakistan
DOI: 10.32474/ANOAJ.2018.01.000119
Abstract
The biosynthesis of nanoparticles put forward a cost-free and eco-friendly method for the synthesis of nanoparticles. In this paper degradation of Malachite green has been carried out using Copper nanoparticles synthesized by Aloe Barbadensis leaf extracts. The green synthesis of the copper nanoparticles has been carried out using an aqueous solution of copper sulphate and extract of Aloe Barbadensis. The colour change of the reaction mixture containing 1mm copper sulphate has been observed from deep blue to colourless and then brick red to dark red indicating the formation of copper nanoparticles. For the characterization of CuO nanoparticles, Ultraviolet (UV), Infrared (IR), X-ray Diffraction (XRD), and Scanning Electron Microscopy (SEM) have been used. The experimental analysis has revealed an average 60n sized synthesized CuO nanoparticles. The shape of copper nanoparticles has been observed as spherical and cubic ranging between 80-120nm. The different functional group of synthesized nanoparticles has been examined usingFourier-Transform Infrared Spectroscopy (FTIR). The UV spectrophotometer has confirmedthe peak of Copper nanoparticles at 265-285nm.The maximum absorbance of Copper oxide nanoparticles has been observed at 280nm. copper oxide /zinc oxide nanocomposite were synthesized using cheap and cost effective sol-gel method. These cuo/zno nanocomposite were characterized by analytical techniques like (SEM), (TEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and ultraviolet-visible spectroscopy (UV-VIS. The Fourier transform IR analysis result confirmed that composite material were formed. SEM images showed the particle size in the range between 15 to 35nm. Nanocomposite exhibited excellent microwave induced catalytic degradation for malachite green (MG) dye. The initial concentration was 1.8 X 10-5M degradation. The microwave induced catalysis reaction gained about 80% degradation of Malachite Green dye.
Keywords: Malachite Green, Green Synthesized Cooper, Nanoparticles, Nanoparticles
Introduction
Explosion of population, industrialization and progress in agricultural techniques have result in the degradation of environment and hazardous materials into the environment [1-3]. Textile industry is one of the important water consumers produces colored waste water [4]. Dyes have a broad spectrum of chemical structures [5,6]. The discharge of effluents of azo dyes into the water is un controlable because the dyes degrade to carcinogenic and toxic products [7,8]. These dyes residence time is greater in the waste water. There are techniques which are used for dye removal like chemical oxidation, microbial, microwave induced degradation, photocatalysis and adsorption. Today microwave induced degradation and photocatalytic degradation of azo dyes is the effective and economic process [9-11]. The advantage of microwave induced degradation for the control of azo dye pollution are less applied in terms of initial cost, quick operation, and recovery of the adsorbents [12]. The microwave induced degradation process leads to oxidation–reduction and production of free radical.
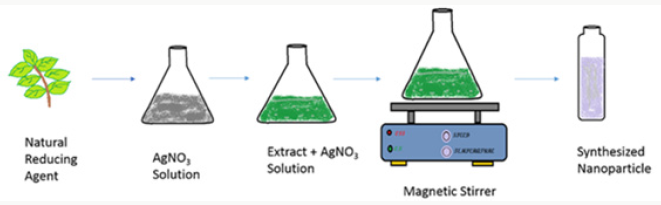
Nanotechnology deals with the manipulation of matter at lower size normally less than 100nm.The preparation of metallic nanoparticles can be carried out using chemical and physical methods. These methods have certain flaws, like emission of toxic chemicals during its chemical reaction can cause serious environmental hazards which can cause pollution and serious issues for the living beings1.The research in green chemistry has played important role in nanotechnology in terms of the benefits to the surface area of society due to which the mass ratio absorption has increased to maximum level 2. Green synthesis has been anxious in the synthesis of highly stabilized nanoparticles. Due to the disadvantages of standard synthesis methods the synthesis of nanoparticles in collaboration with eco-friendly methods has achieved huge attention in the modern era. The nature of particles produced using green synthesis and physical or chemical methods are totally different [3]. In the green synthesis, the chemical reducing agent has been replaced with an extract of leaves of trees and fruits for the synthesis of metal or metal oxide nanoparticles. The green synthesis follows the same bottom-up approach followed by any chemical method but the use of expensive reducing agents has been replaced with the eco-friendly extracts of natural products4. The synthesis process starts with the natural reducing agent and the final result of the process is the synthesized nanoparticles as shown in Figure 1.
For the synthesis of Copper nanoparticles, leaf extract of Aloe Barbadensis (Aloe Vera) plant has been used. The green synthesis of the copper nanoparticles using Aloe Vera plant has been comparatively easier, fast, and environmentally suitable [5]. Aloe Vera plant can be seen in Figure 2.. The phenolic contents in plant extracts are usually dissolved in water, which is degradable and catalyzed for the synthesis of the nanoparticle as capping and reducing agent [6]. The old plant of Aloe Vera is famous for its deeper healing effects. Further, it has been mostly used in the cosmetics and skin creams due to its ability to clean skin and antiageing effects [7]. The folic acid choline and other antioxidant vitamins including A, C and B12 are also present in Aloe Vera plant due to which its gel juice has been used as energy drink [8]. Aloe Vera contains other minerals including copper, chromium, calcium, magnesium, selenium, manganese, potassium and zinc8.The leaves of Aloe Vera also provides anthraquinones [9]. The Copper nanoparticles synthesis using electron microscopy represented their range up to 50nm to 130nm [10].
Copper nanoparticles are important due to their properties with less cost as compared to other expensive metals such as gold and silver. The cost of synthesis for the examination of catalytic activity of Copper nanoparticles by degradation of Malachite green is too much less as compared to the other metals [11]. Copper oxide particles had shown the effective catalytic removal of organic dyes such as Malachite green when particles were added into it [12]. The use of green method has been increased due to the easier preparation of materials and low manufacturing cost. Further, its starting is less toxic due to which the handling of materials is more favourable as compared to physiochemical methods [13]. Malachite green has been used for the dye of leather and textiles since decades further, it has also been used for the control of fish parasites and diseases and in aquaculture industry [14]. The Malachite green has been categorized as class II health hazard due cause of mutagenicity and genotoxicity to the organisms including fish, bacteria and algae. It has been banned by different countries due to its toxic nature [15,16]. The Malachite green dye can be seen in Figure 3. The removal of dyes and organic contaminants in industries had remained a challenging task due to the complexity of decomposition of dye molecules. In this regard researchers have tried to remove various organic and heavy metal pollutants by using nano adsorbents [16-18]. In this paper degradation of Malachite green has been carried out using Copper nanoparticles Aloe Barbadensis leaf extracts. The green synthesis of the copper nanoparticles has been carried out using an aqueous solution of copper sulphate and extract of Aloe Barbadensis. The present work emphesis on the degradation of MG from aqueous media using microwave induced catalysis phenomenon. It is point to be noted, no study has been reported on the application of cuo/zno nano composite with microwave induced catalytic degradation of dye activity and compare it to metal nano oxides [19].
Materials and Methods
The detailed discussion of the materials and methods involved in achieving the target has been reported in subsequent subsections for the better understanding of the experimentation.
Material
Copper sulphate, Aloe barbadensis leaves, sodium borohydride (NaBH4), organic dyes such as Malachite green.
Preparation of Plant Leaf Extract
50g of the Aloe Vera is taken from the nearby garden. The leaves of Aloe Vera are first separated from the gel part of Aloe Vera. The leaves are then washed thoroughly with distilled water to remove soil and dust particles. After washing leaves were dried and finely chopped. These finely chopped leaves were allowed to boil for 15min at 100 °C with 100mL of de-ionized water in a 250mL flask and then allow to cooled down to come at least at room temperature. The resulting solution is passed through a filter paper to remove any solid particles and then again filtered through a Whatman filter paper of pore size 0.2μm. The filtrate is stored at 6 °C as a stock for the synthesis of CuO NPs [18].
Synthesis of CuO-ZnO nanocomposite
For the Synthesis of CuO-ZnO nanocomposite we used the solgel method [11]. In first step, 25mL of ethylene glycol eg was mixed with 5.3g of Zn(CH3COO)2.2H2O zinc acetate and 25mL of distilled water now 6.29g of citric acid with vigorous stirred for about an hour. After an hour, 1.3g of CuSO4.5H2O copper sulphate was added to the suspension at 80 °C for 2 hours and quickly mixed. The solution was kept in the dark for long time and washed with water several times. The resulted gel was dried at 120 °C to and calcined at 500 °C for 4 hours to produce the CuO-ZnO nanocomposites. These nanocomposites further used for characterization and dye degradation.
Green Synthesis of Cuo Nanoparticles
A copper sulphate solution of fifty millilitres was added to 15ml Aloe Vera leaves extract. The solution was stirred on a magnetic stirrer at 120degrees. The colour change was observed. The colour changes from deep blue to colourless and then dark red at saturation. Brick redcolour confirms the nanoparticles formation. The resultant solution was centrifuged for ten mints at speed of 50,000rpm. After discarding supernatant copper oxide nanoparticles were dried in a watch glass. After drying, black colour particlesw ere assembled for further characterization. The process of nanoparticles synthesis can be seen in Figure 4..
Characterization of Green Synthesis Copper Nanoparticles
The morphological, structural and chemical composition of CuO NPs were analyzed by using SEM (jsm-6480) and XRD (XPERTPRO) equipment. Optical properties of synthesized particles are investigated by UV spectrophotometer (DB-20). Size and shape of copper oxide nanoparticles were observed by SEM (jsm-6480). The crystal structure of synthesized nanoparticle is examined by XRD(XPERT-PRO), FTIR analysis is performed for the collection of the functional groups, present in this synthesis of CuONps.
Colour Change Observation:
Colour changes indicate the formation of nanoparticles of copper oxide. The bluecolour solution was turned into red or brick red indicated for the formation of copper nanoparticles synthesis.
Results
X-rays Diffraction Studies
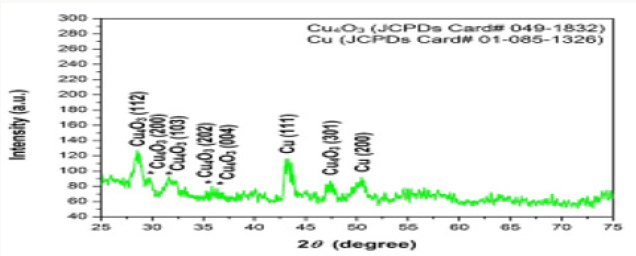
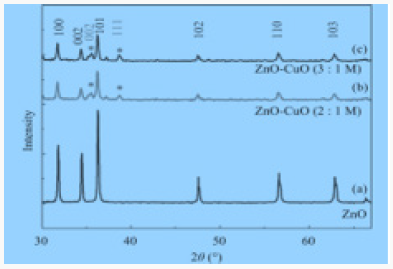
Copper oxide nanoparticles were examined by X-ray diffractometer (XPERT-PRO). Copper oxide powder was put in cubes of XRD for calculation of intensity. The resultant pattern of synthesized nanoparticles was analyzed. The peaks at 2θ correspond to intensity as the peaks at 28, 29.8, 32.1, 35.8, 36, 43.3, 47.5, 51.1, and have 112, 200, 103, 202, 004, 111, 301 and 200, a pattern which is compared to JCPDS card no (049-1832). The pattern of Cu nanoparticles compared to JCPDS card no (01-085-1326), the peaks at 2θ. XRD pattern confirmed that CuO nanoparticles are highly crystalline with the cubic crystal structure. The average size of the particle calculated by Scherrer equation was 60-100nm.The XRD pattern of CuO nanoparticles has been graphically represented in Figure 5..
FTIR Analysis:
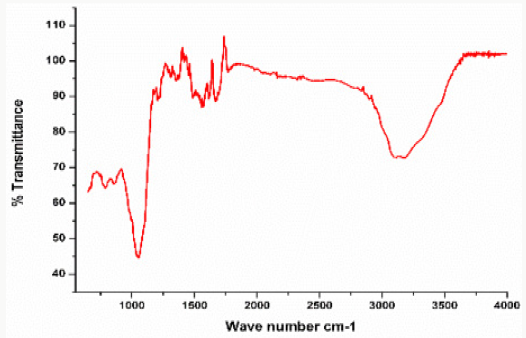
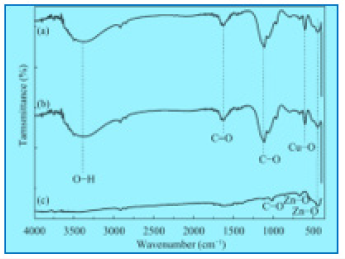
In this analysis, FTIR (IPRrestige-21) spectrum was analyzed. The analyzation confirms the presence of copper nanoparticles. Different peaks were observed at 1100cm-1 confirm the formation of Copper oxide nanoparticles peaks was observe in range of 400-4000cm-1. The FTIR spectrum of Copper oxide nanoparticle exhibits that the broad absorption band at 32cm-1 corresponds to the hydroxyl (OH) functional group in alcohols and phenolic compounds. The peak at 1601.2cm-1 is due C=C aromatic bending. The absorption peak at 1038.0cm-1 stretching vibrations of C–O group of primary and secondary alcohols (C–O), while smaller peaks at 900–700cm-1 were also assigned to the aromatic bending vibration of C–H group [20]. For better elaboration, the FTIR spectra of the Copper oxide nanoparticles is shown in Figure 6..
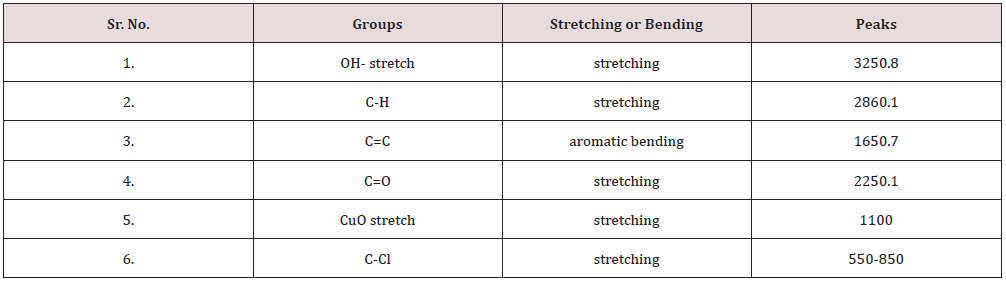
The peaks have been observed in FTIR, the same has been reported in Table 1.
Ultraviolet Spectroscopy:
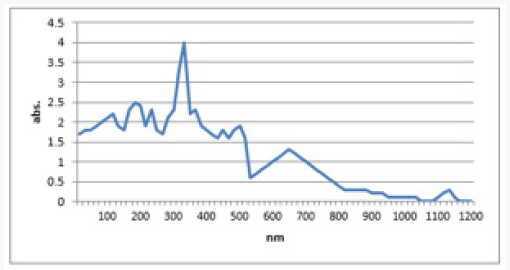
The presence of copper oxide nanoparticles is confirmed at the range of 200-1100nm. The eco-friendly method for the synthesis of copper oxide nanoparticles using Aloe vera leaves extracts proved feasible, coast free and successful method. UV-Vis spectra analysis has apparently shown the formation of copper oxide nanoparticles. Nanoparticles synthesized have a variety of application in the different field. The maximum absorption peak is between 265-285nm.The peak at about 280nm was achieved. This peak confirmed the formation of the copper oxide nanoparticles as shown in Figure 7..
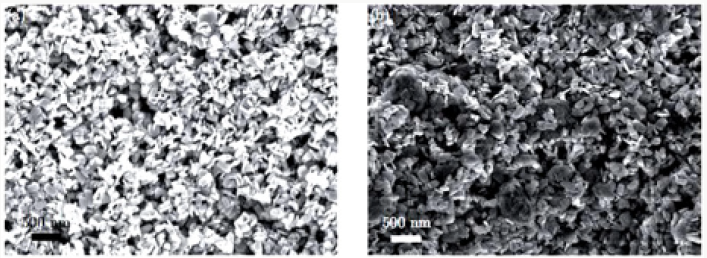
SEM Analysis:
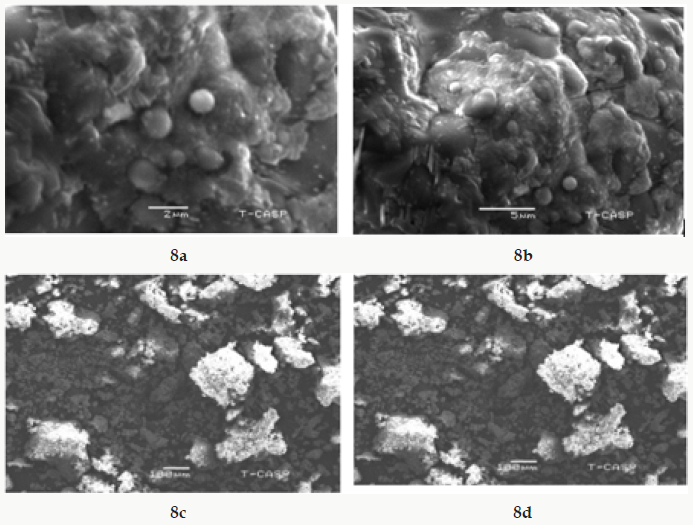
The average particle size of copper nanoparticle was analyzed by SEM model (JSM-6480). The range of grain of copper oxide nanoparticle was calculated about 50.5-130nm by SEM micrograph. It was observed that particles were smooth with a spherical shape. The images of Copper Oxide nanoparticles with different ranges of 2Mm, 5Mm and 100Mm are shown in ( Figure 8a-8c.).
Figure 8a: SEM image of Copper Oxide nanoparticles with 2Mm.
Figure 8b: SEM image of CopperOxide nanoparticles5 Mm.
Figure 8c: SEM image of copper oxidenanoparticles100 Mm.
Figure 8d:
Characterization of Cuo/Zno Nanocomposites
X-ray diffraction analysis (XRD) of the nano composite material was calculated by using Cu Ka radiation (k = 1.54 Å). The SEM (S-3400N, Hitachi) image of metal oxide nanocomposite was recorded at different magnifications. The UV-vis spectra (DB-20) of cuo/zno nanocomposite were recorded for wavelength. The spectrum showed absorption in the range of 200-600nm regions. Fourier transforms infra-red spectra of cuo/zno nanocomposite were taken by KBr disc method. CuO/ZnO nanocomposite exibit the excellent photodegradation of malachite green dye. The adsorption and microwave induced catalysis was more effective. the percentage efficiency of degradation of dye was observed efficiently increase by nanocomposites rather than metal oxide nanoparticles. So, we reviewed comparative potential of copper nano oxide and its composites degradation efficiency, which was observed in nano composite due to reduce size particals.
Preparation of 1000mg/l dye S.S
1000ppm solution of Malachite green dye was prepared by dissolving dye in 1-litre distilled water. Different concentration of dyes was prepared from stock solution. 100ppm solution was prepared from 1000ppm solution after dilution. After that 150,200,250-ppm solution was prepared. 18g of NaBH4 is made up to 10mL and kept aside. Different concentrations of NaBH4 and catalyst are tested on the methylene blue dye. The catalytic degradation of organic dyes was observed by measuring UV-Visible spectra at regular time intervals [19].
Malachite Green
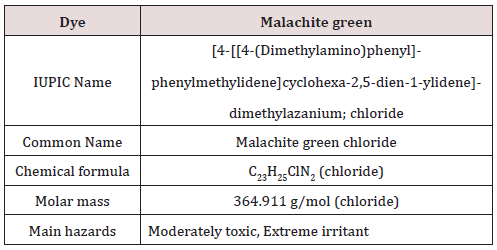
Malachite green is extensively used in many industries as a dye for leather, textiles and also in aquaculture industry to control fish parasites and disease. The use has increased so much because of its easy preparation and low manufacturing cost [17]. The detailed description of the Malachite green dye is provided in Table 2.
Structure:
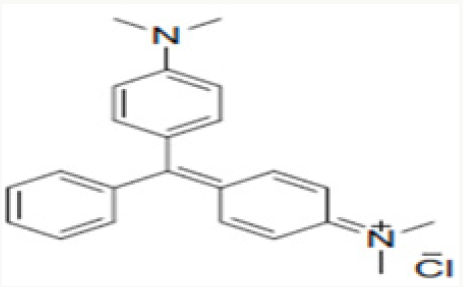
The chemical formula of the Malachite green is C23H25N2, the parasites and disease. The use has increased so much because of basic use of this organic component is in dye industry where it has been used for the dye of materials like silk, leather and paper. The structure of the Malachite green is elaborated in Figure 9..
Dye Degradation
The degradation of malachite green in the absence and presence of CuO NPs were studied spectrophotometrically by using DB-20 UVVis spectrophotometer determining the decrease in the absorbance at 631nm.The reaction was to study spectrophotometrically at room temperature (250C). The colour of the reaction mixtures faded, indicating that degradation had occurred. The same procedure was followed for uncatalyzed reactions, in absence of CuO NPs.
Time Effect on Dye Removal
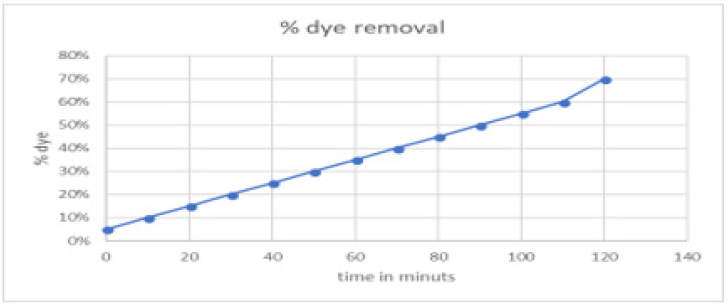
Decolourization of dye Malachite Green at room temperature was analyzed. Initially 20ml dye solution was taken and 1mg of greenly synthesized copper nanoparticles using Aloe Vera leaves extract dissolved in it. 0.1mg of NaBH4 was dissolved as a reducing agent. The solution was heated for 10-20mint at 100 degrees. The time interval was taken in consider gradually during the reaction. The removal percentage of decolourization was calculated and draw graphically. The maximum time was 120mints with 70% colour removal. This confirms the rapid reaction of copper oxide nanoparticles (CuO NPS). The time effect on dye removal has been graphically represented in Figure 10..
pH Effect on Dye Removal
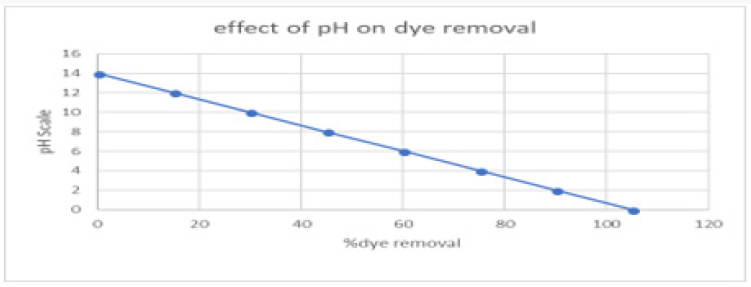
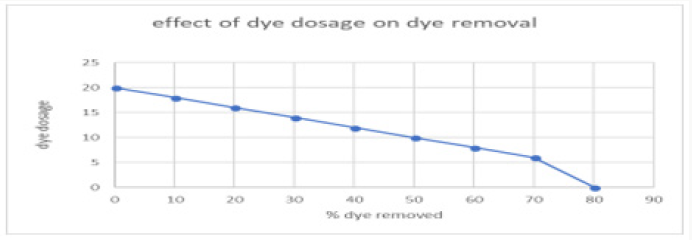
pH of the solution also majorly affected the de-colourization of dye. pH effect on the decolourization of copper oxide nanoparticles was analyzed in this research. Aloe Vera synthesized copper oxide nanoparticles showed maximum percentage de-colourization as pH was increased at a certain limit after more increase has a negative effect. This may be happened due to the formation of more positive ion competition. Maximum decolourization 70% was at pH [5]. The effect of pH on dye removal has been graphically represented in Figure 11..
The Concentration of Dye Effect on Decolourization of Dye:
The increase or decrease in the concentration of Malachite green MG dye is also considerable in decolourization efficiency. The graph was obtained after experimenting with various concentration of dyes. The maximum amount of dye taken was 20mg/l. After increasing concentration, no effect on70decolorization of dye was observed. The concentration of dye effect on decolourization on dye has been graphically represted in Figure 12..
Effect of copper oxide nanoparticles amount on dye removal
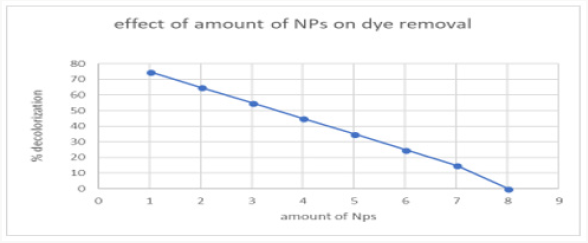
The number of copper oxide nanoparticles exhibits positive results on decolourization. The number of nanoparticles 1 gram was taken, showed maximum de-colourization power. This confirmed from the experiments that increasing the of nanoparticle showed no effect on de-colourization. This concentration of nanoparticles was used in further experimentation of research. The effect of the amount of copper oxide nanoparticles on dye removal has been elaborated in Figure 13..
Figure 13: The catalytic activity of the CuO NPs analyzed by the degradation of malachite green dye.
Microwave Induced Catalytic Studies of Cuo/Zno Nano Composites
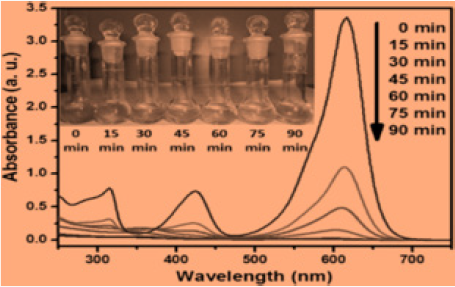
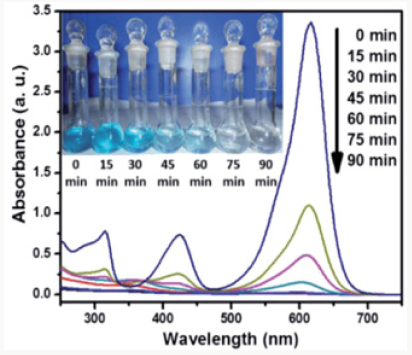
The adsorption and photo catalytic efficiency of cuo/zno nanocomposite were examined for Malachite Green dye degradation. In start 100mg of cuo/zno nano composite were added into 1.8 X 10-5M solution of Malachite green dye [21]. The degradation studies were examined by using a glass beaker and stirred for agitation. During degradation experiments, the suspension was retained in dark to desorption of MG dye, when the equilibrium was gained, the suspension was exposed to microwave for further degradation. The suspension of dye and composite were stirred continuously and exposed to microwave directly. The reaction was compared to study the effect of the conditions with metal nano oxides. In the progress of reaction, 5mL solution was taken at different time intervals and centrifuged. The catalytic degradation of the dye was performed at 620nm uv wavelength. The degradation percentage of dye was calculated using formula as: A-B/A× 100 where A and B are the concentrations of MG dye at equilibrium and at time dye degradation uv graph shown below in Figure 4. microwave induced dye degradation by cuo/zno nanocomposites.
Microwave Induced Degradation of Dye by Nanocomposites
Microwave induced photo degradation of CuO/ZnO nanocomposite were studied at different condition such as adsorption in dark followed by catalysis. in this process of catalysis process, the MG dye solution containing nanocomposite were kept in dark. for next experiment for microwave induced degradation process, the solution was exposed to microwave light to react with dye. The absorption band intensities decrease with irradiation time was observed for malachite green showed that the dye was degraded by nanocomposite. It was examined that only 31% MG dye was degraded in dark while 91% by MG Dye degraded in microwave irradiation. The nano copper oxide nanoparticals efficiency of dye degradation was 70%. The photo degradation of MG dye using cuo/ zno nanocomposite plot of ln Ao/At vs irradiation time predict a linear correlation shown in graph. The MG dyes degradation was pseudo-first-order kinetics. The microwave catalytic degradation of MG dyes by nanocomposite was examined to be 90 % in 90min of microwave irradiation. This reserch indicated the greater degradation of malachite green as compared to MNPs and MNcomposites microwave catalysis. The process generate free radicals to disturb the conjugation in free dye molecules present. The dye adsorption can enhance the degradation process and increases the rate and decrease the time [20].
The supposed mechanism for degradation of dye is shown:
nanocomposite + Dye → nanocomposite -Dye adsorbed (microwave light)
-Dye adsorbed + O2 →cuo/zno composites (O2 • )-Dye O2 -• or OH•
Thus degradation was studied in dark and microwave induced condition. The kinetics of MG dye degradation resulted a linear correlation a. The value of rate constant and rend pseudo first order type. The comparison of degradation of dye by microwave induced catalysis of nano compositees and nanometals undoubtedly prove that the catalysis using metal oxide nanocomposites due to small size has greater percentage removal efficiency than nano oxide metal ( Figure 14.- 19.).
Conclusion
Here, in conclusion, we concluded a method of green synthesis of Cu nanoparticles by leaf extract of Aloe Verabarbadensis plant. This eco-friendly way of synthesis of nanoparticles is more recommended over other methods as green synthesized CuO NPs are cost-effective, biogenic molecules with the capability to serve as dye absorbent. From vast of analyzation on nanotechnology for the synthesis of nanoparticles, it is declared that it is safer and best by using natural plants. With the huge plant variety, much more plants are still not known for the synthesis of nanoparticles. Nanoparticles synthesized can be applicable in the different field of biochemistry, pharma, agriculture and industry. Copper oxide nanoparticles have the ability to remove carcinogenic dyes. In the present study, Malachite green dye was removed by nanoparticles and its time, pH, contact time was observed. The maximum contact time was 120min, pH was observed 5, nanoparticle amount 1mg which proved green synthesized copper nanoparticles, as best removal of carcinogenic dye like Malachite green. CuO/ZnO nanocomposite exibit the excellent photodegradation of malachite green dye. The adsorption and microwave induced catalysis was more effective. the percentage efficiency of degradation of dye was observed efficiently increase by nanocomposites rather than metal oxide nanopartivcals. So, we reviewed comparative potential of copper nano oxide and its composites degradation efficiency, which was observed in nano composite due to reduce size particals.
References
- White RJ, Luque R, Budarin VL, Clark JH, Macquarrie DJ (2009) Supported metal nanoparticles on porous materials. Methods and applications. Chemical Society Reviews 38(2): 481-494.
- Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chemical reviews 104(1): 293- 346.
- Vaidyanathan R, Kalishwaralal K, Gopalram S, Gurunathan S (2009) RETRACTED: Nanosilver-The burgeoning therapeutic molecule and its green synthesis.
- Hussain I, Singh NB, Singh A, Singh H, Singh SC (2016) Green synthesis of nanoparticles and its potential application. Biotechnology letters 38(4): 545-560.
- . Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chemistry 13(10): 2638-2650.
- Dipankar C, Murugan S (2012) The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresineherbstii leaf aqueous extracts. Colloids and Surfaces B: Biointerfaces 98: 112-119.
- Sharma H (2008) Innovative approaches in cosmeceuticals_developing anti_aging topical application forms.
- Joseph B, Raj SJ (2010) Pharmacognostic and phytochemical properties of Aloe veralinn an overview. International journal of pharmaceutical sciences review and research 4(2): 106-110.
- Nandal U, Bhardwaj RL (2012) Aloe vera: A valuable wonder plant for food, medicine and cosmetic use-a review. Int J Pharm Sci Rev Res 13(1): 59-67.
- Agnihotri S, Mukherji S, Mukherji S (2014) Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. Rsc Advances 4(8): 3974-3983.
- Kharissova OV, Dias HR, Kharisov BI, Pérez BO, Pérez VMJ (2013) The greener synthesis of nanoparticles. Trends in biotechnology 31(4): 240- 248
- Sun H, Cao L, Lu L (2011) Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Research 4(6): 550-562.
- Tomasino C (1992) Chemistry & technology of fabric preparation & finishing NC: North Carolina State University pp. 13-30.
- . Alderman DJ (1985) Malachite green: a review. Journal of Fish Diseases 8(3): 289-298.
- Sudova E, Machova, J Svobodova Z, Vesely T (2007) Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a review. Veterinarni Medicina-Praha 52(12): 527.
- https://link.springer.com/article/10.1007%2Fs40097-017-0249-y
- Carmen Z, Daniela S (2012) Textile organic dyes–characteristics, polluting effects and separation/elimination procedures from industrial effluents–a critical overview. In Organic pollutants ten years after the Stockholm convention-environmental and analytical update. In Tech.
- Palajonna Narasaiah, Badal Kumar Mandal, NC Sarada (2017) Biosynthesis of Copper Oxide nanoparticles from Drypetes sepiaria Leaf extract and their catalytic activity to dye degradation. IOP Conf. Series: Materials Science and Engineering 263(2017): 022012.
- . Sadia Saif, Arifa Tahir, Tayyaba Asim, Yongsheng Chen (2016) Plant Mediated Green Synthesis of CuO Nanoparticles: Comparison of Toxicity of Engineered and Plant Mediated CuO Nanoparticles towards Daphnia magna. Nanomaterials 6(11): 205.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...