Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Research Article(ISSN: 2637-6660) 
Recent Development Issues in Nanotechnology for Gas Storage Volume 1 - Issue 5
Dina S Ahmed, Hadeel Adil and Emad Yousif*
- Department of Chemistry, College of Science, Al-Nahrain University, Iraq
Received: September 04, 2018; Published: September 10, 2018
*Corresponding author: Emad Yousif, Department of Chemistry, College of Science, Al-Nahrain University, Baghdad, Iraq
DOI: 10.32474/ANOAJ.2018.01.000121
Abstract
Nanomaterials and their derived sub-groups are emerging into the advanced materials field due to their high free porous volume, structural regularity, robustness, hydrothermal stability, and functional variety. They present high gas uptake capacities and presence of stabilized active functions in the framework. A significant technical challenge has been recognized as the development of a viable method to efficiently trap hydrogen, methane and carbon dioxide gas molecules in a confined space for various applications.
Keywords: Nanomaterials; Gas Storage; Microporous Organic Polymers; Hydrogen; Carbon Dioxide; Methane; Carbon Nanotubes; Clean Energy
Introduction
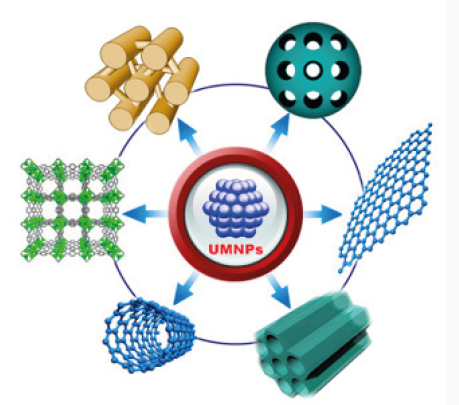
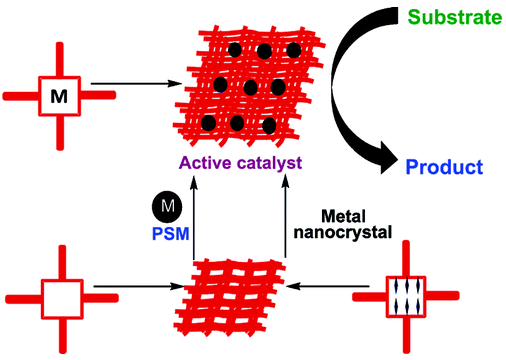
During the past decade, nano-materials have emerged as the new building blocks to construct light energy harvesting assemblies. Organic and inorganic hybrid structures that exhibit improved selectivity and efficiency toward catalytic processes have been designed. Size dependent properties such as size quantization effects in semiconductor nanoparticles and quantized charging effects in metal nanoparticles provide the basis for developing new and effective systems [1,2] (Figure 1). The development of microporous organic polymers (MOPs) with pore size less than 2nm has attracted a great deal of attention [3]. These microporous materials possessing high surface area, low skeleton density, and chemical tunability have opened the door to many potential applications in the area of heterogeneous catalysis [4] (Figure 2). Light harvesting [5], gas separation and storage [6]. For MOPs, the permanent porosity derives from backbone rigidity and spaceinefficient packing of the polymer chains [7]. In this regard, many rigid and contorted monomers such as spirocyclic and tetrahedral compounds have been investigated as successful building blocks in the preparation of soluble polymers of intrinsic microporosity (PIMs) [8] and other insoluble MOPs [9]. The particular advantage of MOPs is to introduce a wide range of useful chemical functionalities into the pores [10]. Furthermore, the embedding of transition metal sites into MOPs could open up second-generation porous materials with useful combined chemical and physical properties [10] and offers potential as heterogeneous catalysts for various organic reactions such as general hydrogenations [11], oxidation of thiols [12], Suzukie Miyaura couplings [13] and so forth [14]. Metal catalytic moieties into functional MOPs has at least two advantages: i) the homogeneous distribution of active metal nanoparticles (NPs) is enabled by the strong interaction with the functional porous supports, which has been believed to give effective catalytic activity and selectivity [15]; ii) the metal leaching could be greatly suppressed in the process of catalyst separation for recycling [16]. Therefore it is highly desirable to investigate methods to incorporate a variety of functionalities into MOPs.
Hydrogen Storage in Nanomaterials
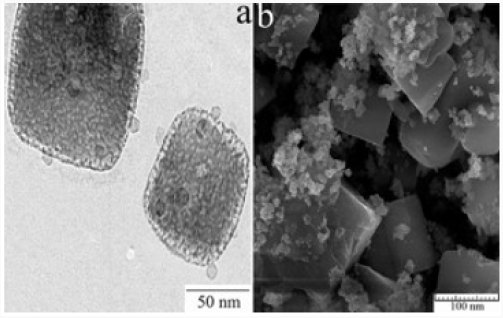
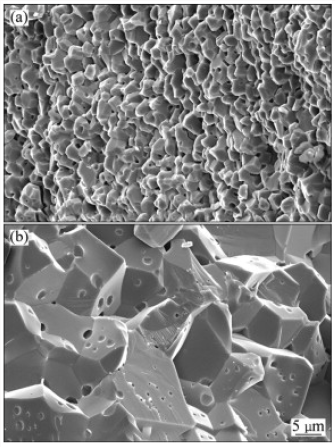
Hydrogen gas is a clean energy source, which can replace fossil fuels while the need for this clean and sustainable energy has been greater [17]. One hopeful way of hydrogen generation increasing is the use of transition metal (0) nanoclusters as catalysts because a large percentage of atoms lie on the surface of activity nanoclusters [18]. Co, Cu, Ni and Fe were chosen as they are among the reactive transition metals toward hydrolysis of boron-based hydrides such as NaBH4. The cheaper transition metal catalysts generally exhibit only moderate catalytic activity. A variety of preparative methods are available for obtaining polymer-stabilized metal nanoclusters [19]. The most widely used synthetic method involves reduction of the metal ion in zero valent state within the polymer medium. Followed by coalescence of the polymer onto the formed nanoclusters a strong stabilizer such as polymer is needed to prevent agglomeration of metal (0) nanoclusters in aqueous solution at high pH medium, acting as a catalyst in the hydrolysis of sodium borohydride [20]. Next attempt was done by investigation of PVP role on size, morphology and catalytic activity of crystalized Co-La-Zr-B nano powder [21] (Figure 3).
Investigate role of oleic acid as stabilizing agent on size and morphology of quaternary nano catalyst [22]. Since their discovery [23,24] carbon nanotubes (CNTs) have continued to attract a huge interest worldwide because of their peculiar physical and chemical properties [25]. In particular, the adsorption behavior of a singlewall carbon nanotube (SWNT), be it chemi- or physisorption, substantially differs from that of graphite or fullerenes, and critically depends on whether the inner or outer surface is exposed and on the tube chirality and diameter. Understanding its characteristics and being able to predict relevant adsorption configurations are important for complementing ongoing experimental efforts in developing covalent sidewall functionalization [26], applications of CNTs as gas sensors [27] and hydrogen storage materials [28].
Carbon Dioxide Storage in Nanomaterials
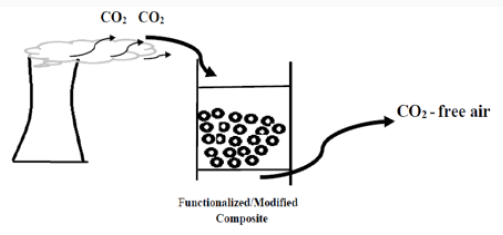
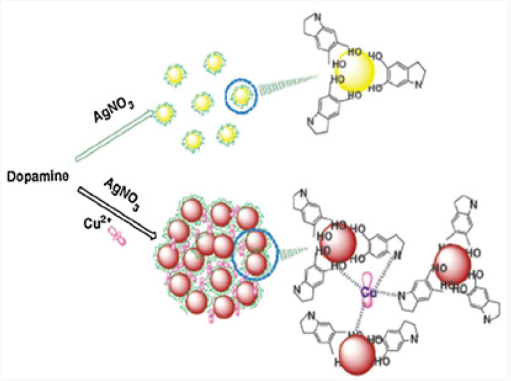
CO2 is one of the major greenhouse gases, and its concentration has been on the rise from 280 ppm to 370 ppm, such that the rising amplitude of the global earth temperature varies from 0.6°C to 1.0°C during the same period of time [29,41,42] (Figure 4). Hydroxyl functionalized MOPs provide the materials with metal ions coordinating ability, ion-exchange as well as reducing properties, and exhibit significant CO2 uptakes at atmospheric pressure even though their surface areas are moderate [30]. For example, a facile route involving phenolic resin inspired chemistry to hyper-cross-linked MOPs with hydroxyl functional groups has been demonstrated by Kanatzidis [30]. Those polymeric frameworks based on the polymerization of phloroglucinol and 1,5-dihydroxynaphthalene with several benzaldehydes under solvothermal conditions capture a maximum of 18 wt% CO2 at 273K and 1 bar, and deposit Ag NPs in situ inside the pores. Catechol and its derivatives have been widely used as ligands or chelating agents in coordination chemistry [31] (Figure 5).
CH4 Storage in Nanomaterials
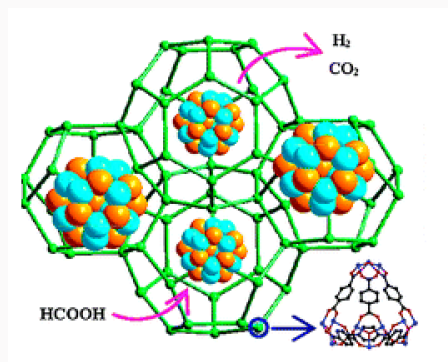
As with hydrogen, methane is also considered a clean energy gas. Compared to petroleum oil, it can provide much more energy because of its higher hydrogen-to-carbon ratio and has much lower carbon emission. In addition, deposits of methanecontaining natural gas are more widespread globally than those of petroleum oil, and its refinement (purification) to an energy fuel is much simpler than that of crude petroleum oil to gasoline or diesel fuels. Methane is also produced by decomposition of organic waste and by bacteria in the guts of ruminants and termites. In terms of near-term practical utilization and innovations necessary for commercialization, methane appears to be a more promising alternative for mobile applications [32]. As an exceptional member of alkaline-earth oxides, beryllium oxide (BeO) can be arisen as an important covalent component in the initial ionic Be-O bond [33]. Synthesized BeO nanoparticles are insulators with a wide band gap about 10.6eV [34] (Figure 6). Recently, the adsorption of some gases such as H2O, CH4, NH3, H2 and CO on BeO nanomaterial has been studied. Nonetheless, several materials, like as aluminum nitride (AlN) nanostructures [35], boron nitride (BN) systems [36] and fullerene clusters [37] boron buckyballs and sheets, B80 [38] have been tested experimentally and theoretically as potential storage materials for hydrogen [39-42] (Figure 7).
Conclusion
Nanomaterials, all suitable morphological characteristics and physicochemical properties to carry out a variety of applications are present: homogeneous porous distribution, high hydrothermal stability and robustness, peculiar hydrophobic–hydrophilic nature, structural regularity, and capability to incorporate different active functions in the planar organic builders. They have shown great promise for the adsorptive storage of hydrogen, methane, and carbon dioxide in clean energy applications.
References
- A Henglein (1989) Small-particle research: Physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem Rev 89(8): 1861-1873.
- ML Steigerwald, LE Brus (1990) Semiconductor crystallites: A class of large molecules. Acc Chem Res 23(6): 183-188.
- R Dawson, AI Cooper, DJ Adams (2012) Nanoporous organic polymer networks. Prog Polym Sci 37(4): 530-563.
- P Kaur, JT Hupp, ST Nguyen (2011) Porous Organic Polymers in Catalysis: Opportunities and Challenges. ACS Catal 1(7): 819-835.
- L Chen, Y Honsho, S Seki, DL Jiang (2010) Light-Harvesting Conjugated Microporous Polymers: Rapid and Highly Efficient Flow of Light Energy with a Porous Polyphenylene Framework as Antenna. J Am Chem Soc 132(19): 6742-6748.
- HB Park, CH Jung, YM Lee, AJ Hill, SJ Pas, et al. (2007) Polymers with Cavities Tuned for Fast Selective Transport of Small Molecules and Ions Science 318: 254-258.
- NB McKeown, PM Budd, KJ Msayib, BS Ghanem, HJ Kingston, et al. (2005) Polymers of Intrinsic Microporosity (PIMs): Bridging the Void between Microporous and Polymeric Materials. Chem Eur J 11(9): 2610-2620.
- PM Budd, NB McKeown, D Fritsch (2005) Free volume and intrinsic microporosity in polymers. J Mater Chem 15: 1977-1986.
- T Ben, H Ren, SQ Ma, DP Cao, JH Lan, et al. (2009) Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area. Angew Chem Int Ed 48(50): 9457-9460.
- JX Jiang, C Wang, A Laybourn, T Hasell, R Clowes, et al. (2011) Metal- Organic Conjugated Microporous Polymers. Angew Chem Int Ed 123: 1104-1107.
- J Schmidt, J Weber, JD Epping, M Antonietti, A Thomas (2009) Microporous Conjugated Poly (thienylene arylene) Networks. Adv Mater 21(6): 702-705.
- L Chen, Y Yang, DL Jiang (2010) CMPs as Scaffolds for Constructing Porous Catalytic Frameworks: A Built-in Heterogeneous Catalyst with High Activity and Selectivity Based on Nanoporous Metalloporphyrin Polymers. J Am Chem Soc 132(26): 9138-9143.
- P Zhang, ZH Weng, J Guo, CC Wang (2011) Solution-Dispersible, Colloidal, Conjugated Porous Polymer Networks with Entrapped Palladium Nanocrystals for Heterogeneous Catalysis of the Suzuki- Miyaura Coupling Reaction. Chem Mater 23(23): 5243-5249.
- Z Xie, C Wang, KE deKrafft, W Lin (2011) Highly Stable and Porous Cross- Linked Polymers for Efficient Photocatalysis. J Am Chem Soc 133(7): 2056-2059.
- CE Chan-Thaw, A Villa, P Katekomol, DS Su, A Thomas, et al. (2010) Covalent Triazine Framework as Catalytic Support for Liquid Phase Reaction. Nano Lett 10(2): 537-541.
- YM Lu, HZ Zhu, WG Li, B Hu, SH Yu (2013) Size-controllable palladium nanoparticles immobilized on carbon nanospheres for nitroaromatic hydrogenation. J Mater Chem A 1: 3783-3788.
- L Schlapbach, A Züttel (2001) Hydrogen-storage materials for mobile applications. Nature 414: 353-358.
- S Ozkar (2009) ChemInform Abstract: Enhancement of catalytic activity by increasing surface area in heterogeneous catalysis. Appl Surf Sci 256(5): 1272-1277.
- ABR Mayer (2001) Colloidal metal nanoparticles dispersed in amphiphilic polymers. Polym Adv Technol 12(1-2): 96-106.
- YT Vu, JE Mark (2004) Polymer-protected palladium nanoparticles. Morphologies and catalytic selectivities. Colloid Polym Sci 282(6): 613- 619.
- MH Loghmani, AF Shojaei (2013) Hydrogen generation from hydrolysis of sodium borohydride by cubic Co-La-Zr-B nano particles as novel catalyst. Int J Hydrogen Energy 38: 10470-10478.
- MH Loghmani, AF Shojaei (2014) Hydrogen production through hydrolysis of sodium borohydride: oleic acid stabilized Co-La-Zr-B nanoparticle as a novel catalyst. Energy 68: 152-159.
- S Iijima (1991) Helical microtubules of graphitic carbon. Nature 354: 56-58.
- A Alwash, H Adil, Z Hussain, E Yousif (2018) Potential of Carbon Nanotubes in Enhance of Photocatalyst Activity. Arch Nano Op Acc J 1(3): 65-70.
- T Tasis, N Tagmatarchis, A Bianco, M Prato (2006) Chemistry of Carbon Nanotubes. Chem Rev 106(3): 1105-1136.
- A Hirsch (2002) Functionalization of Single‐Walled Carbon Nanotubes. Angew Chem Int Ed 41: 1853-1859.
- J Kong, M Chapline, H Dai (2001) Functionalized Carbon Nanotubes for Molecular Hydrogen Sensors. Adv Mater 13(18): 1384-1386.
- F Lamari Darkrim, P Malbrunot, GP Tartaglia (2002) Review of hydrogen storage by adsorption in carbon nanotubes. Int J Hydrogen Energy 27(2): 193-202.
- AL Yaumi, MZA Bakar, BH Hameed (2017) Recent advances in functionalized composite solid materials for carbon dioxide capture. Energy 124(1): 461-480.
- AP Katsoulidis, MG Kanatzidis (2011) Phloroglucinol Based Microporous Polymeric Organic Frameworks with −OH Functional Groups and High CO2 Capture Capacity. Chem Mater 23(7): 1818-1824.
- E Faure, C Falentin-Daudre, C Jerome, J Lyskawa, D Fournier, et al. (2013) Catechols as versatile platforms in polymer chemistry. Prog Polym Sci 38(1): 236-270.
- S Ma (2008) Gas Adsorption Applications of Porous Metal-Organic Frameworks. Degruyter 81(12).
- K Joshi, R Jain, R Pandya, B Ahuja, B Sharma (1999) Compton profile study of bonding in BeO. J Chem Phys 111: 163-167.
- Xf Wang, Rc Wang, Cq Peng, Tt Li, L Bing (2010) Synthesis and sintering of beryllium oxide nanoparticles. Prog Natur Sci Mater Int 20: 81-86.
- Q Wang, Q Sun, P Jena, Y Kawazoe (2009) Potential of AlN nanostructures as hydrogen storage materials. ACS nano 3(3): 621-626.
- AA Peyghan, SA Aslanzadeh, A Samiei (2014) Ammonia borane reaction with a BN nanotube: a hydrogen storage route, Monatshefte für Chemie- Chem. Month 145(7): 1083-1087.
- YH Kim, Y Zhao, A Williamson, MJ Heben, S Zhang (2006) Nondissociative adsorption of H2 molecules in light-element-doped fullerenes. Phys Rev lett 96(1): 016102.
- S Er, GA De Wijs, G Brocks (2009) DFT study of planar boron sheets: a new template for hydrogen storage. J Phys Chem C 113(43): 18962- 18967.
- X Gu, ZH Lu, HL Jiang, T Akita, Q Xu (2011) Synergistic catalysis of metal-organic framework-immobilized Au-Pd nanoparticles in dehydrogenation of formic acid for chemical hydrogen storage. J Am Chem Soc 133(3): 11822-11825.
- Z Qi-Long, X Qiang (2016) Immobilization of Ultrafine Metal Nanoparticles to High-Surface-Area Materials and Their Catalytic Applications. Chem 1(2): 220-245.
- DS Ahmed, GA El-Hiti, E Yousif, AA Ali, AS Hameed (2018) Design and synthesis of porous polymeric materials and their applications in gas capture and storage: a review. Journal of Polymer Research 25: 75.
- DS Ahmed, GA El-Hiti, E Yousif, AS Hameed, M Abdalla (2017) New ecofriendly phosphorus organic polymers as gas storage media. Polymers 9(8): 336.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...