Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Research Article(ISSN: 2637-6660) 
Preparation and Evaluation of Mefenamic Acid Nanoparticles by Nanoprecipitation Technique Volume 2 - Issue 1
Nidha Begum and A Krishna Sailaja*
- Department of pharmaceutics, RBVRR Women’s college of pharmacy, Osmania University, Hyderabad, India
Received:June 27, 2019; Published:July 29, 2019
*Corresponding author:Nidha Begum and A Krishna Sailaja,Department of pharmaceutics, RBVRR Women’s college of pharmacy, Osmania University, Hyderabad, India
DOI: 10.32474/ANOAJ.2019.02.000128
Abstract
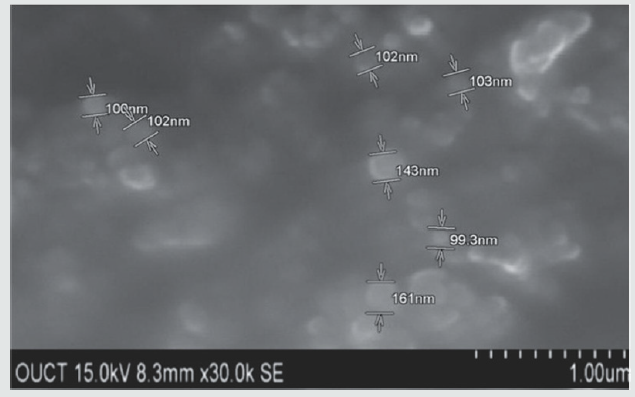
The aim of the present investigation is to prepare mefenamic acid loaded ethyl cellulose nanoparticles by Nano precipitation technique. The obtained nanoparticles were evaluated for product yield, drug content, entrapment efficiency and loading capacity. The drug content was found to be 69.3%. The entrapment efficiency and loading capacity were observed as 88.82%and 60.74% respectively. The SEM images indicates the formation of nanoparticles.
Keywords: Entrapment Efficiency, Loading Capacity, Scanning Electron Microscope
Introduction
Advanced drug delivery systems have numerous advantages over conventional multi dose therapy. Much research effort in developing such drug delivery systems has been focused on controlled release and sustained release dosage forms. Now a day effort is being made to deliver the drug in such a manner so as to get optimum benefits [1,2]. There are numerous strategies in delivering therapeutic agent to the target site in a sustained release fashion. One such strategy is using nanoparticles as drug carrier [3,4]. Nanoparticles have increasing interest from every branch of medicine for the ability to deliver drugs in the optimum dosage range, often resulting in increased therapeutic efficiency of the drug and weakened side effects. Generally, nanoparticles are defined as solid colloidal particles that include both nanospheres and nanocapsules. They can be prepared by both polymerization methods and synthesis from preformed polymers. One of the fundamental characteristics is their size, which is taken around 10nm to1000nm range [5,6]. As stated by various authors they can improve the stability of active substances and can be biodegradable with tissue and cells when synthesized from materials that are either biocompatible or biodegradable. Biocompatible nanoparticles as drug delivery vehicles provide several advantages, including high drug entrapment, protection of the encapsulated therapeutic substance, improved efficacy, weakened adverse effects, controlled release and drug targeting. Copious techniques are available for the preparation of polymeric nanoparticles [7,8]. Nanoprecipitation method is one of the easiest techniques. In the nanoprecipitation process, particle formation is spontaneous, because of the rapid precipitation of preformed drug-polymer organic solution in the aqueous environment in the presence or absence of a surfactant [9,10]. Polymer deposition on the interface between the water and organic solvent, caused by fast diffusion of the solvent leads to the instantaneous formation of a colloidal suspension. This solvent displacement technique allows the preparation of nanocapsules when a small volume of non-toxic oil is incorporated in the organic phase. Considering the oil-based central cavities of nanocapsules, high loading efficiencies are generally reported for lipophilic drugs when nanocapsules are prepared [11,12]. The development of polymeric biomaterials is more or less a recent phenomenon. Polymers are gaining an access over the field of drug delivery. Polymers bear various pharmaceutical applications including their use as binders and coating agent in tablets, viscosity and flow controlling agents in liquid oral formulations, release retarding agent in many novel drug delivery systems and so forth. An appropriate selection of the polymer is necessary in order to develop a successful drug delivery system. The three key advantages that polymeric drug delivery products are: Localized drug delivery, Sustained drug delivery, Stabilization of the drug. The polymers could be biodegradable or non-biodegradable. Following are some of the natural and synthetic polymers used for nanoparticle preparation [13]. The sustained release of drug from the polymeric matrix could occur either by diffusion of the drug from the polymer matrix, or by the erosion of the polymer (due to degradation) or by a combination of the two mechanisms. Therefore, the choice of the polymer is central to optimize the final properties of nanoparticles designed for a specific application. This choice has to be guided by a series of factors such as physico-chemical parameters of the organic compound, chemical composition nanoparticles diameter, structure, morphology, or environmental consideration [14]. this study Mefenamic acid was formulated as nanoparticle drug delivery system using Ethyl cellulose and Eudragit®S100 as polymers. Mefenamic acid is a widely prescribed NSAID and used as first line therapy for the treatment of ailments such as Arthritis and Dysmonorrhoea. Mefenamic acid is a Non-steroidal anti-inflammatory drug (NSAID), with analgesic, and anti-pyretic properties. It is considered to be a BCS Class II drug (low soluble and high permeable). Mefenamic acid binds with prostaglandin synthetase receptors COX-1 and COX-2, inhibiting the action of prostaglandin synthetase. As these receptors have a role as a major mediator of inflammation, the symptoms of pain are temporarily reduced. Mefenamic acid has less biological half life (t1/2) of 2hrs and being an NSAID has a major side effect of gastric irritation. Formulating such drug into nanoparticles using biocompatible polymers is expected to increase the sustain release action and to improve patient compliance with fewer side effects.
Materials and Methodology
Mefenamic acid and Ethyl cellulose were obtained from S.D Fine Chemicals. Pvt.Ltd.. Acetone was supplied by S.D Fine Chemicals. Pvt. Ltd.
Experimental methodology
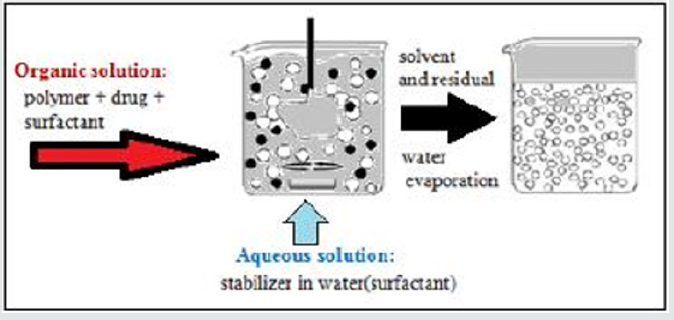
For the preparation of aspirin nanoparticles Nano precipitation technique was adapted. Drug and polymer were dissolved in Acetone and sonicated for 10-15 minutes. The drug-polymer solution was added drop by drop in to 0.6% PVA solution under continuous mechanical stirring at 700 rpm. Spontaneous precipitate formation can be observed. After 4hrs of continuous stirring the solvent from the resultant precipitate is removed by rotary evaporation. Free flowing amorphous nanoparticles were obtained [15] (Figure 1).
Results and Discussion
A. Surface morphology studies using SEM
B. Drug content
C. Entrapment efficiency
D. Loading capacity
Study of surface morphology of nanoparticles by scanning electron microscope (SEM)
Morphological characterization of the nanoparticles was carried using scanning electron microscopy (SEM-S-3700N). For SEM the double – sided sticking tape and coated with gold film (thickness 200nm) under the reduced pressure (0.001torr). The sample for the SEM analysis was prepared by sprinkling the nanoparticles on one side of double adhesive stub. The nanoparticles were viewed at an accelerating voltage of 15-20kv.The prepared amorphous nanoparticles were dispersed in deionised water and sonicated for 30 minutes. A circular metal plate is taken on to which carbon double tape (1mm×1mm) is stickered; a drop of the resultant nano dispersion is placed on to the tape and allowed to dry for a while. Then it is scanned under SEM for morphology [10] (Figure 2).
Drug Content: A known amount of Drug loaded nanoparticles were weighed, then grinded to fine powder and dissolved in a solvent in which the drug is completely soluble i.e., methanol. It was subjected to stirring at 700rpm for 3 hrs. Amount of drug in the supernatant was determined by UV-Spectrophotometric method [11]. The drug content was observed to be 69.3±3%.
Entrapment Efficiency: The nanoparticle formulations were examined for entrapment efficiency. Entrapment efficiency was conducted by taking prepared particles in equivalent quantity of pH 7.4 phosphate buffer [12,13]. The nanoparticles suspension is ultra-centrifuged at 17240rpm and temperature of -4oC for 40 minutes. The entrapment efficiency can be expressed as follow; Entrapment Efficiency = Total amount of the drug entrapped× 100 Total amount of the drug initially taken Loading Capacity = Total amount of the drug entrapped× 100 Total weight of the nanoparticles taken The entrapment efficiency and loading capacity of aspirin loaded ethyl cellulose nanoparticles were observed as 88.82±2%and 60.74±4% respectively.
Conclusion
Mefenamic acid loaded ethyl cellulose nanoparticles were prepared by Nano precipitation technique. The obtained nanoparticles were characterized by Scanning electron microscopy. The images clearly reveals that the particles were in nano range. The drug content was found to be 69±3%. The entrapment efficiency and loading capacity of aspirin loaded ethyl cellulose nanoparticles were observed as 88.82±2%and 60.74±4% respectively. Further studies has to be performed to determine the percentage of drug release.
References
- S Daisy Chella Kumari, CB Tharani, N Narayanan, C Senthil Kumar (2013) Formulation and characterization of Methotrexate loaded sodium alginate chitosan Nanoparticles. Indian Journal of Research in Pharmacy and Biotechnology 1(6): 915-921.
- Sundar Lal Tripathi, Kapil Rana, Vaibhav Rathore, Abhinav Kumar (2013) Development and Optimization of Ranitidine Hydrochloride Nanospheres by 3 2 Factorial Design. International Journal of Pharma Professionals Research 4(4): 928-932.
- Sanjay J Kshirsagar (2013) Nanoparticle for colon specific drug delivery system. Journal of Nanomedicine & Nanotechnology 4(6).
- Sarita Kumari Yadav, Shivani Mishra, Brahmeshwar Mishra (2012) Eudragit-Based Nanosuspension of Poorly Water-Soluble Drug: Formulation and In Vitro–In Vivo AAPS Pharm Sci Technology 13(4): 1031-1044.
- Sahu S, Saraf S, Kaur CD (2013) Biocompatible nanoparticles for sustained topical delivery of anticancer phytoconstituent quercetin. Pakistan Journal of Biological Science 16(13): 601-609.
- Simar preet Kaur, Rekha Rao, Afzal Hussain (2011) Preparation and Characterization of Rivastigmine Loaded Chitosan Nanoparticles. Journal of Pharmaceutical Science & Research 3(5): 1227-1232.
- Sarei F, Dounighi NM, Zolfagharian H, Khaki P, Bidhendi SM (2013) Alginate Nanoparticles as a Promising Adjuvant and Vaccine Delivery System. Indian Journal of Pharmaceutical Science 75(4): 442-449.
- Ji Yeon Jun, Hoang Hai Nguyen, Sae-Yeol-Rim Paik, Hyang Sook Chun, Byeong-Cheol Kang (2011) Preparation of Size-controlled Bovine serum albumun (BSA) Nanoparticles By a modified desolvation method. Food Chemistry 127(4): 1892-1898.
- M Sivabalan (2011) Formulation and evaluation of 5-Flurouracil loaded chitosan and Eudragit R nanoparticles for cancer therapy. International journal of Comprehensive Pharmacy. Pharmacie Globale 1(7): 1-4.
- Swarnali Das, Preeti K Suresh, Deshmukh R (2010) Design of Eudragit RL 100 nanoparticles by Nanoprecipitation method for ocular drug delivery. Nanomedicine: Nanotechnology 6(2): 318-323.
- Zhang HM, Lou K, Cao J, Wang (2014) Interaction of a hydrophobic-functionalized PAMAM dendrimer with bovine serum albumin: Thermodynamic and structural changes. Langmuir 30(19): 5536–5544.
- Naheed Begum, Krishna A (2015) Sailaja Effect of formulation variables on the Preparation of Ibuprofen loaded polymeric nanoparticles. Pharmaceutical Nanotechnology 3(2): 111-121.
- Abbaraju Krishna Sailaja, Marneni Shreya (2018) Preparation and Characterization of Naproxen Loaded Niosomes by Ether Injection Method. Nano Biomedicine and Engineering 10(2): 174-180.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...